-
Accolate Tablets (Astrazeneca)
DESCRIPTION
Zafirlukast is a synthetic, selective peptide leukotriene receptor antagonist (LTRA), with the chemical name 4-(5-cyclopentyloxy-carbonylamino-1-methyl-indol-3-ylmethyl)-3-methoxy-N-o-tolylsulfonylbenzamide. The molecular weight of zafirlukast is 575.7 and the structural formula is:
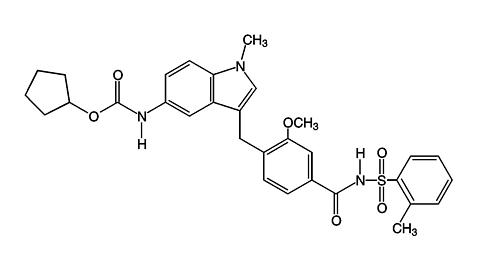
The empirical formula is: C 31 H 33 N 3 O 6 S
Zafirlukast, a fine white to pale yellow amorphous powder, is practically insoluble in water. It is slightly soluble in methanol and freely soluble in tetrahydrofuran, dimethylsulfoxide, and acetone.
ACCOLATE is supplied as 10 and 20 mg tablets for oral administration.
Inactive Ingredients: Film-coated tablets containing croscarmellose sodium, lactose, magnesium stearate, microcrystalline cellulose, povidone, hypromellose, and titanium dioxide.
CLINICAL PHARMACOLOGY
Mechanism of Action
Zafirlukast is a selective and competitive receptor antagonist of leukotriene D 4 and E 4 (LTD 4 and LTE 4 ), components of slow-reacting substance of anaphylaxis (SRSA). Cysteinyl leukotriene production and receptor occupation have been correlated with the pathophysiology of asthma, including airway edema, smooth muscle constriction, and altered cellular activity associated with the inflammatory process, which contribute to the signs and symptoms of asthma. Patients with asthma were found in one study to be 25-100 times more sensitive to the bronchoconstricting activity of inhaled LTD 4 than nonasthmatic subjects.
In vitro studies demonstrated that zafirlukast antagonized the contractile activity of three leukotrienes (LTC 4 , LTD 4 and LTE 4 ) in conducting airway smooth muscle from laboratory animals and humans. Zafirlukast prevented intradermal LTD 4 -induced increases in cutaneous vascular permeability and inhibited inhaled LTD 4 -induced influx of eosinophils into animal lungs. Inhalational challenge studies in sensitized sheep showed that zafirlukast suppressed the airway responses to antigen; this included both the early- and late-phase response and the nonspecific hyperresponsiveness.
In humans, zafirlukast inhibited bronchoconstriction caused by several kinds of inhalational challenges. Pretreatment with single oral doses of zafirlukast inhibited the bronchoconstriction caused by sulfur dioxide and cold air in patients with asthma. Pretreatment with single doses of zafirlukast attenuated the early- and late-phase reaction caused by inhalation of various antigens such as grass, cat dander, ragweed, and mixed antigens in patients with asthma. Zafirlukast also attenuated the increase in bronchial hyperresponsiveness to inhaled histamine that followed inhaled allergen challenge.
Clinical Pharmacokinetics and Bioavailability:
Absorption
Zafirlukast is rapidly absorbed following oral administration. Peak plasma concentrations are generally achieved 3 hours after oral administration. The absolute bioavailability of zafirlukast is unknown. In two separate studies, one using a high fat and the other a high protein meal, administration of zafirlukast with food reduced the mean bioavailability by approximately 40%.
Distribution
Zafirlukast is more than 99% bound to plasma proteins, predominantly albumin. The degree of binding was independent of concentration in the clinically relevant range. The apparent steady-state volume of distribution (V SS /F) is approximately 70 L, suggesting moderate distribution into tissues. Studies in rats using radiolabeled zafirlukast indicate minimal distribution across the blood-brain barrier.
Metabolism
Zafirlukast is extensively metabolized. The most common metabolic products are hydroxylated metabolites which are excreted in the feces. The metabolites of zafirlukast identified in plasma are at least 90 times less potent as LTD 4 receptor antagonists than zafirlukast in a standard in vitro test of activity. In vitro studies using human liver microsomes showed that the hydroxylated metabolites of zafirlukast excreted in the feces are formed through the cytochrome P450 2C9 (CYP2C9) pathway. Additional in vitro studies utilizing human liver microsomes show that zafirlukast inhibits the cytochrome P450 CYP3A4 and CYP2C9 isoenzymes at concentrations close to the clinically achieved total plasma concentrations (see Drug Interactions ).
Excretion
The apparent oral clearance (CL/f) of zafirlukast is approximately 20 L/h. Studies in the rat and dog suggest that biliary excretion is the primary route of excretion. Following oral administration of radiolabeled zafirlukast to volunteers, urinary excretion accounts for approximately 10% of the dose and the remainder is excreted in feces. Zafirlukast is not detected in urine.
In the pivotal bioequivalence study, the mean terminal half-life of zafirlukast is approximately 10 hours in both normal adult subjects and patients with asthma. In other studies, the mean plasma half-life of zafirlukast ranged from approximately 8 to 16 hours in both normal subjects and patients with asthma. The pharmacokinetics of zafirlukast are approximately linear over the range from 5 mg to 80 mg. Steady-state plasma concentrations of zafirlukast are proportional to the dose and predictable from single-dose pharmacokinetic data. Accumulation of zafirlukast in the plasma following twice-daily dosing is approximately 45%.
The pharmacokinetic parameters of zafirlukast 20 mg administered as a single dose to 36 male volunteers are shown with the table below.
Mean (% Coefficient of Variation) pharmacokinetic
parameters of zafirlukast following single 20 mg
oral dose administration to male volunteers (n=36)C max
ng/mLt max hAUC
ng·h/mLt 1/2
hCL/f
L/h326 (31.0)2 (0.5-5.0)1137 (34)13.3 (75.6)19.4 (32)1 Median and range
Special Populations
Gender: The pharmacokinetics of zafirlukast are similar in males and females. Weight-adjusted apparent oral clearance does not differ due to gender.
Race: No differences in the pharmacokinetics of zafirlukast due to race have been observed.
Elderly: The apparent oral clearance of zafirlukast decreases with age. In patients above 65 years of age, there is an approximately 2-3 fold greater C max and AUC compared to young adult patients.
Children: Following administration of a single 20 mg dose of zafirlukast to 20 boys and girls between 7 and 11 years of age, and in a second study, to 29 boys and girls between 5 and 6 years of age, the following pharmacokinetic parameters were obtained:
ParameterChildren age
5-6 years
Mean (% Coefficient
of Variation)Children age
7-11 years
Mean (% Coefficient
of Variation)C max (ng/mL)756 (39%) 601 (45%) AUC (ng·h/mL)2458 (34%) 2027 (38%) t max (h)2.1 (61%) 2.5 (55%) CL/f (L/h)9.2 (37%) 11.4 (42%) Weight unadjusted apparent clearance was 11.4 L/h (42%) in the 7-11 year old children and 9.2 L/h (37%) in the 5-6 year old children, which resulted in greater systemic drug exposures than that obtained in adults for an identical dose. To maintain similar exposure levels in children compared to adults, a dose of 10 mg twice daily is recommended in children 5-11 years of age (see DOSAGE AND ADMINISTRATION ).
Zafirlukast disposition was unchanged after multiple dosing (20 mg twice daily) in children and the degree of accumulation in plasma was similar to that observed in adults.
Hepatic Insufficiency: In a study of patients with hepatic impairment (biopsy-proven cirrhosis), there was a reduced clearance of zafirlukast resulting in a 50-60% greater C max and AUC compared to normal subjects.
Renal Insufficiency: Based on a cross-study comparison, there are no apparent differences in the pharmacokinetics of zafirlukast between renally-impaired patients and normal subjects.
Drug-Drug Interactions
The following drug interaction studies have been conducted with zafirlukast (see PRECAUTIONS , Drug Interactions ).
- Coadministration of multiple doses of zafirlukast (160 mg/day) to steady-state with a single 25 mg dose of warfarin (a substrate of CYP2C9) resulted in a significant increase in the mean AUC (+63%) and half-life (+36%) of S-warfarin. The mean prothrombin time increased by approximately 35%. The pharmacokinetics of zafirlukast were unaffected by coadministration with warfarin.
- Coadministration of zafirlukast (80 mg/day) at steady-state with a single dose of a liquid theophylline preparation (6 mg/kg) in 13 asthmatic patients, 18 to 44 years of age, resulted in decreased mean plasma concentrations of zafirlukast by approximately 30%, but no effect on plasma theophylline concentrations was observed.
- Coadministration of zafirlukast (20 mg/day) or placebo at steady-state with a single dose of sustained release theophylline preparation (16 mg/kg) in 16 healthy boys and girls (6 through 11 years of age) resulted in no significant differences in the pharmacokinetic parameters of theophylline.
- Coadministration of zafirlukast dosed at 40 mg twice daily in a single-blind, parallel-group, 3-week study in 39 healthy female subjects taking oral contraceptives, resulted in no significant effect on ethinyl estradiol plasma concentrations or contraceptive efficacy.
- Coadministration of zafirlukast (40 mg/day) with aspirin (650 mg four times daily) resulted in mean increased plasma concentrations of zafirlukast by approximately 45%.
- Coadministration of a single dose of zafirlukast (40 mg) with erythromycin (500 mg three times daily for 5 days) to steady-state in 11 asthmatic patients resulted in decreased mean plasma concentrations of zafirlukast by approximately 40% due to a decrease in zafirlukast bioavailability.
Clinical Studies
Three U.S. double-blind, randomized, placebo-controlled, 13-week clinical trials in 1380 adults and children 12 years of age and older with mild-to-moderate asthma demonstrated that ACCOLATE improved daytime asthma symptoms, nightime awakenings, mornings with asthma symptoms, rescue beta 2 -agonist use, FEV 1 , and morning peak expiratory flow rate. In these studies, the patients had a mean baseline FEV 1 of approximately 75% of predicted normal and a mean baseline beta 2 -agonist requirement of approximately 4-5 puffs of albuterol per day. The results of the largest of the trials are shown in the table below.
Mean Change from Baseline at Study End Point ACCOLATE
20 mg twice
daily
N=514Placebo
N=248Daytime Asthma symptom score
(0-3 scale)-0.44 *-0.25Nighttime Awakenings
(number per week)-1.27 *-0.43Mornings with Asthma Symptoms
(days per week)-1.32 *-0.75Rescue (beta) 2 -agonist use
(puffs per day)-1.15 *-0.24FEV 1 (L)+0.15 *+0.05Morning PEFR (L/min)+22.06 *+7.63Evening PEFR (L/min)+13.12+10.14*p<0.05, compared to placebo
In a second and smaller study, the effect of ACCOLATE on most efficacy parameters was comparable to the active control (inhaled cromolyn sodium 1600 mcg four times per day) and superior to placebo at end point for decreasing rescue beta 2 -agonist use (figure below).
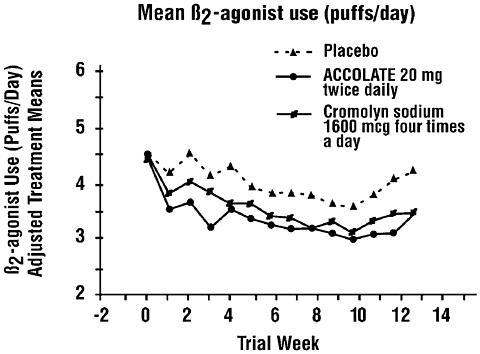
In these trials, improvement in asthma symptoms occurred within one week of initiating treatment with ACCOLATE. The role of ACCOLATE in the management of patients with more severe asthma, patients receiving antiasthma therapy other than as-needed, inhaled beta 2 -agonists, or as an oral or inhaled corticosteroid-sparing agent remains to be fully characterized.
INDICATIONS AND USAGE
ACCOLATE is indicated for the prophylaxis and chronic treatment of asthma in adults and children 5 years of age and older.
CONTRAINDICATIONS
ACCOLATE is contraindicated in patients who are hypersensitive to zafirlukast or any of its inactive ingredients.
WARNINGS
Hepatotoxicity:
Cases of life-threatening hepatic failure have been reported in patients treated with ACCOLATE. Cases of liver injury without other attributable cause have been reported from post-marketing adverse event surveillance of patients who have received the recommended dose of ACCOLATE (40 mg/day). In most, but not all post-marketing reports, the patient's symptoms abated and the liver enzymes returned to normal or near normal after stopping ACCOLATE. In rare cases, patients have either presented with fulminant hepatitis or progressed to hepatic failure, liver transplantation and death.
Physicians may consider the value of liver function testing. Periodic serum transaminase testing has not proven to prevent serious injury but it is generally believed that early detection of drug-induced hepatic injury along with immediate withdrawal of the suspect drug enhances the likelihood for recovery.
Patients should be advised to be alert for signs and symptoms of liver dysfunction (eg, right upper quadrant abdominal pain, nausea, fatigue, lethargy, pruritus, jaundice, flu-like symptoms, and anorexia)) and to contact their physician immediately if they occur. Ongoing clinical assessment of patients should govern physician interventions, including diagnostic evaluations and treatment.
If liver dysfunction is suspected based upon clinical signs or symptoms (eg, right upper quadrant abdominal pain, nausea, fatigue, lethargy, pruritus, jaundice, flu-like symptoms, anorexia, and enlarged liver), ACCOLATE should be discontinued. Liver function tests, in particular serum ALT, should be measured immediately and the patient managed accordingly. If liver function tests are consistent with hepatic dysfunction. ACCOLATE therapy should not be resumed. Patients in whom ACCOLATE was withdrawn because of hepatic dysfunction where no other attributable cause is identified should not be re-exposed to ACCOLATE (see PRECAUTIONS , Information for Patients and ADVERSE REACTIONS ).
Bronchospasm:
ACCOLATE is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmaticus. Therapy with ACCOLATE can be continued during acute exacerbations of asthma.
Concomitant Warfarin Administration:
Coadministration of zafirlukast with warfarin results in a clinically significant increase in prothrombin time (PT). Patients on oral warfarin anticoagulant therapy and ACCOLATE should have their prothrombin times monitored closely and anticoagulant dose adjusted accordingly (see PRECAUTIONS , Drug Interactions ).
PRECAUTIONS
Information for Patients
Patients should be told that a rare side effect of ACCOLATE is hepatic dysfunction, and to contact their physician immediately if they experience symptoms of hepatic dysfunction (eg, right upper quadrant abdominal pain, nausea, fatigue, lethargy, pruritus, jaundice, flu-like symptoms, and anorexia). Liver failure resulting in liver transplantation and death has occurred in patients taking zafirlukast (see WARNINGS , Hepatotoxicity and ADVERSE REACTIONS ).
ACCOLATE is indicated for the chronic treatment of asthma and should be taken regularly as prescribed, even during symptom-free periods. ACCOLATE is not a bronchodilator and should not be used to treat acute episodes of asthma. Patients receiving ACCOLATE should be instructed not to decrease the dose or stop taking any other antiasthma medications unless instructed by a physician. Women who are breast-feeding should be instructed not to take ACCOLATE (see PRECAUTIONS , Nursing Mothers ). Alternative antiasthma medication should be considered in such patients.
The bioavailability of ACCOLATE may be decreased when taken with food. Patients should be instructed to take ACCOLATE at least 1 hour before or 2 hours after meals.
Eosinophilic Conditions
In rare cases, patients on ACCOLATE therapy may present with systemic eosinophilia, eosinophilic pneumonia, or clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic steroid therapy. These events usually, but not always, have been associated with the reduction of oral steroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between ACCOLATE and these underlying conditions has not been established (see ADVERSE REACTIONS ).
Drug Interactions
In a drug interaction study in 16 healthy male volunteers, coadministration of multiple doses of zafirlukast (160 mg/day) to steady-state with a single 25 mg dose of warfarin resulted in a significant increase in the mean AUC (+63%) and half-life (+36%) of S-warfarin. The mean prothrombin time (PT) increased by approximately 35%. This interaction is probably due to an inhibition by zafirlukast of the cytochrome P450 2C9 isoenzyme system. Patients on oral warfarin anticoagulant therapy and ACCOLATE should have their prothrombin times monitored closely and anticoagulant dose adjusted accordingly (see WARNINGS , Concomitant Warfarin Administration ). No formal drug-drug interaction studies with ACCOLATE and other drugs known to be metabolized by the cytochrome P450 2C9 isoenzyme (eg, tolbutamide, phenytoin, carbamazepine) have been conducted; however, care should be exercised when ACCOLATE is coadministered with these drugs.
In a drug interaction study in 11 asthmatic patients, coadministration of a single dose of zafirlukast (40 mg) with erythromycin (500 mg three times daily for 5 days) to steady-state resulted in decreased mean plasma levels of zafirlukast by approximately 40% due to a decrease in zafirlukast bioavailability.
Coadministration of zafirlukast (20 mg/day) or placebo at steady-state with a single dose of sustained release theophylline preparation (16 mg/kg) in 16 healthy boys and girls (6 through 11 years of age) resulted in no significant differences in the pharmacokinetic parameters of theophylline.
Coadministration of zafirlukast (80 mg/day) at steady-state with a single dose of a liquid theophylline preparation (6 mg/kg) in 13 asthmatic patients, 18 to 44 years of age, resulted in decreased mean plasma levels of zafirlukast by approximately 30%, but no effect on plasma theophylline levels was observed.
Rare cases of patients experiencing increased theophylline levels with or without clinical signs or symptoms of theophylline toxicity after the addition of ACCOLATE to an existing theophylline regimen have been reported. The mechanism of the interaction between ACCOLATE and theophylline in these patients is unknown (see ADVERSE REACTIONS ).
Coadministration of zafirlukast (40 mg/day) with aspirin (650 mg four times daily) resulted in mean increased plasma levels of zafirlukast by approximately 45%.
In a single-blind, parallel-group, 3-week study in 39 healthy female subjects taking oral contraceptives, 40 mg twice daily of zafirlukast had no significant effect on ethinyl estradiol plasma concentrations or contraceptive efficacy.
No formal drug-drug interaction studies between ACCOLATE and marketed drugs known to be metabolized by the P450 3A4 (CYP3A4) isoenzyme (eg, dihydropyridine calcium-channel blockers, cyclosporin, cisapride) have been conducted. As ACCOLATE is known to be an inhibitor of CYP3A4 in vitro , it is reasonable to employ appropriate clinical monitoring when these drugs are coadministered with ACCOLATE.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In two-year carcinogenicity studies, zafirlukast was administered at dietary doses of 10, 100, and 300 mg/kg to mice and 40, 400, and 2000 mg/kg to rats. Male mice at an oral dose of 300 mg/kg/day (approximately 30 times the maximum recommended daily oral dose in adults and in children on a mg/m 2 basis) showed an increased incidence of hepatocellular adenomas; female mice at this dose showed a greater incidence of whole body histocytic sarcomas. Male and female rats at an oral dose of 2000 mg/kg/day (resulting in approximately 160 times the exposure to drug plus metabolites from the maximum recommended daily oral dose in adults and in children based on a comparison of the plasma area-under the curve [AUC] values) of zafirlukast showed an increased incidence of urinary bladder transitional cell papillomas. Zafirlukast was not tumorigenic at oral doses up to 100 mg/kg (approximately 10 times the maximum recommended daily oral dose in adults and in children on a mg/m 2 basis) in mice and at oral doses up to 400 mg/kg (resulting in approximately 140 times the exposure to drug plus metabolites from the maximum recommended daily oral dose in adults and in children based on a comparison of the plasma AUC values) in rats. The clinical significance of these findings for the long-term use of ACCOLATE is unknown.
Zafirlukast showed no evidence of mutagenic potential in the reverse microbial assay, in 2 forward point mutation (CHO-HGPRT and mouse lymphoma) assays or in two assays for chromosomal aberrations (the in vitro human peripheral blood lymphocyte clastogenic assay and the in vivo rat bone marrow micronucleus assay).
No evidence of impairment of fertility and reproduction was seen in male and female rats treated with zafirlukast at oral doses up to 2000 mg/kg (approximately 410 times the maximum recommended daily oral dose in adults on a mg/m 2 basis).
Pregnancy Category B
No teratogenicity was observed at oral doses up to 1600 mg/kg/day in mice (approximately 160 times the maximum recommended daily oral dose in adults on a mg/m 2 basis), up to 2000 mg/kg/day in rats (approximately 410 times the maximum recommended daily oral dose in adults on a mg/m 2 basis) and up to 2000 mg/kg/day in cynomolgus monkeys (which resulted in approximately 20 times the exposure to drug plus metabolites compared to that from the maximum recommended daily oral dose in adults based on comparison of the AUC values). At an oral dose of 2000 mg/kg/day in rats, maternal toxicity and deaths were seen with increased incidence of early fetal resorption. Spontaneous abortions occurred in cynomolgus monkeys at the maternally toxic oral dose of 2000 mg/kg/day. There are no adequate and well-controlled trials in pregnant women. Because animal reproductive studies are not always predictive of human response, ACCOLATE should be used during pregnancy only if clearly needed.
Nursing Mothers
Zafirlukast is excreted in breast milk. Following repeated 40 mg twice-a-day dosing in healthy women, average steady-state concentrations of zafirlukast in breast milk were 50 ng/mL compared to 255 ng/mL in plasma. Because of the potential for tumorigenicity shown for zafirlukast in mouse and rat studies and the enhanced sensitivity of neonatal rats and dogs to the adverse effects of zafirlukast, ACCOLATE should not be administered to mothers who are breast-feeding.
Pediatric Use
The safety of ACCOLATE at doses of 10 mg twice daily has been demonstrated in 205 pediatric patients 5 through 11 years of age in placebo-controlled trials lasting up to six weeks and with 179 patients in this age range participating in 52 weeks of treatment in an open label extension.
The effectiveness of ACCOLATE for the prophylaxis and chronic treatment of asthma in pediatric patients 5 through 11 years of age is based on an extrapolation of the demonstrated efficacy of ACCOLATE in adults with asthma and the likelihood that the disease course, and pathophysiology and the drug's effect are substantially similar between the two populations. The recommended dose for the patients 5 through 11 years of age is based upon a cross-study comparison of the pharmacokinetics of zafirlukast in adults and pediatric subjects, and on the safety profile of zafirlukast in both adult and pediatric patients at doses equal to or higher than the recommended dose.
The safety and effectiveness of zafirlukast for pediatric patients less than 5 years of age has not been established. The effect of ACCOLATE on growth in children has not been determined.
Geriatric Use
Based on cross-study comparison, the clearance of zafirlukast is reduced in patients 65 years of age and older such that C max and AUC are approximately 2- to 3-fold greater than those of younger patients (see DOSAGE AND ADMINISTRATION and CLINICAL PHARMACOLOGY ).
A total of 8094 patients were exposed to zafirlukast in North American and European short-term placebo-controlled clinical trials. Of these, 243 patients were elderly (age 65 years and older). No overall difference in adverse events was seen in the elderly patients, except for an increase in the frequency of infections among zafirlukast-treated elderly patients compared to placebo-treated elderly patients (7.0% vs. 2.9%). The infections were not severe, occurred mostly in the lower respiratory tract, and did not necessitate withdrawal of therapy.
An open-label, uncontrolled, 4-week trial of 3759 asthma patients compared the safety and efficacy of ACCOLATE 20 mg given twice daily in three patient age groups, adolescents (12-17 years), adults (18-65 years), and elderly (greater than 65 years). A higher percentage of elderly patients (n=384) reported adverse events when compared to adults and adolescents. These elderly patients showed less improvement in efficacy measures. In the elderly patients, adverse events occurring in greater than 1% of the population included headache (4.7%), diarrhea and nausea (1.8%), and pharyngitis (1.3%). The elderly reported the lowest percentage of infections of all three age groups in this study.
ADVERSE REACTIONS
Adults and Children 12 years of age and older
The safety database for ACCOLATE consists of more than 4000 healthy volunteers and patients who received ACCOLATE, of which 1723 were asthmatics enrolled in trials of 13 weeks duration or longer. A total of 671 patients received ACCOLATE for 1 year or longer. The majority of the patients were 18 years of age or older; however, 222 patients between the age of 12 and 18 years received ACCOLATE.
A comparison of adverse events reported by >/=1% of zafirlukast-treated patients, and at rates numerically greater than in placebo-treated patients, is shown for all trials in the table below.
Adverse EventACCOLATE
N=4058PLACEBO
N=2032Headache12.9% 11.7% Infection3.5% 3.4% Nausea3.1% 2.0% Diarrhea2.8% 2.1% Pain (generalized)1.9% 1.7% Asthenia1.8% 1.6% Abdominal Pain1.8% 1.1% Accidental Injury1.6% 1.5% Dizziness1.6% 1.5% Myalgia1.6% 1.5% Fever1.6% 1.1% Back Pain1.5% 1.2% Vomiting1.5% 1.1% SGPT Elevation1.5% 1.1% Dyspepsia1.3% 1.2% The frequency of less common adverse events was comparable between ACCOLATE and placebo.
Rarely, elevations of one or more liver enzymes have occurred in patients receiving ACCOLATE in controlled clinical trials. In clinical trials, most of these have been observed at doses four times higher than the recommended dose. The following hepatic events (which have occurred predominantly in females) have been reported from postmarketing adverse event surveillance of patients who have received the recommended dose of ACCOLATE (40 mg/day): cases of symptomatic hepatitis (with or without hyperbilirubinemia) without other attributable cause; and rarely, hyperbilirubinemia without other elevated liver function tests. In most, but not all postmarketing reports, the patient's symptoms abated and the liver enzymes returned to normal or near normal after stopping ACCOLATE. In rare cases, patients have presented with fulminant hepatitis or progressed to hepatic failure, liver transplantation and death (see WARNINGS , Hepatotoxicity and PRECAUTIONS , Information for Patients .)
In clinical trials, an increased proportion of zafirlukast patients over the age of 55 years reported infections as compared to placebo-treated patients. A similar finding was not observed in other age groups studied. These infections were mostly mild or moderate in intensity and predominantly affected the respiratory tract. Infections occurred equally in both sexes, were dose-proportional to total milligrams of zafirlukast exposure, and were associated with coadministration of inhaled corticosteroids. The clinical significance of this finding is unknown.
In rare cases, patients on ACCOLATE therapy may present with systemic eosinophilia, eosinophilic pneumonia, or clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic steroid therapy. These events usually, but not always, have been associated with the reduction of oral steroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between ACCOLATE and these underlying conditions has not been established (see PRECAUTIONS , Eosinophilic Conditions ).
Hypersensitivity reactions, including urticaria, angioedema and rashes, with or without blistering, have been reported in association with ACCOLATE therapy. Additionally, there have been reports of patients experiencing agranulocytosis, bleeding, bruising, or edema, arthralgia, myalgia, insomnia, malaise, and pruritus in association with ACCOLATE therapy.
Rare cases of patients experiencing increased theophylline levels with or without clinical signs or symptoms of theophylline toxicity after the addition of ACCOLATE to an existing theophylline regimen have been reported. The mechanism of the interaction between ACCOLATE and theophylline in these patients is unknown and not predicted by available in vitro metabolism data and the results of two clinical drug interaction studies (see CLINICAL PHARMACOLOGY and PRECAUTIONS , Drug Interactions ).
Pediatric Patients 5 through 11 years of age
ACCOLATE has been evaluated for safety in 788 pediatric patients 5 through 11 years of age. Cumulatively, 313 pediatric patients were treated with ACCOLATE 10 mg twice daily or higher for at least 6 months, and 113 of them were treated for one year or longer in clinical trials. The safety profile of ACCOLATE 10 mg twice daily-versus placebo in the 4- and 6-week double-blind trials was generally similar to that observed in the adult clinical trials with ACCOLATE 20 mg twice daily.
In pediatric patients receiving ACCOLATE in multi-dose clinical trials, the following events occurred with a frequency of >/=2% and more frequently than in pediatric patients who received placebo, regardless of causality assessment: headache (4.5 vs. 4.2%) and abdominal pain (2.8 vs. 2.3%).
The post-marketing experience in this age group is similar to that seen in adults, including hepatic dysfunction, which may lead to liver failure.
OVERDOSAGE
No deaths occurred at oral zafirlukast doses of 2000 mg/kg in mice (approximately 210 times the maximum recommended daily oral dose in adults and children on a mg/m 2 basis), 2000 mg/kg in rats (approximately 420 times the maximum recommended daily oral dose in adults and children on a mg/m 2 basis), and 500 mg/kg in dogs (approximately 350 times the maximum recommended daily oral dose in adults and children on a mg/m 2 basis).
Overdosage with ACCOLATE has been reported in four patients surviving reported doses as high as 200 mg. The predominant symptoms reported following ACCOLATE overdose were rash and upset stomach. There were no acute toxic effects in humans that could be consistently ascribed to the administration of ACCOLATE. It is reasonable to employ the usual supportive measures in the event of an overdose; eg, remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive therapy, if required.
DOSAGE AND ADMINISTRATION
Because food can reduce the bioavailability of zafirlukast, ACCOLATE should be taken at least 1 hour before or 2 hours after meals.
Adults and Children 12 years of age and older
The recommended dose of ACCOLATE in adults and children 12 years and older is 20 mg twice daily.
Pediatric Patients 5 through 11 years of age
The recommended dose of ACCOLATE in children 5 through 11 years of age is 10 mg twice daily.
Elderly Patients: Based on cross-study comparisons, the clearance of zafirlukast is reduced in elderly patients (65 years of age and older), such that C max and AUC are approximately twice those of younger adults. In clinical trials, a dose of 20 mg twice daily was not associated with an increase in the overall incidence of adverse events or withdrawals because of adverse events in elderly patients.
Patients with Hepatic Impairment: The clearance of zafirlukast is reduced in patients with stable alcoholic cirrhosis such that the C max and AUC are approximately 50-60% greater than those of normal adults. ACCOLATE has not been evaluated in patients with hepatitis or in long-term studies of patients with cirrhosis.
Patients with Renal Impairment: Dosage adjustment is not required for patients with renal impairment.
HOW SUPPLIED
ACCOLATE 10 mg Tablets, (NDC 0310-0401) white, unflavored, round, biconvex, film-coated, mini-tablets identified with "ACCOLATE 10" debossed on one side are supplied in opaque HDPE bottles of 60 tablets and Hospital Unit Dose blister packages of 100 tablets.
ACCOLATE 20 mg Tablets, (NDC 0310-0402) white, round, biconvex, coated tablets identified with "ACCOLATE 20" debossed on one side are supplied in opaque HDPE bottles of 60 tablets and Hospital Unit Dose blister packages of 100 tablets.
Store at controlled room temperature, 20-25°C (68-77°F) [see USP]. Protect from light and moisture. Dispense in the original air-tight container.
ACCOLATE is a trademark of the Astrazeneca group of companies
©Astrazeneca 2001, 2004
Manufactured for:
Astrazeneca Pharmaceuticals LP
Wilmington, DE 19850
By: IPR Pharmaceuticals, Inc.
Carolina, PR 00984
30013-00 Rev 07/04
Subscribe to the "News" RSS Feed 
TOP ۞
