-
Ammonul Injection (Ucyclyd)
DESCRIPTION
AMMONUL® (sodium phenylacetate and sodium benzoate) Injection 10% / 10% is a sterile, concentrated, aqueous solution of sodium phenylacetate and sodium benzoate, used for the treatment of hyperammonemia in urea cycle disorders. The pH of the solution is between 6 and 8. Sodium phenylacetate is a crystalline, white to off-white powder with a strong, offensive odor. It is soluble in water. Sodium benzoate is a white and odorless, crystalline powder that is readily soluble in water.
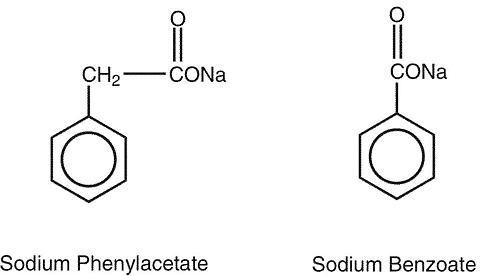
Sodium phenylacetate has a molecular weight of 158.13 and the molecular formula C 8 H 7 NaO 2 . Sodium benzoate has a molecular weight of 144.11 and the molecular formula C 7 H 5 NaO 2 .
Each mL of AMMONUL® contains 100 mg of sodium phenylacetate and 100 mg of sodium benzoate, and Water for Injection. Sodium hydroxide and/or hydrochloric acid may have been used for pH adjustment.
AMMONUL® injection is a sterile, concentrated solution intended for intravenous administration via a central line only after dilution (see DOSAGE AND ADMINISTRATION ). AMMONUL® is packaged in single-use vials.
CLINICAL PHARMACOLOGY
Sodium phenylacetate and sodium benzoate are metabolically active compounds that can serve as alternatives to urea for the excretion of waste nitrogen. Phenylacetate conjugates with glutamine in the liver and kidneys to form phenylacetylglutamine, via acetylation. Phenylacetylglutamine is excreted by the kidneys via glomerular filtration and tubular secretion. The nitrogen content of phenylacetylglutamine per mole is identical to that of urea (both contain two moles of nitrogen). Similarly, preceded by acylation, benzoate conjugates with glycine to form hippuric acid, which is rapidly excreted by the kidneys by glomerular filtration and tubular secretion. One mole of hippuric acid contains one mole of waste nitrogen. It has been shown that phenylacetylglutamine and hippurate can serve as alternative vehicles to effectively reduce waste nitrogen levels in patients with deficiencies of urea cycle enzymes and, thus, attenuate the risk of ammonia and glutamine-induced neurotoxicity.
Urea cycle disorders can result from decreased activity of any of the following enzymes: N -acetylglutamate synthetase (NAGS), carbamyl phosphate synthetase (CPS), argininosuccinate synthetase (ASS), ornithine transcarbamylase (OTC), argininosuccinate lyase (ASL), or arginase (ARG). The most frequently observed initial presenting symptoms in neonates include lethargy, seizures, poor feeding, neurologic changes, edema, and respiratory distress. Patients with milder forms of enzyme deficiencies may not present until late childhood, adolescence, or adulthood. Hyperammonemic crisis with lethargy, delirium, and coma, in these patients, are often precipitated by viral illness, high protein diet, stress, or trauma.
Plasma and urine amino acid analyses are used to diagnose ASS and ASL and to provide a preliminary diagnosis of CPS, OTC, or ARG. Blood citrulline levels are very low or absent in OTC and CPS, very high in ASS, and normal to moderately high in ASL and ARG. ASL may be distinguished by the presence of high levels of the unusual amino acid argininosuccinic acid (ASA) in the urine. It should be noted, however, that ASA tends to co-elute initially with other amino acids (such as leucine and isoleucine) in chromatographs, and may be missed on initial examination. ARG is characterized by high urine levels of arginine. A definitive diagnosis of CPS and OTC require a liver biopsy, and red blood cell enzyme analysis is needed to confirm a diagnosis of ARG. Patients suspected of having a urea cycle disorder, based on family history, should have documented hyperammonemia prior to administration of AMMONUL®.
Mechanism of Action
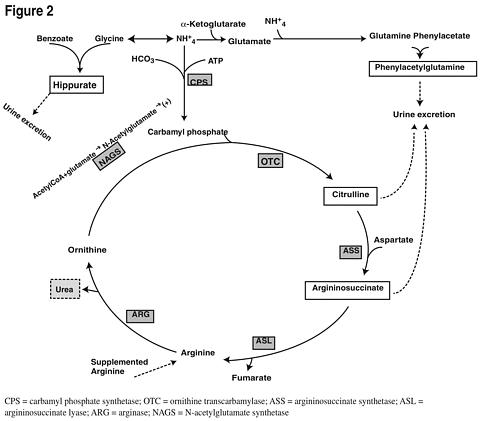
Figure 2 is a schematic illustrating how the components of AMMONUL®, phenylacetate and benzoate, provide an alternative pathway for nitrogen disposal in patients without a fully functioning urea cycle. Two moles of nitrogen are removed per mole of phenylacetate when it is conjugated with glutamine, and one mole of nitrogen is removed per mole of benzoate when it is conjugated with glycine.
Pharmacokinetics
The pharmacokinetics of intravenously administered AMMONUL® were characterized in healthy adult volunteers. Both benzoate and phenylacetate exhibited nonlinear kinetics. Following 90 minute intravenous infusion mean AUC last for benzoate was 20.3, 114.9, 564.6, 562.8, and 1599.1 mcg/mL following doses of 1, 2, 3.75, 4, and 5.5 g/m 2 , respectively. The total clearance decreased from 5.19 to 3.62 L/h/m 2 at the 3.75 and 5.5 g/m 2 doses, respectively.
Similarly, phenylacetate exhibited nonlinear kinetics following the priming dose regimens. AUC last was 175.6, 713.8, 2040.6, 2181.6, and 3829.2 mcg·h/mL following doses of 1, 2, 3.75, 4, and 5.5 g/m 2 , respectively. The total clearance decreased from 1.82 to 0.89 mcg·h/mL with increasing dose (3.75 and 4 g/m 2 , respectively).
During the sequence of 90 minute priming infusion followed by a 24 hour maintenance infusion, phenylacetate was detected in the plasma at the end of infusion (T max of 2 hr at 3.75 g/m 2 ) whereas, benzoate concentrations declined rapidly (T max of 1.5 hr at 3.75 g/m 2 ) and were undetectable at 14 and 26 hours following the 3.75 and 4 g/m 2 dose, respectively.
A difference in the metabolic rates for phenylacetate and benzoate was noted. The formation of hippurate from benzoate occurred more rapidly than that of phenylacetylglutamine from phenylacetate, and the rate of elimination for hippurate appeared to be more rapid than that for phenylacetylglutamine.
Pharmacokinetic observations have also been reported from twelve episodes of hyperammonemic encephalopathy in seven children diagnosed (age 3 to 26 months) with urea cycle disorders who had been administered AMMONUL® intravenously. These data showed peak plasma levels of phenylacetate and benzoate at approximately the same times as were observed in adults. As in adults, the plasma levels of phenylacetate were higher than benzoate and were present for a longer time [1] .
The pharmacokinetics of intravenous phenylacetate have been reported following administration to adult patients with advanced solid tumors. The decline in serum phenylacetate concentrations following a loading infusion of 150 mg/kg was consistent with saturable enzyme kinetics. Ninety-nine percent of administered phenylacetate was excreted as phenylacetylglutamine [2,3] .
Special Populations
Gender:
Pharmacokinetic parameters of AMMONUL® were compared in healthy males and females. Bioavailability of both benzoate and phenylacetate was slightly higher in females than in males. However, conclusions cannot be drawn due to the limited number of subjects in this study.
Hepatic Insufficiency:
Limited information is available on the metabolism and excretion of sodium phenylacetate and sodium benzoate in patients with impaired hepatic function. However, as the liver is one of the two organs (the other is the kidney) in which the metabolic conjugation of sodium phenylacetate and sodium benzoate is known to take place, care should be used in administering AMMONUL® to patients with hepatic insufficiency.
Renal Impairment:
For effective AMMONUL® drug therapy, renal clearance of the drug metabolites and subsequently ammonia is required. Therefore, patients with impaired renal function should be closely monitored.
Dialysis:
Intravenous use of AMMONUL® is complementary with the use of dialysis [4,5] . In the non-neonatal study patient population treated with AMMONUL® , dialysis (standard hemodialysis, peritoneal dialysis, arteriovenous hemofiltration, or other dialysis) was required in 13% of hyperammonemic episodes. Standard hemodialysis was the most frequently used dialysis method. High levels of ammonia can be reduced quickly when AMMONUL® is used with dialysis, as the ammonia-scavenging of AMMONUL® suppresses the production of ammonia from catabolism of endogenous protein [6] and dialysis eliminates the ammonia and ammonia conjugates.
Drug Interactions:
Formal drug interaction studies have not been performed with AMMONUL®.
Pharmacodynamics
In patients with hyperammonemia due to deficiencies in enzymes of the urea cycle, AMMONUL® has been shown to decrease elevated plasma ammonia levels and improve encephalopathy and survival outcome compared to historical controls. These effects are considered to be the result of reduction in nitrogen overload through glutamine and glycine scavenging by AMMONUL® in combination with appropriate dietary and other supportive measures.
Clinical Data
The efficacy of AMMONUL® in improving patient survival of acute hyperammonemic episodes was demonstrated in an analysis of 316 patients (1045 episodes of hospitalization) treated between 1981 and 2003.
The demographic characteristics and diagnoses of the patient population are shown in Table 1.
Table 1 Baseline Characteristics and Diagnoses of Study Population Patients *
N=316GenderMale158 (51%) Female150 (49%) Age (years)N310 Mean (SD)6.2 (8.54) Min-Max0.0-53.0 Age groups0-30 days104 (34%) 31 days-2 years55 (18%) > 2-12 years90 (29%) > 12-16 years30 (10%) > 16 years31 (10%) Enzyme deficiencyOTC146 (46%) ASS71 (22%) CPS38 (12%) ASL7 (2%) ARG2 (< 1%) THN2 (< 1%) Other **56 (18%) OTC = ornithine transcarbamylase deficiency; ASS = argininosuccinate synthetase deficiency; CPS = carbamyl phosphate synthetase deficiency; ASL = argininosuccinate lyase deficiency; ARG = arginase deficiency; THN = transient hyperammonemia of the newborn*For the summary at the patient level, data obtained at first episode used.**Diagnosis unknown or pending (33 episodes), acidemia (14 episodes), HHH syndrome (6 episodes), carnitine translocase deficiency (4 episodes), liver disease (3 episodes), HMG CoA lyase deficiency (1 episode), non-ketotic hyperglycinemia (1 episode), suspected fatty acid oxidation deficiency (1 episode), and valproic-acid-induced hyperammonemia (1 episode).
On admission to the hospital, patients with hyperammonemia or a potential urea cycle disorder (UCD) were treated with a bolus dose of 0.25 g/kg (or 5.5 g/m 2 ) sodium phenylacetate + 0.25 g/kg (or 5.5 g/m 2 ) sodium benzoate over a period of 90 minutes to 6 hours, depending on the specific UCD. Infusions also contained arginine; the dose of arginine depended on the specific UCD. After completion of the bolus dose, maintenance infusions of the same dose over 24 hours were continued until the patient was no longer hyperammonemic or oral therapy could be tolerated. The mean (SD) duration of treatment was 4.6 (6.45) days per episode, and ranged from 1 to 72 days.
Survival was substantially improved after AMMONUL® treatment compared with historical values (estimated 14% 1-year survival rate with dietary therapy alone) [10] and with dialysis (estimated 43% survival of acute hyperammonemia) [11] .
Ninety-four percent (981 of 1045) of hyperammonemic episodes treated with AMMONUL® resulted in patients being discharged from the hospital. Eighty percent of patients (252 of 316) survived their last episode. Of the 64 patients who died, 53 (83%) died during their first hyperammonemic episode. Of the 104 neonates (<30d) treated with AMMONUL®, 34 (33%) died during the first hyperammonemic episode.
Ammonia levels decreased from very high levels (> 4 times the upper limit of normal [ULN]) to lower levels in 91% of episodes after treatment. In patients responding to therapy, mean ammonia concentrations decreased significantly within four hours of initiation of AMMONUL® therapy and were maintained. Dialysis is recommended for those patients who fail to have a significant reduction in plasma ammonia levels within 4 to 8 hours after receiving AMMONUL®. A shift from high (</= 4 times ULN) to very high (> 4 times ULN) levels was observed in only 4% of the episodes.
Improvements in neurological status endpoints were observed in most episodes and patients. Overall, investigators rated neurological status as improved, much improved, or the same in 93% of episodes, and overall status in response to treatment as improved, much improved, or the same in 97% of episodes. Recovery from coma was observed in 97% of episodes where coma was present at admission (111 of 114 episodes).
INDICATIONS AND USAGE
AMMONUL® is indicated as adjunctive therapy for the treatment of acute hyperammonemia and associated encephalopathy in patients with deficiencies in enzymes of the urea cycle. In acute neonatal hyperammonemic coma, in moderate to severe episodes of hyperammonemic encephalopathy, and in episodes of hyperammonemia which fail to respond to an initial course of AMMONUL® therapy, he-modialysis is the most rapid and effective technique for re-moving ammonia [12,13] . In such cases, the concomitant administration of AMMONUL® can help prevent the re-accumulation of ammonia by increasing waste nitrogen excretion [4,5,13] .
CONTRAINDICATIONS
AMMONUL® should not be administered to patients with known hypersensitivity to sodium phenylacetate or sodium benzoate.
WARNINGS
Any episode of acute symptomatic hyperammonemia should be treated as a life-threatening emergency. Treatment of hyperammonemia may require dialysis, preferably hemodialysis, to remove a large burden of ammonia. Uncontrolled hyperammonemia can rapidly result in brain damage or death, and prompt use of all therapies necessary to reduce ammonia levels is essential.
Management of hyperammonemia due to inborn errors of metabolism should be done in coordination with medical personnel familiar with these diseases. The severity of the disorder may necessitate the use of hemodialysis combined with nutritional management and medical support. The multidisciplinary nature of the treatment usually requires the facilities of a tertiary or quaternary care center.
Ongoing monitoring of plasma ammonia levels, neurological status, laboratory tests, and clinical response in patients receiving AMMONUL® is crucial to assess patient response to treatment. Because urine potassium loss is enhanced by the excretion of the nonreabsorbable anions, phenylacetylglutamine and hippurate, plasma potassium levels should be carefully monitored and appropriate treatment given when necessary. Serum electrolyte levels should be monitored and maintained within the normal range.
AMMONUL® contains 30.5 mg of sodium per mL of undiluted product. Thus, AMMONUL® should be used with great care, if at all, in patients with congestive heart failure or severe renal insufficiency, and in clinical states in which there is sodium retention with edema. If an adverse reaction does occur, discontinue administration of AMMONUL®, evaluate the patient, and institute appropriate therapeutic countermeasures.
Administration must be through a central line. Administration through a peripheral line may cause burns.
Bolus infusion flow rates are relatively high, especially for infants (see DOSAGE AND ADMINISTRATION ). Extravasation of AMMONUL® into the perivenous tissues may lead to skin necrosis. If extravasation is suspected, discontinue the infusion and resume at a different infusion site, if necessary. Standard treatment for extravasation can include aspiration of residual drug from the catheter, limb elevation, and intermittent cooling using cold packs [14] . The infusion site must be monitored closely for possible infiltration during drug administration. Do not administer undiluted product.
Due to structural similarities between phenylacetate and benzoate to salicylate, AMMONUL® may cause side effects typically associated with salicylate overdose, such as hyperventilation and metabolic acidosis. The clinician is advised to perform blood chemistry profiles, and frequent blood pH and pCO 2 monitoring.
PRECAUTIONS
General:
AMMONUL® is a concentrated solution and must be diluted before administration via a central line. Because sodium phenylacetate and sodium benzoate are metabolized in the liver and kidney, and since phenylacetylglutamine and hippurate are primarily excreted by the kidney, use caution when administering AMMONUL® to patients with hepatic or renal insufficiency. AMMONUL® infusion has been associated with nausea and vomiting. An antiemetic may be administered during AMMONUL® infusion.
Because of prolonged plasma levels achieved by phenylacetate in pharmacokinetic studies, repeat loading doses of AMMONUL® should not be administered.
Use of corticosteroids may cause the breakdown of body protein and, thereby, potentially increase plasma ammonia levels in patients with impaired ability to form urea.
Neurotoxicity of Phenylacetate:
Neurotoxicity was reported in cancer patients receiving intravenous phenylacetate, 250-300 mg/kg/day for 14 days, repeated at 4-week intervals. Manifestations were predominantly somnolence, fatigue, and lightheadedness, with less frequent headaches, dysgeusia, hypoacusis, disorientation, impaired memory, and exacerbation of a preexisting neuropathy. These adverse events were mainly mild. The acute onset of symptoms upon initiation of treatment and reversibility of symptoms when the phenylacetate was discontinued suggest a drug effect [2,3] .
In animal studies, subcutaneous administration to rat pups of 190-474 mg/kg of phenylacetate caused decreased proliferation and increased loss of neurons, and reduced central nervous system (CNS) myelin. Cerebral synapse maturation was retarded, and the number of functioning nerve terminals in the cerebrum was reduced, which resulted in impaired brain growth [15] . Pregnant rats were given phenylacetate at 3.5 µmol/g/day subcutaneous from gestation day 7 through normal delivery. Prenatal exposure of rat pups to phenylacetate produced lesions in layer 5 cortical pyramidal cells; dendritic spines were longer and thinner than normal and reduced in number [16] .
Drug Interactions:
Some antibiotics such as penicillin may compete with phenylacetylglutamine and hippurate for active secretion by renal tubules, which may affect the overall disposition of the infused drug.
Probenecid is known to inhibit the renal transport of many organic compounds, including aminohippuric acid, and may affect renal excretion of phenylacetylglutamine and hippurate [13] .
There have been reports that valproic acid can induce hyperammonemia through inhibition of the synthesis of N-acetylglutamate, a co-factor for carbamyl phosphate synthetase [14] . Therefore, administration of valproic acid to patients with urea cycle disorders may exacerbate their condition and antagonize the efficacy of AMMONUL® [15] .
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Carcinogenicity, mutagenicity and fertility studies of sodium phenylacetate have not been conducted. Sodium benzoate has been extensively tested as a food preservative. Results indicate that sodium benzoate is not mutagenic or carcinogenic, and does not impair fertility.
Pregnancy:
Pregnancy Category C. Animal reproduction studies have not been conducted with AMMONUL®. It is not known whether AMMONUL® can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Thus, AMMONUL® should be given to a pregnant woman only if clearly needed.
Labor and Delivery:
The effects of AMMONUL® on labor and delivery are unknown.
Nursing Mothers:
It is not known whether sodium phenylacetate, sodium benzoate, or their conjugation products are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when AMMONUL® is administered to a nursing woman.
Pediatric:
AMMONUL® has been used as a treatment for acute hyperammonemia in pediatric patients including patients in the early neonatal period (see DOSAGE AND ADMINISTRATION ).
ADVERSE REACTIONS
The safety data were obtained from 316 patients who received AMMONUL® as emergency (rescue) or prospective treatment for hyperammonemia as part of an uncontrolled, open-label study. The study population included patients between the ages of 0 to 53 years with a mean (SD) of 6.2 (8.54) years; 51% were male and 49% were female who had the following diagnoses: OTC (46%), ASS (22%), CPS (12%), ASL (2%), ARG (< 1%), THN (< 1%), and other (18%).
Table 2 Adverse Events Occurring in >/= 3% of Patients Treated with AMMONUL® Patients
N=316No. patients with any adverse event163 (52%) Blood and lymphatic system disorders35 (11%) Anemia NOS12 (4%) Disseminated intravascular
coagulation11 (3%) Cardiac disorders28 (9%) Gastrointestinal disorders42 (13%) Diarrhea NOS10 (3%) Nausea9 (3%) Vomiting NOS29 (9%) General disorders and administration-site conditions45 (14%) Injection-site reaction NOS11 (3%) Pyrexia17 (5%) Infections39 (12%) Urinary tract infection NOS9 (3%) Injury, poisoning and procedural complications12 (4%) Investigations32 (10%) Metabolism and nutrition disorders67 (21%) Acidosis NOS8 (3%) Hyperammonemia17 (5%) Hyperglycemia NOS22 (7%) Hypocalcemia8 (3%) Hypokalemia23 (7%) Metabolic acidosis NOS13 (4%) Nervous system disorders71 (22%) Brain edema17 (5%) Coma10 (3%) Convulsions NOS19 (6%) Mental impairment NOS18 (6%) Psychiatric disorders16 (5%) Agitation8 (3%) Renal and urinary disorders14 (4%) Respiratory, thoracic and mediastinal disorders47 (15%) Respiratory distress9 (3%) Skin and subcutaneous tissue disorders19 (6%) Vascular disorders19 (6%) Hypotension NOS14 (4%) Clinically Important Adverse Reactions
Adverse events occurred most frequently in the following system organ classes: nervous system disorders (22% of patients), metabolism and nutrition disorders (21% of patients), and respiratory, thoracic and mediastinal disorders (15% of patients). The most frequently reported adverse events were vomiting (9% of patients), hyperglycemia (7% of patients), hypokalemia (7% of patients), convulsions (6% of patients), and mental impairment (6% of patients).
Adverse events leading to study drug discontinuation occurred in 4% of patients. Metabolic acidosis and injection-site reactions each led to discontinuation in 2 patients (< 1%). Adverse events leading to discontinuation in 1 patient included bradycardia, abdominal distension, injection-site extravasation, injection-site hemorrhage, blister, overdose, subdural hematoma, hyperammonemia, hypoglycemia, clonus, coma, increased intercranial pressure, hypercapnia, Kussmaul respiration, respiratory distress, respiratory failure, pruritis, and maculo-papular rash.
Subpopulation and Risk Factor Data
Adverse events were reported with similar frequency in patients with OTC, ASS, CPS, and diagnoses categorized as "other." Nervous system disorders were more frequent in patients with OTC and CPS, compared with patients with ASS and patients with "other" diagnoses. Convulsions and mental impairment were reported in patients with OTC and CPS. These observations are consistent with literature reports that patients with enzyme deficiencies occurring earlier in the urea cycle (i.e., OTC and CPS) tend to be more severely affected.
Adverse event profiles did differ by age group. Patients </= 30 days of age had more blood and lymphatic system disorders and vascular disorders (specifically hypotension), while patients > 30 days of age had more gastrointestinal disorders (specifically nausea, vomiting and diarrhea).
Other Less Common Adverse Events Occurring in < 3% of Patients
Less common adverse events that could represent drug-induced reactions or are characterized as severe are listed below by body system.
BLOOD AND LYMPHATIC SYSTEM DISORDERS: coagulopathy, pancytopenia, thrombocytopenia
CARDIAC DISORDERS: atrial rupture, cardiac or cardiopulmonary arrest/failure, cardiogenic shock, cardiomyopathy, pericardial effusion
EYE DISORDERS: blindness
GASTROINTESTINAL DISORDERS: gastrointestinal hemorrhage
GENERAL DISORDERS AND ADMINISTRATION-SITE CONDITIONS: asthenia, brain death, chest pain, multiorgan failure, edema
HEPATOBILIARY DISORDERS: cholestasis, hepatic artery stenosis, hepatic failure/ hepatotoxicity, jaundice
INFECTIONS AND INFESTATIONS: sepsis/septic shock
INJURY, POISONING AND PROCEDURAL COMPLICATIONS: brain herniation, subdural hematoma
INVESTIGATIONS: blood carbon dioxide changes, blood glucose changes, blood pH increased, cardiac output decreased, pCO 2 changes, respiratory rate increased
METABOLISM AND NUTRITION DISORDERS: alkalosis, dehydration, fluid overload/retention, hyperkalemia, hypernatremia, alkalosis, tetany
NEOPLASMS BENIGN, MALIGNANT AND UNSPECIFIED: hemangioma acquired
NERVOUS SYSTEM DISORDERS: areflexia, ataxia, brain infarction, brain hemorrhage, cerebral atrophy, clonus, depressed level of consciousness, encephalopathy, nerve paralysis, intracranial pressure increased, tremor
PSYCHIATRIC DISORDERS: acute psychosis, aggression, confusional state, hallucinations
RENAL AND URINARY DISORDERS: anuria, renal failure, urinary retention
RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERS: acute respiratory distress syndrome, dyspnea, hypercapnia, hyperventilation, Kussmaul respiration, pneumonia aspiration, pneumothorax, pulmonary hemorrhage, pulmonary edema, respiratory acidosis or alkalosis, respiratory arrest/failure
SKIN AND SUBCUTANEOUS TISSUE DISORDERS: alopecia, pruritis generalized, rash, urticaria
VASCULAR DISORDERS: flushing, hemorrhage, hypertension, phlebothrombosis/thrombosis
OVERDOSAGE
Overdosage has been reported during AMMONUL® treatment in urea cycle-deficient patients [17] . All patients in the uncontrolled open-label study were to be treated at the same dose of AMMONUL®. However, some patients received more than the dose level specified in the protocol. In 16 of the 64 deaths, the patient received a known overdose of AMMONUL®. Causes of death in these patients included cardiorespiratory failure/arrest (6 patients), hyperammonemia (3 patients), increased intracranial pressure (2 patients), pneumonitis with septic shock and coagulopathy (1 patient), error in dialysis procedure (1 patient), respiratory failure (1 patient), intractable hypotension and probable sepsis (1 patient), and unknown (1 patient). Additionally, other signs of intoxication may include obtundation (in the absence of hyperammonemia), hyperventilation, a severe compensated metabolic acidosis, perhaps with a respiratory component, large anion gap, hypernatremia and hyperosmolarity, progressive encephalopathy, cardiovascular collapse, and death.
In case of overdose of AMMONUL®, discontinue the drug and institute appropriate emergency medical monitoring and procedures. In severe cases, the latter may include hemodialysis (procedure of choice) or peritoneal dialysis (when hemodialysis is unavailable) [17] .
DOSAGE AND ADMINISTRATION
Administration must be through a central line. Administration through a peripheral line may cause burns.
General
AMMONUL® is administered intravenously as a loading dose infusion administered over 90 to 120 minutes, followed by an equivalent maintenance dose infusion administered over 24 hours. AMMONUL® may not be administered by any other route. Administration of analogous oral drugs, such as Buphenyl® (sodium phenylbutyrate), should be terminated prior to AMMONUL® infusion.
Hyperammonemic coma (regardless of cause) in the newborn infant should be aggressively treated while the specific diagnosis is pursued. All patients should be promptly hemodialyzed as the procedure of choice using the largest catheters consistent with the patient's size. A target blood flow of 150 mL/min/m 2 may be attained using a 7F catheter. (Ammonia clearance [mL/min] is similar to the blood flow rate [mL/min] through the dialyzer). Clearance of ammonia is approximately ten times greater by hemodialysis than by peritoneal dialysis or hemofiltration. Exchange transfusion is ineffective in the management of hyperammonemia. Hemodialysis may be repeated until the plasma ammonia level is stable at normal or near normal levels.
AMMONUL® infusion should be started as soon as the diagnosis of hyperammonemia is made. Treatment of hyperammonemia also requires caloric supplementation and restriction of dietary protein. Non-protein calories should be supplied principally as glucose (8-10 mg/kg/min) with Intralipid added. Attempts should be made to maintain a caloric intake of greater than 80 cal/kg/d. During and after infusion of AMMONUL®, ongoing monitoring of neurological status, plasma ammonia levels, clinical laboratory values, and clinical responses are crucial to assess patient response to treatment. The need for other interventions to control hyperammonemia must be considered throughout the course of treatment. Patients with a large ammonia burden or who are not responsive to AMMONUL® administration require aggressive therapy including hemodialysis (see WARNINGS ).
AMMONUL® must be diluted with sterile Dextrose Injection, 10% (D10W) before administration. The dilution and dosage of AMMONUL® are determined by weight for neonates, infants and young children, and by body surface area for larger patients, including older children, adolescents, and adults (Table 3). Maintenance infusions may be continued until elevated plasma ammonia levels have been normalized or the patient can tolerate oral nutrition and medications.
AMMONUL® solutions are physically and chemically stable for up to 24 hours at room temperature and room lighting conditions. No compatibility information is presently available for AMMONUL® infusion solutions except for Arginine HCl Injection, 10%, which may be mixed in the same container as AMMONUL®. Other infusion solutions and drug products should not be administered together with AMMONUL® infusion solution. AMMONUL® solutions may be prepared in glass and PVC containers. AMMONUL® solutions should be inspected visually for particulate matter and discoloration before administration.
Table 3. Dosage and Administration Patient Population Components of Infusion Solution
AMMONUL® must be diluted with
sterile dextrose injection 10% at
>/= 25 mL/Kg before administration.Dosage Provided AMMONUL® Arginine HCl
Injection, 10%Sodium Phenylacetate Sodium Benzoate Arginine HCl 0 to 20 kg: CPS and OTC Deficiency Dose Loading: over 90 to 120 minutes
Maintenance: over 24 hours2.5 mL/kg 2.0 mL/kg 250 mg/kg 250 mg/kg 200 mg/kg ASS and ASL Deficiency Dose Loading: over 90 to 120 minutes
Maintenance: over 24 hours2.5 mL/kg 6.0 mL/kg 250 mg/kg 250 mg/kg 600 mg/kg > 20 kg:CPS and OTC Deficiency Dose Loading: over 90 to 120 minutes
Maintenance: over 24 hours55 mL/m 2 2.0 mL/kg 5.5 g/m 2 5.5 g/m 2 200 mg/kg ASS and ASL Deficiency Dose Loading: over 90 to 120 minutes
Maintenance: over 24 hours55 mL/m 2 6.0 mL/kg 5.5 g/m 2 5.5 g/m 2 600 mg/kg Arginine Administration:
Intravenous arginine is an essential component of therapy for patients with carbamyl phosphate synthetase (CPS), ornithine transcarbamylase (OTC), argininosuccinate synthetase (ASS), or argininosuccinate lyase (ASL) deficiency. Because a hyperchloremic acidosis may ensue after high-dose arginine hydrochloride administration, plasma levels of chloride and bicarbonate should be monitored and appropriate amounts of bicarbonate administered.
Pending a specific diagnosis, intravenous arginine (6 mL/kg of Arginine HCl Injection, 10%, over 90 minutes followed by the same dose over 24 hours) should be given to hyperammonemic infants suspected of having a urea cycle disorder for two reasons: 1) infants with deficiencies in enzymes of the urea cycle (apart from arginase deficiency) are usually arginine-deficient; 2) hyperammonemia in infants with ASS or ASL deficiency usually respond favorably to arginine administration. If deficiencies of ASS or ASL are excluded as diagnostic possibilities, the intravenous dose of arginine HCl should be reduced to 2 mL/kg/d Arginine HCl Injection, 10%.
Converting To Oral Treatment:
Once elevated ammonia levels have been reduced to the normal range, oral therapy, such as sodium phenylbutyrate, dietary management and protein restrictions should be started or reinitiated.
HOW SUPPLIED
AMMONUL® (sodium phenylacetate and sodium benzoate) Injection 10% / 10% is supplied in single-use glass vials.
NDC-62592-720-50 single use vial containing 50 mL of sodium phenylacetate and sodium benzoate injection 10% / 10%.
Storage: Store at 25°C (77°F), excursions permitted to 15°-30°C (59°-86°F).
KEEP OUT OF REACH OF CHILDREN
Non-pyrogenic.
Rx Only
REFERENCES
- Brusilow SW, Danney M, Waber LJ, Batshaw M, Burton B, Levitsky L, Roth K, McKeethren C, Ward J. Treatment of episodic hyperammonemia in children with inborn errors of urea synthesis. N Engl J Med 1984; 310:1630-1634.
- Thibault A, Cooper MR, Figg WD, Venzon DJ, Sartor AO, Tompkins AC, Weinberger MS, Headlee DJ, McCall NA, Samid D, Myers CE. A Phase I and pharmacokinetic study of intravenous phenylacetate in patients with cancer. Cancer Research 1994; 54:1690-1694.
- Thibault A, Samid D, Cooper MR, Figg WD, Tompkins AC, Patronas N, Headlee DJ, Kohler DR, Venzon DJ, Myers CE. Phase I study of phenylacetate administered twice daily to patients with cancer. Cancer 1995; 75:2932-2938.
- Batshaw M, MacArthur RB, Tuchman M. Alternative pathway therapy for urea cycle disorders: Twenty years later. Proceedings of a Consensus Conference for the Management of Patients with Urea Cycle Disorders. J. Pediatr 2001; 138:S46-S55.
- The Urea Cycle Disorders Conference Group. Consensus statement from a conference for the management of patients with urea cycle disorders. Proceedings of a Consensus Conference for the Management of Patients with Urea Cycle Disorders. J. Pediatr 2001; 138:Sl-S5
- Legras A, Labarthe F, Maillot F, Garrigue MA, Kouatchet A, Ogier D. Late diagnosis of ornithine transcarbamylase defect in three related female patients: polymorphic presentations. Crit Care Med 2002 Jan;30(1)241-4.
- Tsuji A. Transporter-mediated drug interactions. Drug Metabol Pharmacokin 2002; 17(4):253-274.
- Williams CA, Tiefenbach S, McReynolds JW. Valproic acid-induced hyperammonemia in mentally retarded adults. Neurol 1984; 34: 550-553
- Batshaw ML, Brusilow SW. Valproate-induced hyperammonemia. Ann Neurol 1982; 11: 319-321.
- Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis: outcome of urea-cycle enzymopathies. N Engl J Med 1984 Jun 7;310:1500-5.
- Schaefer F, Straube E, Oh J, Mehls O, Mayatepek E. Dialysis in neonates with inborn errors of metabolism. Nephrol Dial Transplant 1999;14:910-8.
- Neu AM, Christenson MJ, Brusilow SW. Hemodialysis for inborn errors of metabolism. In: Nissenson RA, Fine RN, editors. Dialysis therapy. 2nd ed. Philadelphia (PA): Hanley & Belfus; 1992. p. 371-372.
- Summar M. Current strategies for the management of neonatal urea cycle disorders. J Pediatr 2001; 138; S30-S39.
- Camp-Sorrell D. Developing extravasation protocols and monitoring outcomes. J lntrav Nurse 1998; 21:232-239.
- Wen GY, Wisniewski HM, Shek JW, Loo YH, Fulton TR. Neuropathology of phenylacetate poisoning in rats: An experimental model of phenylketonuria. Ann Neurol 1980; 7:557-566.
- Lacey DJ. Cortical dendritic spine loss in rat pups whose mothers were prenatally injected with phenylacetate ('maternal PKU' model). Dev Brain Res 1986; 27:283-285.
- Maestri NE, Hauser ER, Bartholomew D, Brusilow SW. Prospective treatment of urea cycle disorders. J. Pediatr 1991; 119:923-928.
Manufactured by:
Chesapeake Biological Laboratories, Inc., 1111 South Paca Street, Baltimore MD 21230.
Manufactured for:
Ucyclyd Pharma, Inc., a wholly-owned subsidiary of Medicis Pharmaceutical Corp., 8125 North Hayden Road, Scottsdale, AZ 85258
Revision: February 2005
Subscribe to the "News" RSS Feed 
TOP ۞
