-
Boniva Tablets (Roche Laboratories)
DESCRIPTION
BONIVA (ibandronate sodium) is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. The chemical name for ibandronate sodium is 3-(N-methyl-N-pentyl) amino-1-hydroxypropane-1,1-diphosphonic acid, monosodium salt, monohydrate with the molecular formula C 9 H 22 NO 7 P 2 Na·H 2 O and a molecular weight of 359.24. Ibandronate sodium is a white- to off-white powder. It is freely soluble in water and practically insoluble in organic solvents.
BONIVA is available as a white, oblong, 2.5-mg film-coated tablet for daily oral administration or as a white, oblong, 150-mg film-coated tablet for once-monthly oral administration. One 2.5-mg film-coated tablet contains 2.813 mg ibandronate monosodium monohydrate, equivalent to 2.5 mg free acid. One 150-mg film-coated tablet contains 168.75 mg ibandronate monosodium monohydrate, equivalent to 150 mg free acid. BONIVA also contains the following inactive ingredients: lactose monohydrate, povidone, microcrystalline cellulose, crospovidone, purified stearic acid, colloidal silicon dioxide, and purified water. The tablet film coating contains hypromellose, titanium dioxide, talc, polyethylene glycol 6000, and purified water.
CLINICAL PHARMACOLOGY
Mechanism of Action
The action of ibandronate on bone tissue is based on its affinity for hydroxyapatite, which is part of the mineral matrix of bone. Ibandronate inhibits osteoclast activity and reduces bone resorption and turnover. In postmenopausal women, it reduces the elevated rate of bone turnover, leading to, on average, a net gain in bone mass.
Pharmacokinetics
Absorption
The absorption of oral ibandronate occurs in the upper gastrointestinal tract. Plasma concentrations increase in a dose-linear manner up to 50 mg oral intake and increases nonlinearly above this dose.
Following oral dosing, the time to maximum observed plasma ibandronate concentrations ranged from 0.5 to 2 hours (median 1 hour) in fasted healthy post-menopausal women. The mean oral bioavailability of 2.5 mg ibandronate was about 0.6% compared to intravenous dosing. The extent of absorption is impaired by food or beverages (other than plain water). The oral bioavailability of ibandronate is reduced by about 90% when BONIVA is administered concomitantly with a standard breakfast in comparison with bioavailability observed in fasted subjects. There is no meaningful reduction in bioavailability when ibandronate is taken at least 60 minutes before a meal. However, both bioavailability and the effect on bone mineral density (BMD) are reduced when food or beverages are taken less than 60 minutes following an ibandronate dose.
Distribution
After absorption, ibandronate either rapidly binds to bone or is excreted into urine. In humans, the apparent terminal volume of distribution is at least 90 L, and the amount of dose removed from the circulation via the bone is estimated to be 40% to 50% of the circulating dose. In vitro protein binding in human serum was 99.5% to 90.9% over an ibandronate concentration range of 2 to 10 ng/mL in one study and approximately 85.7% over a concentration range of 0.5 to 10 ng/mL in another study.
Metabolism
There is no evidence that ibandronate is metabolized in humans.
Elimination
The portion of ibandronate that is not removed from the circulation via bone absorption is eliminated unchanged by the kidney (approximately 50% to 60% of the absorbed dose). Unabsorbed ibandronate is eliminated unchanged in the feces.
The plasma elimination of ibandronate is multiphasic. Its renal clearance and distribution into bone accounts for a rapid and early decline in plasma concentrations, reaching 10% of the C max within 3 or 8 hours after intravenous or oral administration, respectively. This is followed by a slower clearance phase as ibandronate redistributes back into the blood from bone. The observed apparent terminal half-life for ibandronate is generally dependent on the dose studied and on assay sensitivity. The observed apparent terminal half-life for the 150 mg ibandronate tablet upon oral administration to healthy postmenopausal women ranges from 37 to 157 hours.
Total clearance of ibandronate is low, with average values in the range 84 to 160 mL/min. Renal clearance (about 60 mL/min in healthy postmenopausal females) accounts for 50% to 60% of total clearance and is related to creatinine clearance. The difference between the apparent total and renal clearances likely reflects bone uptake of the drug.
Special Populations
Pediatrics
The pharmacokinetics of ibandronate has not been studied in patients <18 years of age.
Gender
The bioavailability and pharmacokinetics of ibandronate are similar in both men and women.
Geriatric
Since ibandronate is not known to be metabolized, the only difference in ibandronate elimination for geriatric patients versus younger patients is expected to relate to progressive age-related changes in renal function (see Special Populations : Renal Impairment ).
Race
Pharmacokinetic differences due to race have not been studied.
Renal Impairment
Renal clearance of ibandronate in patients with various degrees of renal impairment is linearly related to creatinine clearance (CLcr).
Following a single dose of 0.5 mg ibandronate by intravenous administration, patients with CLcr 40 to 70 mL/min had 55% higher exposure (AUC (infinity) ) than the exposure observed in subjects with CLcr >90 mL/min. Patients with CLcr <30 mL/min had more than a two-fold increase in exposure compared to the exposure for healthy subjects (see DOSAGE AND ADMINISTRATION : Patients with Renal Impairment ).
Hepatic Impairment
No studies have been performed to assess the pharmacokinetics of ibandronate in patients with hepatic impairment since ibandronate is not metabolized in the human liver.
Drug Interactions
Ibandronate does not undergo hepatic metabolism and does not inhibit the hepatic cytochrome P450 system. Ibandronate is eliminated by renal excretion. Based on a rat study, the ibandronate secretory pathway does not appear to include known acidic or basic transport systems involved in the excretion of other drugs.
Products containing calcium and other multivalent cations (such as aluminum, magnesium, iron), including milk, food, and antacids are likely to interfere with absorption of ibandronate, which is consistent with findings in animal studies.
H2 Blockers and Proton Pump Inhibitors (PPIs)
A pharmacokinetic interaction study in healthy volunteers demonstrated that 75 mg ranitidine (25 mg injected intravenously 90 and 15 minutes before and 30 minutes after ibandronate administration) increased the oral bioavailability of 10 mg ibandronate by about 20%. This degree of increase is not considered to be clinically relevant.
Tamoxifen
A pharmacokinetic interaction study in healthy postmenopausal women demonstrated that there was no interaction between oral 30 mg tamoxifen and intravenous 2 mg ibandronate.
Pharmacodynamics
Osteoporosis is characterized by decreased bone mass and increased fracture risk, most commonly at the spine, hip, and wrist. The diagnosis can be confirmed by a finding of low bone mass, evidence of fracture on x-ray, a history of osteoporotic fracture, or height loss or kyphosis indicative of vertebral fracture. While osteoporosis occurs in both men and women, it is most common among women following menopause. In healthy humans, bone formation and resorption are closely linked; old bone is resorbed and replaced by newly formed bone. In postmenopausal osteoporosis, bone resorption exceeds bone formation, leading to bone loss and increased risk of fracture. After menopause, the risk of fractures of the spine and hip increases; approximately 40% of 50-year-old women will experience an osteoporosis-related fracture during their remaining lifetimes.
BONIVA produced biochemical changes indicative of dose-dependent inhibition of bone resorption, including decreases of biochemical markers of bone collagen degradation (such as deoxypyridinoline, and cross-linked C-telopeptide of Type I collagen) in the daily dose range of 0.25 to 5.0 mg and once-monthly doses from 100 mg to 150 mg in postmenopausal women.
Treatment with 2.5 mg daily BONIVA resulted in decreases in biochemical markers of bone turnover, including urinary C-terminal telopeptide of Type I collagen (uCTX) and serum osteocalcin, to levels similar to those in premenopausal women. Changes in markers of bone formation were observed later than changes in resorption markers, as expected, due to the coupled nature of bone resorption and formation. Treatment with 2.5 mg daily BONIVA decreased levels of uCTX within 1 month of starting treatment and decreased levels of osteocalcin within 3 months. Bone turnover markers reached a nadir of approximately 64% below baseline values by 6 months of treatment and remained stable with continued treatment for up to 3 years. Following treatment discontinuation, there is a return to pretreatment baseline rates of elevated bone resorption associated with postmenopausal osteoporosis.
In a 1-year, Phase 3 study comparing once-monthly vs. once-daily oral dosing regimens, the median decrease from baseline in serum CTX values was -76% for patients treated with the 150 mg once-monthly regimen and -67% for patients treated with the 2.5 mg daily regimen.
CLINICAL STUDIES
Treatment of Postmenopausal Osteoporosis
The effectiveness and safety of BONIVA were demonstrated in a randomized, double-blind, placebo-controlled, multinational study (Treatment Study) of 2946 women aged 55 to 80 years, who were on average 21 years post-menopause, who had lumbar spine BMD 2 to 5 SD below the premenopausal mean (T-score) in at least one vertebra [L1-L4], and who had 1 to 4 prevalent vertebral fractures. BONIVA was evaluated at oral doses of 2.5 mg daily and 20 mg intermittently. The main outcome measure was the occurrence of new radiographically diagnosed vertebral fractures after 3 years of treatment. The diagnosis of an incident vertebral fracture was based on both qualitative diagnosis by the radiologist and quantitative morphometric criterion. The morphometric criterion required the dual occurrence of 2 events: a relative height ratio or relative height reduction in a vertebral body of at least 20%, together with at least a 4 mm absolute decrease in height. All women received 400 IU vitamin D and 500 mg calcium supplementation per day.
The effectiveness and safety of BONIVA once monthly were demonstrated in a randomized, double-blind, multinational, noninferiority trial in 1602 women aged 54 to 81 years, who were on average 18 years postmenopause, and had L2-L4 lumbar spine BMD T-score below -2.5 SD at baseline. The main outcome measure was the comparison of the percentage change from baseline in lumbar spine BMD after 1 year of treatment with once-monthly ibandronate (100 mg, 150 mg) to daily ibandronate (2.5 mg). All patients received 400 IU vitamin D and 500 mg calcium supplementation per day.
Effect on Vertebral Fracture
BONIVA 2.5 mg daily significantly reduced the incidence of new vertebral and of new and worsening vertebral fractures. Over the course of the 3-year study, the risk for vertebral fracture was 9.6% in the placebo-treated women and 4.7% in the women treated with BONIVA 2.5 mg (p<0.001) (see Table 1 ).
Table 1 Effect of BONIVA on the Incidence of Vertebral Fracture in the 3-Year Osteoporosis Treatment Study *Proportion of Patients with Fracture (%) Placebo
n=975BONIVA 2.5 mg Daily
n=977Absolute Risk Reduction (%)
95% CIRelative Risk Reduction (%)
95% CINew Vertebral Fracture
0-3 Year9.6 4.7 4.9
(2.3, 7.4)52 **
(29, 68)New and Worsening Vertebral Fracture
0-3 Year10.4 5.1 5.3
(2.6, 7.9)52
(30, 67)Clinical (Symptomatic) Vertebral Fracture
0-3 Year5.3 2.8 2.5
(0.6, 4.5)49
(14, 69)*The endpoint value is the value at the study's last time point, 3 years, for all patients who had a fracture identified at that time; otherwise, the last post-baseline value prior to the study's last time point is used. **p=0.0003 vs. placebo Effect on Nonvertebral Fractures
There was a similar number of nonvertebral osteoporotic fractures at 3 years reported in women treated with BONIVA 2.5 mg daily [9.1%, (95% CI: 7.1%, 11.1%)] and placebo [8.2%, (95% CI: 6.3%, 10.2%)]. The two treatment groups were also similar with regard to the number of fractures reported at the individual nonvertebral sites: pelvis, femur, wrist, forearm, rib, and hip.
Effect on Bone Mineral Density (BMD)
BONIVA significantly increased BMD at the lumbar spine and hip relative to treatment with placebo. In the 3-year osteoporosis treatment study, BONIVA 2.5 mg daily produced increases in lumbar spine BMD that were progressive over 3 years of treatment and were statistically significant relative to placebo at 6 months and at all later time points. Lumbar spine BMD increased by 6.4% after 3 years of treatment with 2.5 mg daily BONIVA compared with 1.4% in the placebo group. Table 2 displays the significant increases in BMD seen at the lumbar spine, total hip, femoral neck, and trochanter compared to placebo. Thus, overall BONIVA reverses the loss of BMD, a central factor in the progression of osteoporosis.
Table 2 Mean Percent Change in BMD from Baseline to Endpoint in Patients Treated Daily with BONIVA 2.5 mg or Placebo in the 3-Year Osteoporosis Treatment Study *Placebo BONIVA 2.5 mg Daily Lumbar Spine1.4
(n=693)6.4
(n=712)Total Hip-0.7
(n=638)3.1
(n=654)Femoral Neck-0.7
(n=683)2.6
(n=699)Trochanter0.2
(n=683)5.3
(n=699)*The endpoint value is the value at the study's last time point, 3 years, for all patients who had BMD measured at that time; otherwise, the last postbaseline value prior to the study's last time point is used. BONIVA 150 mg once-monthly (n=327) was shown to be noninferior to BONIVA 2.5 mg daily (n=318) in lumbar spine BMD in a 1-year, double-blind, multicenter study of women with postmenopausal osteoporosis. In the primary efficacy analysis (per-protocol population), the mean increases from baseline in lumbar spine BMD at 1 year were 3.86% (95% CI: 3.40%, 4.32%) in the 2.5-mg daily group and 4.85% (95% CI: 4.41%, 5.29%) in the 150-mg once-monthly group; the mean difference between 2.5 mg daily and 150 mg once monthly was 0.99% (95% CI: 0.38%, 1.60%), which was statistically significant (p=0.002). The results of the intent-to-treat analysis were consistent with the primary efficacy analysis. The 150 mg once-monthly group also had consistently higher BMD increases at the other skeletal sites compared to the 2.5 mg daily group.
Bone Histology
The effects of BONIVA 2.5 mg daily on bone histology were evaluated in iliac crest biopsies from 16 women after 22 months of treatment and 20 women after 34 months of treatment.
The histological analysis of bone biopsies showed bone of normal quality and no indication of osteomalacia or a mineralization defect.
Prevention of Postmenopausal Osteoporosis
BONIVA 2.5 mg daily prevented bone loss in a majority of women in a randomized, double-blind, placebo-controlled 2-year study (Prevention Study) of 653 postmenopausal women without osteoporosis at baseline. Women were aged 41 to 82 years, were on average 8.5 years postmenopause, and had lumbar spine BMD T-scores >-2.5. Women were stratified according to time since menopause (1 to 3 years, >3 years) and baseline lumbar spine BMD (T-score: >-1, -1 to -2.5). The study compared daily BONIVA at three dose levels (0.5 mg, 1.0 mg, 2.5 mg) with placebo. All women received 500 mg of supplemental calcium per day.
The primary efficacy measure was the change in BMD of lumbar spine after 2 years of treatment. BONIVA 2.5 mg daily resulted in a mean increase in lumbar spine BMD of 3.1% compared with placebo following 2 years of treatment (see Figure 1 ). Increases in BMD were seen at 6 months and at all later time points. Irrespective of the time since menopause or the degree of pre-existing bone loss, treatment with BONIVA resulted in a higher BMD response at the lumbar spine compared with placebo across all four baseline strata [time since menopause (1 to 3 years, >3 years) and baseline lumbar spine BMD (T-score: >-1, -1 to -2.5)]. Compared with placebo, treatment with BONIVA 2.5 mg daily increased BMD of the total hip by 1.8%, the femoral neck by 2.0%, and the trochanter by 2.1% (see Figure 1 ).
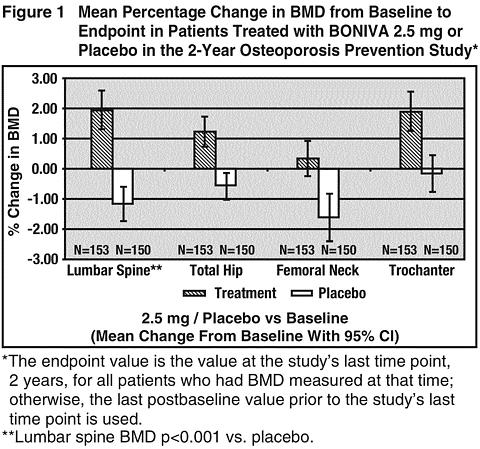
The safety and efficacy of once-monthly BONIVA 150 mg in postmenopausal women without osteoporosis are currently being studied, but data are not yet available.
Animal Pharmacology
Animal studies have shown that ibandronate is an inhibitor of osteoclast-mediated bone resorption. In the Schenk assay in growing rats, ibandronate inhibited bone resorption and increased bone volume, based on histologic examination of the tibial metaphyses. There was no evidence of impaired mineralization at the highest dose of 5 mg/kg/day (sub-cutaneously), which is 1000 times the lowest antiresorptive dose of 0.005 mg/kg/day in this model, and 5000 times the optimal antiresorptive dose of 0.001 mg/kg/day in the aged ovariectomized rat. This indicates that BONIVA ad-ministered at therapeutic doses is unlikely to induce osteomalacia.
Long-term daily or once-monthly intermittent administration of ibandronate to ovariectomized rats or monkeys was associated with suppression of bone turnover and increases in bone mass. In both rats and monkeys, vertebral BMD, trabecular density, and biomechanical strength were increased dose-dependently at doses up to 15 times the recommended human daily oral dose of 2.5 mg, or cumulative monthly doses up to 8 times (rat) or 6 times (monkey) the recommended human once-monthly oral dose of 150 mg, based on body surface area (mg/m 2 ) or AUC comparison. In monkeys, ibandronate maintained the positive correlation between bone mass and strength at the ulna and femoral neck. New bone formed in the presence of ibandronate had normal histologic structure and did not show mineralization defects.
INDICATIONS AND USAGE
BONIVA is indicated for the treatment and prevention of osteoporosis in postmenopausal women.
Treatment of Postmenopausal Osteoporosis
In postmenopausal women with osteoporosis, BONIVA increases BMD and reduces the incidence of vertebral fractures (see CLINICAL STUDIES ). Osteoporosis may be confirmed by the presence or history of osteoporotic fracture or by a finding of low bone mass (BMD more than 2 standard deviations below the premenopausal mean [ie, T-score]).
Prevention of Postmenopausal Osteoporosis
BONIVA may be considered in postmenopausal women who are at risk of developing osteoporosis and for whom the desired clinical outcome is to maintain bone mass and to reduce the risk of fracture.
Factors such as family history of osteoporosis, early menopause, previous fracture, high bone turnover, reduced BMD (at least 1.0 SD below the premenopausal mean), thin body frame, Caucasian or Asian race, and smoking, are associated with an increased risk of developing osteoporosis and fractures. The presence of these risk factors may be important when considering the use of BONIVA for preventing osteoporosis.
CONTRAINDICATIONS
- Known hypersensitivity to BONIVA or to any of its excipients
- Uncorrected hypocalcemia (see PRECAUTIONS : General )
- Inability to stand or sit upright for at least 60 minutes (see DOSAGE AND ADMINISTRATION )
WARNINGS
BONIVA, like other bisphosphonates administered orally may cause upper gastrointestinal disorders such as dysphagia, esophagitis, and esophageal or gastric ulcer (see PRECAUTIONS ).
PRECAUTIONS
General
Mineral Metabolism
Hypocalcemia and other disturbances of bone and mineral metabolism should be effectively treated before starting BONIVA therapy. Adequate intake of calcium and vitamin D is important in all patients.
Upper Gastrointestinal Effects
Bisphosphonates administered orally have been associated with dysphagia, esophagitis, and esophageal or gastric ulcers. This association has been reported for bisphosphonates in postmarketing experience but has not been found in most preapproval clinical trials, including those conducted with BONIVA. Therefore, patients should be advised to pay particular attention to and be able to comply with the dosing instructions to minimize the risk of these effects (see DOSAGE AND ADMINISTRATION ).
Severe Renal Impairment
BONIVA is not recommended for use in patients with severe renal impairment (creatinine clearance <30 mL/min).
Jaw Osteonecrosis
Osteonecrosis, primarily in the jaw, has been reported in patients treated with bisphosphonates. Most cases have been in cancer patients undergoing dental procedures, but some have occurred in patients with postmenopausal osteoporosis or other diagnoses. Known risk factors for osteonecrosis include a diagnosis of cancer, concomitant therapies (eg, chemotherapy, radiotherapy, corticosteroids), and co-morbid disorders (eg, anemia, coagulopathy, infection, pre-existing dental disease). Most reported cases have been in patients treated with bisphosphonates intravenously but some have been in patients treated orally.
For patients who develop osteonecrosis of the jaw (ONJ) while on bisphosphonate therapy, dental surgery may exacerbate the condition. For patients requiring dental procedures, there are no data available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of ONJ. Clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment.
Musculoskeletal Pain
In postmarketing experience, severe and occasionally incapacitating bone, joint, and/or muscle pain has been reported in patients taking bisphosphonates that are approved for the prevention and treatment of osteoporosis (see ADVERSE REACTIONS ). However, such reports have been infrequent. This category of drugs include BONIVA (ibandronate sodium) Tablets. Most of the patients were postmenopausal women. The time to onset of symptoms varied from one day to several months after starting the drug. Most patients had relief of symptoms after stopping. A subset had recurrence of symptoms when rechallenged with the same drug or another bisphosphonate.
In placebo-controlled studies with BONIVA, the percentages of patients with these symptoms were similar in the BONIVA and placebo groups.
Information for Patients
Patients should be instructed to read the Patient Information Leaflet carefully before taking BONIVA, to re-read it each time the prescription is renewed and to pay particular attention to the dosing instructions in order to maximize absorption and clinical benefit.
- BONIVA should be taken at least 60 minutes before the first food or drink (other than water) of the day and before taking any oral medications containing multivalent cations (including antacids, supplements or vitamins).
- To facilitate delivery to the stomach, and thus reduce the potential for esophageal irritation, BONIVA tablets should be swallowed whole with a full glass of plain water (6 to 8 oz) while the patient is standing or sitting in an upright position. Patients should not lie down for 60 minutes after taking BONIVA.
- Plain water is the only drink that should be taken with BONIVA. Please note that some mineral waters may have a higher concentration of calcium and therefore should not be used.
- Patients should not chew or suck the tablet because of a potential for oropharyngeal ulceration.
- The BONIVA 150-mg tablet should be taken on the same date each month (ie, the patient's BONIVA day).
- If the once-monthly dose is missed, and the patient's next scheduled BONIVA day is more than 7 days away, the patient should be instructed to take one BONIVA 150-mg tablet in the morning following the date that it is remembered (see DOSAGE AND ADMINISTRATION ). The patient should then return to taking one BONIVA 150-mg tablet every month in the morning of their chosen day, according to their original schedule.
- The patient must not take two 150-mg tablets within the same week. If the patient's next scheduled BONIVA day is only 1 to 7 days away, the patient must wait until their next scheduled BONIVA day to take their tablet. The patient should then return to taking one BONIVA 150-mg tablet every month in the morning of their chosen day, according to their original schedule.
Patients should receive supplemental calcium and vitamin D if dietary intake is inadequate. Intake of supplemental calcium and vitamin D should be delayed for at least 60 minutes following oral administration of BONIVA in order to maximize absorption of BONIVA.
Physicians should be alert to signs or symptoms signaling a possible esophageal reaction during therapy, and patients should be instructed to discontinue BONIVA and seek medical attention if they develop symptoms of esophageal irritation such as new or worsening dysphagia, pain on swallowing, retrosternal pain, or heartburn.
Drug Interactions
See CLINICAL PHARMACOLOGY : Pharmacokinetics : Drug Interactions .
Calcium Supplements/Antacids
Products containing calcium and other multivalent cations (such as aluminum, magnesium, iron) are likely to interfere with absorption of BONIVA. BONIVA should be taken at least 60 minutes before any oral medications containing multivalent cations (including antacids, supplements or vitamins) (see PRECAUTIONS : Information for Patients ).
H2 Blockers and Proton Pump Inhibitors (PPIs)
Of over 3500 patients enrolled in the BONIVA osteoporosis Treatment and Prevention Studies, 15% used anti-peptic agents (primarily H2 blockers and PPIs). Among these patients, the incidence of upper gastrointestinal adverse experiences in the patients treated with BONIVA was similar to that in placebo-treated patients. Similarly, of over 1600 patients enrolled in a study comparing once-monthly with daily dosing regimens of ibandronate, 14% of patients used anti-peptic agents. Among these patients, the incidence of upper gastrointestinal adverse experiences in the patients treated with BONIVA 150 mg once monthly was similar to that in patients treated with BONIVA 2.5 mg once daily.
Aspirin/Nonsteroidal Antiinflammatory Drugs (NSAIDs)
In the large, placebo-controlled osteoporosis Treatment Study, aspirin and non-steroidal antiinflammatory drugs were taken by 62% of the 2946 patients. Among aspirin or NSAID users, the incidence of upper gastrointestinal adverse events in patients treated with ibandronate 2.5 mg daily (28.9%) was similar to that in placebo-treated patients (30.7%). Similarly, in the 1-year monthly comparison study, aspirin and nonsteroidal antiinflammatory drugs were taken by 39% of the 1602 patients. The incidence of upper gastrointestinal events in patients concomitantly taking aspirin or NSAIDs was similar in patients taking ibandronate 2.5 mg daily (21.7%) and 150 mg once monthly (22.0%). However, since aspirin, NSAIDs, and bisphosphonates are all associated with gastrointestinal irritation, caution should be exercised in the concomitant use of aspirin or NSAIDs with BONIVA.
Drug/Laboratory Test Interactions
Bisphosphonates are known to interfere with the use of bone-imaging agents. Specific studies with ibandronate have not been performed.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 104-week carcinogenicity study, doses of 3, 7, or 15 mg/kg/day were administered by oral gavage to male and female Wistar rats (systemic exposures up to 12 and 7 times, respectively, human exposure at the recommended daily oral dose of 2.5 mg, and cumulative exposures up to 3.5 and 2 times, respectively, human exposure at the recommended once-monthly oral dose of 150 mg, based on AUC comparison). There were no significant drug-related tumor findings in male or female rats. In a 78-week carcinogenicity study, doses of 5, 20, or 40 mg/kg/day were administered by oral gavage to male and female NMRI mice (exposures up to 475 and 70 times, respectively, human exposure at the recommended daily oral dose of 2.5 mg and cumulative exposures up to 135 and 20 times, respectively, human exposure at the recommended once-monthly oral dose of 150 mg, based on AUC comparison). There were no significant drug-related tumor findings in male or female mice. In a 90-week carcinogenicity study, doses of 5, 20, or 80 mg/kg/day were administered in the drinking water to NMRI mice (cumulative monthly exposures in males and females up to 70 and 115 times, respectively, human exposure at the recommended dose of 150 mg, based on AUC comparison). A dose-related increased incidence of adrenal sub-capsular adenoma/carcinoma was observed in female mice, which was statistically significant at 80 mg/kg/day (220 to 400 times human exposure at the recommended daily oral dose of 2.5 mg and 115 times human exposure at the recommended once-monthly oral dose of 150 mg, based on AUC comparison). The relevance of these findings to humans is unknown.
Mutagenesis
There was no evidence for a mutagenic or clastogenic potential of ibandronate in the following assays: in vitro bacterial mutagenesis assay in Salmonella typhimurium and Escherichia coli (Ames test), mammalian cell mutagenesis assay in Chinese hamster V79 cells, and chromosomal aberration test in human peripheral lymphocytes, each with and without metabolic activation. Ibandronate was not genotoxic in the in vivo mouse micronucleus tests for chromosomal damage.
Impairment of Fertility
In female rats treated from 14 days prior to mating through gestation, decreases in fertility, corpora lutea, and implantation sites were observed at an oral dose of 16 mg/kg/day (45 times human exposure at the recommended daily oral dose of 2.5 mg and 13 times human exposure at the recommended once-monthly oral dose of 150 mg, based on AUC comparison).
Pregnancy
Pregnancy Category C
In female rats given oral doses of 1, 4, or 16 mg/kg/day beginning 14 days before mating and continuing through lactation, maternal deaths were observed at the time of delivery in all dose groups (>/=3 times human exposure at the recommended daily oral dose of 2.5 mg or >/=1 times human exposure at the recommended once-monthly oral dose of 150 mg, based on AUC comparison). Perinatal pup loss in dams given 16 mg/kg/day (45 times human exposure at the recommended daily oral dose of 2.5 mg and 13 times human exposure at the recommended once-monthly oral dose of 150 mg, based on AUC comparison) was likely related to maternal dystocia. In pregnant rats given oral doses of 6, 20, or 60 mg/kg/day during gestation, calcium supplementation (32 mg/kg/day by subcutaneous injection from gestation day 18 to parturition) did not completely prevent dystocia and periparturient mortality in any of the treated groups (>/=16 times human exposure at the recommended daily oral dose of 2.5 mg and >/=4.6 times human exposure at the recommended once-monthly oral dose of 150 mg, based on AUC comparison). A low incidence of postimplantation loss was observed in rats treated from 14 days before mating throughout lactation or during gestation, only at doses causing maternal dystocia and periparturient mortality. In pregnant rats dosed orally with 1, 5, or 20 mg/kg/day from gestation day 17 through lactation day 21 (following closure of the hard palate through weaning), maternal toxicity, including dystocia and mortality, fetal perinatal and postnatal mortality, were observed at doses >/=5 mg/kg/day (equivalent to human exposure at the recommended daily oral dose of 2.5 mg and >/=4 times human exposure at the recommended once-monthly oral dose of 150 mg, based on AUC comparison). Periparturient mortality has also been observed with other bisphosphonates and appears to be a class effect related to inhibition of skeletal calcium mobilization resulting in hypocalcemia and dystocia.
Exposure of pregnant rats during the period of organogenesis resulted in an increased fetal incidence of RPU (renal pelvis ureter) syndrome at oral doses >/=10 mg/kg/day (>/=30 times human exposure at the recommended daily oral dose of 2.5 mg and >/=9 times human exposure at the recommended once-monthly oral dose of 150 mg, based on AUC comparison). Impaired pup neuromuscular development (cliff avoidance test) was observed at 16 mg/kg/day when dams were dosed from 14 days before mating through lactation (45 times human exposure at the recommended daily oral dose of 2.5 mg and 13 times human exposure at the recommended once-monthly oral dose of 150 mg, based on AUC comparison).
In pregnant rabbits given oral doses of 1, 4, or 20 mg/kg/day during gestation, dose-related maternal mortality was observed in all treatment groups (>/=8 times the recommended human daily oral dose of 2.5 mg and >/=4 times the recommended human once-monthly oral dose of 150 mg, based on body surface area comparison, mg/m 2 ). The deaths occurred prior to parturition and were associated with lung edema and hemorrhage. No significant fetal anomalies were observed.
Bisphosphonates are incorporated into the bone matrix, from where they are gradually released over periods of weeks to years. The extent of bisphosphonate incorporation into adult bone, and hence, the amount available for release back into the systemic circulation, is directly related to the total dose and duration of bisphosphonate use. Although there are no data on fetal risk in humans, bisphosphonates do cause fetal harm in animals, and animal data suggest that uptake of bisphosphonates into fetal bone is greater than into maternal bone. Therefore, there is a theoretical risk of fetal harm (eg, skeletal and other abnormalities) if a woman becomes pregnant after completing a course of bisphosphonate therapy. The impact of variables such as time between cessation of bisphosphonate therapy to conception, the particular bisphosphonate used, and the route of administration (intravenous versus oral) on this risk has not been established.
There are no adequate and well-controlled studies in pregnant women. BONIVA should be used during pregnancy only if the potential benefit justifies the potential risk to the mother and fetus.
Nursing Mothers
In lactating rats treated with intravenous doses of 0.08 mg/kg, ibandronate was present in breast milk at concentrations of 8.1 to 0.4 ng/mL from 2 to 24 hours after dose administration. Concentrations in milk averaged 1.5 times plasma concentrations. It is not known whether BONIVA is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when BONIVA is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Of the patients receiving BONIVA 2.5 mg daily in postmenopausal osteoporosis studies, 52% were over 65 years of age, and 10% were over 75 years of age. Of the patients receiving BONIVA 150 mg once monthly in the postmenopausal osteoporosis 1-year study, 52% were over 65 years of age, and 9% were over 75 years of age. No overall differences in effectiveness or safety were observed between these patients and younger patients but greater sensitivity in some older individuals cannot be ruled out.
ADVERSE REACTIONS
Daily Dosing
Daily treatment with oral BONIVA was studied in over 3900 patients in postmenopausal osteoporosis trials of up to 3 years duration. The overall adverse event profile of BONIVA 2.5 mg once daily in these studies was similar to that of placebo.
Treatment and Prevention of Postmenopausal Osteoporosis
Most adverse events were mild or moderate and did not lead to discontinuation. The incidence of serious adverse events was 20% in the placebo group and 23% in the BONIVA 2.5 mg daily group. The percentage of patients who withdrew from treatment due to adverse events was approximately 17% in both the BONIVA 2.5 mg daily group and the placebo group. Overall, and according to body system, there was no difference between BONIVA and placebo, with adverse events of the digestive system being the most common reason for withdrawal.
Table 3 lists adverse events from the Treatment and Prevention Studies reported in >/=2% of patients and in more patients treated daily with BONIVA than patients treated with placebo. Adverse events are shown without attribution of causality.
Table 3 Adverse Events Occurring at a Frequency >/=2% and in More Patients Treated with BONIVA than in Patients Treated with Placebo Daily in the Osteoporosis Treatment and Prevention StudiesBody SystemPlacebo
%
(n=1134)BONIVA 2.5 mg
%
(n=1140)Body as a WholeBack Pain12.2 13.5 Pain in Extremity6.4 7.8 Infection3.4 4.3 Asthenia2.3 3.5 Allergic Reaction1.9 2.5 Digestive SystemDyspepsia9.8 11.9 Diarrhea5.0 6.8 Tooth Disorder2.3 3.5 Vomiting2.1 2.7 Gastritis1.9 2.2 Metabolic and Nutritional DisordersHypercholesterolemia4.2 4.8 Musculoskeletal SystemMyalgia5.1 5.7 Joint Disorder3.3 3.6 Arthritis2.7 3.2 Nervous SystemHeadache5.8 6.5 Dizziness2.6 3.7 Vertigo2.5 3.0 Nerve Root Lesion1.9 2.2 Respiratory SystemUpper Respiratory
Infection33.2 33.7 Bronchitis6.8 10.0 Pneumonia4.3 5.9 Pharyngitis1.5 2.5 Urogenital SystemUrinary Tract Infection4.2 5.5 Once-Monthly Dosing
In a 1-year, double-blind, multicenter study comparing BONIVA 2.5 mg once daily and BONIVA 150 mg once monthly in women with postmenopausal osteoporosis, the overall safety and tolerability profiles of the two oral dosing regimens were similar. The incidence of serious adverse events was 4.8% in the BONIVA 2.5 mg daily group and 7.1% in the BONIVA 150 mg once-monthly group. The percentage of patients who withdrew from treatment due to adverse events was approximately 8.9% in the BONIVA 2.5 mg daily group and 7.8% in the BONIVA 150 mg once-monthly group. Table 4 lists the adverse events reported in >/=2% of patients without attribution of causality.
Tabl e 4 Adverse Events With an Incidence of at Least 2% in Patients Treated with BONIVA 150 mg Once Monthly or 2.5 mg DailyBody System/Adverse Event BONIVA 2.5 mg Daily
%
(n=395)BONIVA 150 mg Monthly
%
(n=396)Vascular DisordersHypertension7.3 6.3 Gastrointestinal DisordersDyspepsia7.1 5.6 Nausea4.8 5.1 Diarrhea4.1 5.1 Constipation2.5 4.0 Abdominal Pain a5.3 7.8 Musculoskeletal and Connective Tissue DisordersArthralgia3.5 5.6 Back Pain4.3 4.5 Pain in Extremity1.3 4.0 Localized Osteoarthritis1.3 3.0 Myalgia0.8 2.0 Muscle Cramp2.0 1.8 Infections and InfestationsInfluenza3.8 4.0 Nasopharyngitis4.3 3.5 Bronchitis3.5 2.5 Urinary Tract Infection1.8 2.3 Upper Respiratory Tract
Infection2.0 2.0 Nervous System DisordersHeadache4.1 3.3 Dizziness1.0 2.3 General Disorders and Administration Site ConditionsInfluenza-like Illness b0.8 3.3 Skin and Subcutaneous Tissue DisordersRash c1.3 2.3 Psychiatric DisordersInsomnia0.8 2.0 a Combination of abdominal pain and abdominal pain upper b Combination of influenza-like illness and acute phase reaction c Combination of rash pruritic, rash macular, rash papular, rash generalized, rash erythematous, dermatitis, dermatitis allergic, dermatitis medicamentosa, erythema and exanthem Patients with a previous history of gastrointestinal disease, including patients with peptic ulcer without recent bleeding or hospitalization and patients with dyspepsia or reflux controlled by medication, were included in the once-monthly treatment study. For these patients, there was no difference in upper gastrointestinal adverse events with the 150 mg once-monthly regimen compared to the 2.5 mg once-daily regimen.
Ocular Adverse Events
Reports in the medical literature indicate that bisphosphonates may be associated with ocular inflammation such as uveitis and scleritis. In some cases, these events did not resolve until the bisphosphonate was discontinued. There were no reports of ocular inflammation in studies with BONIVA 2.5 mg daily. Two patients who received BONIVA once monthly experienced ocular inflammation, one was a case of uveitis and the other scleritis.
Laboratory Test Findings
In the 3-year treatment study with BONIVA 2.5 mg daily, there were no clinically significant changes from baseline values or shifts in any laboratory variable for each of the treatment groups. As expected with bisphosphonate treatment, a decrease in total alkaline phosphatase levels was seen in the active treatment groups compared to placebo. There was no difference compared with placebo for laboratory abnormalities indicative of hepatic or renal dysfunction, hypocalcemia, or hypophosphatemia. Similarly, no changes were noted for the 150 mg once-monthly administration in the 1-year study.
OVERDOSAGE
No specific information is available on the treatment of overdosage with BONIVA. However, based on knowledge of this class of compounds, oral overdosage may result in hypocalcemia, hypophosphatemia, and upper gastrointestinal adverse events, such as upset stomach, dyspepsia, esophagitis, gastritis, or ulcer. Milk or antacids should be given to bind BONIVA. Due to the risk of esophageal irritation, vomiting should not be induced, and the patient should remain fully upright. Dialysis would not be beneficial.
DOSAGE AND ADMINISTRATION
The recommended dose of BONIVA for treatment of postmenopausal osteoporosis is one 2.5-mg tablet taken once daily or one 150-mg tablet taken once monthly on the same date each month (see INDICATIONS AND USAGE ).
The recommended dose of BONIVA for the prevention of postmenopausal osteoporosis is one 2.5-mg tablet taken once daily. Alternatively, one 150-mg tablet taken once monthly on the same date each month may be considered (see INDICATIONS AND USAGE ).
- To maximize absorption and clinical benefit, BONIVA should be taken at least 60 minutes before the first food or drink (other than water) of the day or before taking any oral medication or supplementation, including calcium, antacids, or vitamins (see PRECAUTIONS : Information for Patients and Drug Interactions ).
- To facilitate delivery to the stomach and thus reduce the potential for esophageal irritation, BONIVA tablets should be swallowed whole with a full glass of plain water (6 to 8 oz) while the patient is standing or sitting in an upright position. Patients should not lie down for 60 minutes after taking BONIVA (see PRECAUTIONS : General and Information for Patients ).
- Plain water is the only drink that should be taken with BONIVA. Please note that some mineral waters may have a higher concentration of calcium and therefore should not be used.
- Patients should not chew or suck the tablet because of a potential for oropharyngeal ulceration.
- The BONIVA 150-mg tablet should be taken on the same date each month (ie, the patient's BONIVA day).
- If the once-monthly dose is missed, and the patient's next scheduled BONIVA day is more than 7 days away, the patient should be instructed to take one BONIVA 150-mg tablet in the morning following the date that it is remembered. The patient should then return to taking one BONIVA 150-mg tablet every month in the morning of their chosen day, according to their original schedule.
- The patient must not take two 150-mg tablets within the same week. If the patient's next scheduled BONIVA day is only 1 to 7 days away, the patient must wait until their next scheduled BONIVA day to take their tablet. The patient should then return to taking one BONIVA 150-mg tablet every month in the morning of their chosen day, according to their original schedule.
Patients should receive supplemental calcium or vitamin D if dietary intake is inadequate (see PRECAUTIONS : Information for Patients ).
Patients with Hepatic Impairment
No dose adjustment is necessary (see CLINICAL PHARMACOLOGY : Special Populations ).
Patients with Renal Impairment
No dose adjustment is necessary for patients with mild or moderate renal impairment where creatinine clearance is equal to or greater than 30 mL/min.
BONIVA is not recommended for use in patients with severe renal impairment (creatinine clearance of <30 mL/min) (see CLINICAL PHARMACOLOGY : Special Populations ).
Geriatric Patients
No dosage adjustment is necessary in the elderly (see PRECAUTIONS : Geriatric Use ).
HOW SUPPLIED
BONIVA 2.5-mg tablets: supplied as white, oblong, film-coated tablets, engraved with "IT" on one side and "L3" on the other side and packaged in bottles of 30 tablets (NDC 0004-0185-23).
BONIVA 150-mg tablets: supplied as white, oblong, film-coated tablets, engraved with "BNVA" on one side and "150" on the other side. Packaged in boxes of 3 blister packs containing 1 tablet each (NDC 0004-0186-82).
Storage
Store at 25°C (77°F); excursions permitted between 15° and 30°C (59° and 86°F) [see USP Controlled Room Temperature].
PATIENT INFORMATION
Read this patient information carefully before you start taking BONIVA. Read this patient information each time you get a refill for BONIVA. There may be new information. This information is not everything you need to know about BONIVA. It does not take the place of talking with your health care provider about your condition or your treatment. Talk about BONIVA with your health care provider before you start taking it, and at your regular check-ups.
What is the most important information I should know about BONIVA?
BONIVA may cause serious problems in the stomach and the esophagus (the tube that connects your mouth and stomach) such as trouble swallowing, heartburn, and ulcers (see " What are the possible side effects of BONIVA ? ").
You must take BONIVA exactly as prescribed for BONIVA to work for you and to lower the chance of serious side effects (see " How should I take BONIVA ?").
What is BONIVA?
BONIVA is a prescription medicine used to treat or prevent osteoporosis in women after menopause (see the end of this leaflet for " What is osteoporosis ? ").
BONIVA may reverse bone loss by stopping more loss of bone and increasing bone mass in most women who take it, even though they won't be able to see or feel a difference. BONIVA may help lower the chances of breaking bones (fractures).
For BONIVA to treat or prevent osteoporosis, you have to take it as prescribed. BONIVA will not work if you stop taking it.
Who should not take BONIVA?
Do not take BONIVA if you:
- have low blood calcium (hypocalcemia)
- cannot sit or stand up for at least 1 hour (60 minutes)
- have kidneys that work very poorly
- are allergic to ibandronate sodium or any of the other ingredients of BONIVA (see the end of this leaflet for a list of all the ingredients in BONIVA)
Tell your health care provider before using BONIVA:
- if you are pregnant or planning to become pregnant. It is not known if BONIVA can harm your unborn baby.
- if you are breast-feeding. It is not known if BONIVA passes into your milk and if it can harm your baby.
- have swallowing problems or other problems with your esophagus (the tube that connects your mouth and stomach)
- if you have kidney problems
- about all the medicines you take including prescription and non-prescription medicines, vitamins and supplements. Some medicines, especially certain vitamins, supplements, and antacids can stop BONIVA from getting to your bones. This can happen if you take other medicines too close to the time that you take BONIVA (see " How should I take BONIVA ? ").
How should I take BONIVA?
- Take BONIVA exactly as instructed by your health care provider.
- Take BONIVA first thing in the morning at least 1 hour (60 minutes) before you eat, drink anything other than plain water, or take any other oral medicine.
- Take BONIVA with 6 to 8 ounces (about 1 full cup) of plain water. Do not take it with any other drink besides plain water. Do not take it with other drinks, such as mineral water, sparkling water, coffee, tea, dairy drinks (such as milk), or juice.
- Swallow BONIVA whole. Do not chew or suck the tablet or keep it in your mouth to melt or dissolve.
-
After taking BONIVA you must wait at least 1 hour (60 minutes) before:
- Lying down. You may sit, stand, or do normal activities like read the newspaper or take a walk.
- Eating or drinking anything except for plain water.
- Taking other oral medicines including vitamins, calcium, or antacids. Take your vitamins, calcium, and antacids at a different time of the day from the time when you take BONIVA.
- If you take too much BONIVA, drink a full glass of milk and call your local poison control center or emergency room right away. Do not make yourself vomit. Do not lie down.
- Keep taking BONIVA for as long as your health care provider tells you. BONIVA will not work if you stop taking it.
- Your health care provider may tell you to exercise and take calcium and vitamin supplements to help your osteoporosis.
- Your health care provider may do a test to measure the thickness (density) of your bones or do other tests to check your progress.
What is my BONIVA schedule?
Schedule for taking BONIVA 150 mg once monthly:
- Take one BONIVA 150-mg tablet once a month.
- Choose one date of the month (your BONIVA day) that you will remember and that best fits your schedule to take your BONIVA 150-mg tablet.
- Take one BONIVA 150-mg tablet in the morning of your chosen day (see " How should I take BONIVA ? ").
What to do if I miss a monthly dose:
- If your next scheduled BONIVA day is more than 7 days away, take one BONIVA 150-mg tablet in the morning following the day that you remember (see " How should I take BONIVA ? "). Then return to taking one BONIVA 150-mg tablet every month in the morning of your chosen day, according to your original schedule.
- Do not take two 150-mg tablets within the same week. If your next scheduled BONIVA day is only 1 to 7 days away, wait until your next scheduled BONIVA day to take your tablet. Then return to taking one BONIVA 150-mg tablet every month in the morning of your chosen day, according to your original schedule.
- If you are not sure what to do if you miss a dose, contact your health care provider who will be able to advise you.
Schedule for taking BONIVA 2.5 mg once daily:
- Take one BONIVA 2.5-mg tablet once a day first thing in the morning at least 1 hour (60 minutes) before you eat, drink anything other than plain water, or take any other oral medicine (see " How should I take BONIVA ? ").
What to do if I miss a daily dose:
- If you forget to take your BONIVA 2.5-mg tablet in the morning, do not take it later in the day. Just return to your normal schedule and take 1 tablet the next morning. Do not take two tablets on the same day.
- If you are not sure what to do if you miss a dose, contact your health care provider who will be able to advise you.
What should I avoid while taking BONIVA?
- Do not take other medicines, or eat or drink anything but plain water before you take BONIVA and for at least 1 hour (60 minutes) after you take it.
- Do not lie down for at least 1 hour (60 minutes) after you take BONIVA.
What are the possible side effects of BONIVA?
Stop taking BONIVA and call your health care provider right away if you have:
- pain or trouble with swallowing
- chest pain
- very bad heartburn or heartburn that does not get better
BONIVA MAY CAUSE:
- pain or trouble swallowing (dysphagia)
- heartburn (esophagitis)
- ulcers in your stomach or esophagus (the tube that connects your mouth and stomach)
Common side effects with BONIVA are:
- diarrhea
- pain in extremities (arms or legs)
- dyspepsia (upset stomach)
Less common side effects with BONIVA are short-lasting, mild flu-like symptoms (usually improve after the first dose). These are not all the possible side effects of BONIVA. For more information ask your health care provider or pharmacist.
Rarely, patients have reported severe bone, joint, and/or muscle pain starting within one day to several months after beginning to take, by mouth, bisphosphonate drugs to treat osteoporosis (thin bones). This group of drugs includes BONIVA. Most patients experienced relief after stopping the drug. Contact your health care provider if you develop these symptoms after starting BONIVA.
What is osteoporosis?
Osteoporosis is a disease that causes bones to become thinner. Thin bones can break easily. Most people think of their bones as being solid like a rock. Actually, bone is living tissue, just like other parts of the body, such as your heart, brain, or skin. Bone just happens to be a harder type of tissue. Bone is always changing. Your body keeps your bones strong and healthy by replacing old bone with new bone.
Osteoporosis causes the body to remove more bone than it replaces. This means that bones get weaker. Weak bones are more likely to break. Osteoporosis is a bone disease that is quite common in women after menopause. At first, osteoporosis has no symptoms, but people with osteoporosis may develop loss of height and are more likely to break (fracture) their bones, especially the back (spine), wrist, and hip bones.
Osteoporosis can be prevented, and with proper therapy it can be treated.
Who is at risk for osteoporosis?
Talk to your health care provider about your chances for getting osteoporosis.
Many things put people at risk for osteoporosis. The following people have a higher chance of getting osteoporosis:
Women who:
- are going through or who are past menopause ("the change")
- are white (Caucasian) or Oriental (Asian)
People who:
- are thin
- have a family member with osteoporosis
- do not get enough calcium or vitamin D
- do not exercise
- smoke
- drink alcohol often
- take bone thinning medicines (like prednisone) for a long time
General information about BONIVA
Medicines are sometimes prescribed for conditions that are not mentioned in patient information. Do not use BONIVA for a condition for which it was not prescribed. Do not give BONIVA to other people, even if they have the same symptoms you have. It may harm them.
Store BONIVA at 77°F (25°C) or at room temperature between 59°F and 86°F (15°C and 30°C).
Keep BONIVA and all medicines out of the reach of children.
This summarizes the most important information about BONIVA. If you would like more information, talk with your health care provider. You can ask your health care provider or pharmacist for information about BONIVA that is written for health professionals.
For more information about BONIVA, call 1-888-MY-BONIVA or visit www.myboniva.com.
What are the ingredients of BONIVA?
BONIVA (active ingredient): ibandronate sodium
BONIVA (inactive ingredients): lactose monohydrate, povidone, microcrystalline cellulose, crospovidone, purified stearic acid, colloidal silicon dioxide, and purified water. The tablet film coating contains hypromellose, titanium dioxide, talc, polyethylene glycol 6000 and purified water.
BONIVA is a registered trademark of Roche Therapeutics Inc.
Distributed by:
Roche Laboratories Inc.
340 Kingsland Street
Nutley, New Jersey 07110-1199
Co-promoted by Roche Laboratories Inc. and
Research Triangle Park, NC 27709
Issued: March 2005
Subscribe to the "News" RSS Feed 
TOP ۞
