-
Crestor Tablets (Astrazeneca)
DESCRIPTION
CRESTOR ® (rosuvastatin calcium) is a synthetic lipid-lowering agent. Rosuvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, an early and rate-limiting step in cholesterol biosynthesis.
Rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid] calcium salt. The empirical formula for rosuvastatin calcium is (C 22 H 27 FN 3 O 6 S) 2 Ca. Its molecular weight is 1001.14. Its structural formula is:
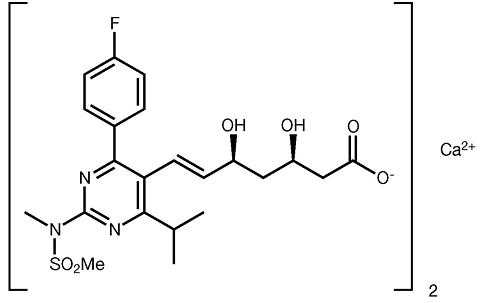
Rosuvastatin calcium is a white amorphous powder that is sparingly soluble in water and methanol, and slightly soluble in ethanol. Rosuvastatin is a hydrophilic compound with a partition coefficient (octanol/water) of 0.13 at pH of 7.0.
CRESTOR Tablets for oral administration contain 5, 10, 20, or 40 mg of rosuvastatin and the following inactive ingredients: microcrystalline cellulose NF, lactose monohydrate NF, tribasic calcium phosphate NF, crospovidone NF, magnesium stearate NF, hypromellose NF, triacetin NF, titanium dioxide USP, yellow ferric oxide, and red ferric oxide NF.
CLINICAL PHARMACOLOGY
General: In the bloodstream, cholesterol and triglycerides (TG) circulate as part of lipoprotein complexes. With ultracentrifugation, these complexes separate into very-low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), and low-density lipoprotein (LDL) fractions that contain apolipoprotein B-100 (ApoB-100) and high-density lipoprotein (HDL) fractions.
Cholesterol and TG synthesized in the liver are incorporated into VLDL and secreted into the circulation for delivery to peripheral tissues. TG are removed by the action of lipases, and in a series of steps, the modified VLDL is transformed first into IDL and then into cholesterol-rich LDL. IDL and LDL are removed from the circulation mainly by high affinity ApoB/E receptors, which are expressed to the greatest extent on liver cells. HDL is hypothesized to participate in the reverse transport of cholesterol from tissues back to the liver.
Epidemiologic, experimental, and clinical studies have established that high LDL cholesterol (LDL-C), low HDL cholesterol (HDL-C), and high plasma TG promote human atherosclerosis and are risk factors for developing cardiovascular disease. In contrast, higher levels of HDL-C are associated with decreased cardiovascular risk.
Like LDL, cholesterol-enriched triglyceride-rich lipoproteins, including VLDL, IDL, and remnants, can also promote atherosclerosis. Elevated plasma triglycerides are frequently found with low HDL-C levels and small LDL particles, as well as in association with non-lipid metabolic risk factors for coronary heart disease (CHD). As such, total plasma TG has not consistently been shown to be an independent risk factor for CHD. Furthermore, the independent effect of raising HDL or lowering TG on the risk of coronary and cardiovascular morbidity and mortality has not been determined.
Mechanism of Action: Rosuvastatin is a selective and competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl coenzyme A to mevalonate, a precursor of cholesterol. In vivo studies in animals, and in vitro studies in cultured animal and human cells have shown rosuvastatin to have a high uptake into, and selectivity for, action in the liver, the target organ for cholesterol lowering. In in vivo and in vitro studies, rosuvastatin produces its lipid-modifying effects in two ways. First, it increases the number of hepatic LDL receptors on the cell-surface to enhance uptake and catabolism of LDL. Second, rosuvastatin inhibits hepatic synthesis of VLDL, which reduces the total number of VLDL and LDL particles.
Rosuvastatin reduces total cholesterol (total-C), LDL-C, ApoB, and nonHDL-C (total cholesterol minus HDL-C) in patients with homozygous and heterozygous familial hypercholesterolemia (FH), nonfamilial forms of hypercholesterolemia, and mixed dyslipidemia. Rosuvastatin also reduces TG and produces increases in HDL-C. Rosuvastatin reduces total-C, LDL-C, VLDL-cholesterol (VLDL-C), ApoB, nonHDL-C, and TG, and increases HDL-C in patients with isolated hypertriglyceridemia. The effect of rosuvastatin on cardiovascular morbidity and mortality has not been determined.
Pharmacokinetics and Drug Metabolism
Absorption: In clinical pharmacology studies in man, peak plasma concentrations of rosuvastatin were reached 3 to 5 hours following oral dosing. Both peak concentration (C max ) and area under the plasma concentration-time curve (AUC) increased in approximate proportion to rosuvastatin dose. The absolute bioavailability of rosuvastatin is approximately 20%.
Administration of rosuvastatin with food decreased the rate of drug absorption by 20% as assessed by C max , but there was no effect on the extent of absorption as assessed by AUC.
Plasma concentrations of rosuvastatin do not differ following evening or morning drug administration.
Significant LDL-C reductions are seen when rosuvastatin is given with or without food, and regardless of the time of day of drug administration.
Distribution: Mean volume of distribution at steady-state of rosuvastatin is approximately 134 liters. Rosuvastatin is 88% bound to plasma proteins, mostly albumin. This binding is reversible and independent of plasma concentrations.
Metabolism: Rosuvastatin is not extensively metabolized; approximately 10% of a radiolabeled dose is recovered as metabolite. The major metabolite is N-desmethyl rosuvastatin, which is formed principally by cytochrome P450 2C9, and in vitro studies have demonstrated that N-desmethyl rosuvastatin has approximately one-sixth to one-half the HMG-CoA reductase inhibitory activity of rosuvastatin. Overall, greater than 90% of active plasma HMG-CoA reductase inhibitory activity is accounted for by rosuvastatin.
Excretion: Following oral administration, rosuvastatin and its metabolites are primarily excreted in the feces (90%). The elimination half-life (t 1/2 ) of rosuvastatin is approximately 19 hours.
After an intravenous dose, approximately 28% of total body clearance was via the renal route, and 72% by the hepatic route.
Special Populations
Race: A population pharmacokinetic analysis revealed no clinically relevant differences in pharmacokinetics among Caucasian, Hispanic, and Black or Afro-Caribbean groups. However, pharmacokinetic studies, including one conducted in the US, have demonstrated an approximate 2-fold elevation in median exposure (AUC and C max ) in Asian subjects when compared with a Caucasian control group (see WARNINGS, Myopathy/Rhabdomyolysis , PRECAUTIONS , General and DOSAGE AND ADMINISTRATION ).
Gender: There were no differences in plasma concentrations of rosuvastatin between men and women.
Geriatric: There were no differences in plasma concentrations of rosuvastatin between the nonelderly and elderly populations (age >/=65 years).
Pediatric: In a pharmacokinetic study, 18 patients (9 boys and 9 girls) 10 to 17 years of age with heterozygous FH received single and multiple oral doses of rosuvastatin. Both C max and AUC of rosuvastatin were similar to values observed in adult subjects administered the same doses.
Renal Insufficiency: Mild to moderate renal impairment (creatinine clearance >/= 30 mL/min/1.73m 2 ) had no influence on plasma concentrations of rosuvastatin when oral doses of 20 mg rosuvastatin were administered for 14 days. However, plasma concentrations of rosuvastatin increased to a clinically significant extent (about 3-fold) in patients with severe renal impairment (CL cr < 30 mL/min/1.73m 2 ) compared with healthy subjects (CL cr > 80 mL/min/1.73m 2 ) (see PRECAUTIONS , General ).
Hemodialysis: Steady-state plasma concentrations of rosuvastatin in patients on chronic hemodialysis were approximately 50% greater compared with healthy volunteer subjects with normal renal function.
Hepatic Insufficiency: In patients with chronic alcohol liver disease, plasma concentrations of rosuvastatin were modestly increased. In patients with Child-Pugh A disease, C max and AUC were increased by 60% and 5%, respectively, as compared with patients with normal liver function. In patients with Child-Pugh B disease, C max and AUC were increased 100% and 21%, respectively, compared with patients with normal liver function (see CONTRAINDICATIONS and WARNINGS , Liver Enzymes ).
Drug-Drug Interactions
Cytochrome P450 3A4: In vitro and in vivo data indicate that rosuvastatin clearance is not dependent on metabolism by cytochrome P450 3A4 to a clinically significant ex-tent. This has been confirmed in studies with known cyto-chrome P450 3A4 inhibitors (ketoconazole, erythromycin, itraconazole).
Ketoconazole: Coadministration of ketoconazole (200 mg twice daily for 7 days) with rosuvastatin (80 mg) resulted in no change in plasma concentrations of rosuvastatin.
Erythromycin: Coadministration of erythromycin (500 mg four times daily for 7 days) with rosuvastatin (80 mg) decreased AUC and C max of rosuvastatin by 20% and 31%, respectively. These reductions are not considered clinically significant.
Itraconazole: Itraconazole (200 mg once daily for 5 days) resulted in a 39% and 28% increase in AUC of rosuvastatin after 10 mg and 80 mg dosing, respectively. These increases are not considered clinically significant.
Fluconazole: Coadministration of fluconazole (200 mg once daily for 11 days) with rosuvastatin (80 mg) resulted in a 14% increase in AUC of rosuvastatin. This increase is not considered clinically significant.
Cyclosporine: Coadministration of cyclosporine with rosuvastatin resulted in no significant changes in cyclosporine plasma concentrations. However, C max and AUC of rosuva-statin increased 11- and 7-fold, respectively, compared with historical data in healthy subjects. These increases are considered to be clinically significant (see PRECAUTIONS Drug Interactions , WARNINGS , Myopathy/Rhabdomyolysis , and DOSAGE AND ADMINISTRATION ).
Warfarin: Coadministration of warfarin (25 mg) with rosuvastatin (40 mg) did not change warfarin plasma concentrations but increased the International Normalized Ratio (INR) (see PRECAUTIONS , Drug Interactions ).
Digoxin: Coadministration of digoxin (0.5 mg) with rosuvastatin (40 mg) resulted in no change to digoxin plasma concentrations.
Fenofibrate: Coadministration of fenofibrate (67 mg three times daily) with rosuvastatin (10 mg) resulted in no significant changes in plasma concentrations of rosuvastatin or fenofibrate (see PRECAUTIONS , Drug Interactions , and WARNINGS , Myopathy/Rhabdomyolysis ).
Gemfibrozil: Coadministration of gemfibrozil (600 mg twice daily for 7 days) with rosuvastatin (80 mg) resulted in a 90% and 120% increase for AUC and C max of rosuvastatin, respectively. This increase is considered to be clinically significant (see PRECAUTIONS , Drug Interactions , WARNINGS , Myopathy/Rhabdomyolysis , DOSAGE AND ADMINISTRATION ).
Antacid: Coadministration of an antacid (aluminum and magnesium hydroxide combination) with rosuvastatin (40 mg) resulted in a decrease in plasma concentrations of rosuvastatin by 54%. However, when the antacid was given 2 hours after rosuvastatin, there were no clinically significant changes in plasma concentrations of rosuvastatin (see PRECAUTIONS , Information for Patients ).
Oral contraceptives: Coadministration of oral contraceptives (ethinyl estradiol and norgestrel) with rosuvastatin resulted in an increase in plasma concentrations of ethinyl estradiol and norgestrel by 26% and 34%, respectively.
Clinical Studies
Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia (Fredrickson Type IIa and IIb)
CRESTOR reduces total-C, LDL-C, ApoB, nonHDL-C, and TG, and increases HDL-C, in patients with hypercholesterolemia and mixed dyslipidemia. Therapeutic response is seen within 1 week, and maximum response is usually achieved within 4 weeks and maintained during long-term therapy.
CRESTOR is effective in a wide variety of adult patient populations with hypercholesterolemia, with and without hypertriglyceridemia, regardless of race, gender, or age and in special populations such as diabetics or patients with heterozygous FH. Experience in pediatric patients has been limited to patients with homozygous FH.
Dose-Ranging Study: In a multicenter, double-blind, placebo-controlled, dose-ranging study in patients with hypercholesterolemia, CRESTOR given as a single daily dose for 6 weeks significantly reduced total-C, LDL-C, nonHDL-C, and ApoB, across the dose range (Table 1).
Table 1.
Dose-Response in Patients With
Primary Hypercholesterolemia (Adjusted Mean
% Change From Baseline at Week 6)DoseN Total-C LDL-C Non
HDL-CApoB TG HDL-C Placebo13 -5 -7 -7 -3 -3 3 517 -33 -45 -44 -38 -35 13 1017 -36 -52 -48 -42 -10 14 2017 -40 -55 -51 -46 -23 8 4018 -46 -63 -60 -54 -28 10 Active-Controlled Study: CRESTOR was compared with the HMG-CoA reductase inhibitors atorvastatin, simvastatin, and pravastatin in a multicenter, open-label, dose-ranging study of 2,240 patients with Type IIa and IIb hypercholesterolemia. After randomization, patients were treated for 6 weeks with a single daily dose of either CRESTOR, atorvastatin, simvastatin, or pravastatin (Figure 1 and Table 2).
Figure 1.
Percent LDL-C Change by Dose of CRESTOR,
Atorvastatin, Simvastatin, and Pravastatin at
Week 6 in Patients With Type IIa/IIb Dyslipidemia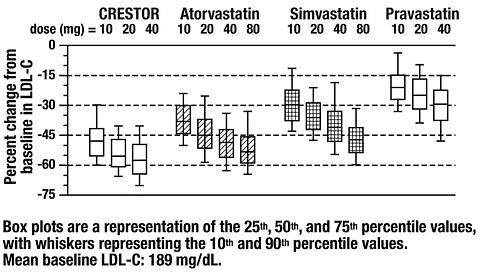
Table 2.
Percent Change in LDL-C From
Baseline to Week 6 (LS means § ) by
Treatment Group (sample sizes ranging
from 156-157 patients per group)Treatment Daily Dose Treatment10 mg 20 mg 40 mg 80 mg CRESTOR-46 * -52 **/* -55 **/** -- Atorvastatin-37 -43 -48 -51 Pravastatin-20 -24 -30 -- Simvastatin-28 -35 -39 -46 *CRESTOR 10 mg reduced LDL-C significantly more than atorvastatin 10 mg; pravastatin 10 mg, 20 mg, and 40 mg; simvastatin 10 mg, 20 mg, and 40 mg. (p<0.002) **/* CRESTOR 20 mg reduced LDL-C significantly more than atorvastatin 20 mg and 40 mg; pravastatin 20 mg and 40 mg; simvastatin 20 mg, 40 mg, and 80 mg. (p<0.002) **/** CRESTOR 40 mg reduced LDL-C significantly more than atorvastatin 40 mg; pravastatin 40 mg; simvastatin 40 mg and 80 mg. (p<0.002) § Corresponding standard errors are approximately 1.00 Heterozygous Familial Hypercholesterolemia
In a study of patients with heterozygous FH (baseline mean LDL of 291), patients were randomized to CRESTOR 20 mg or atorvastatin 20 mg. The dose was increased by 6-week intervals. Significant LDL-C reductions from baseline were seen at each dose in both treatment groups (Table 3).
Table 3.
Mean LDL-C Percentage Change from BaselineCRESTOR
(n=435)
LS Mean * (95% CI)Atorvastatin
(n=187)
LS Mean (95% CI)Week 620 mg -47% (-49%, -46%) -38% (-40%, -36%) Week 1240 mg -55% (-57%, -54%) -47% (-49%, -45%) Week 1880 mg NA -52% (-54%, -50%) *LS Means are least square means adjusted for baseline LDL. Hypertriglyceridemia
(Fredrickson Type IIb & IV)In a double-blind, placebo-controlled dose-response study in patients with baseline TG levels from 273 to 817 mg/dL, CRESTOR given as a single daily dose (5 to 40 mg) over 6 weeks significantly reduced serum TG levels (Table 4).
Table 4.
Dose-Response in Patients With Primary Hypertriglyceridemia Over 6 Weeks
Dosing Median (Min, Max) Percent Change From BaselineDosePlacebo
N=26CRESTOR
5 mg
N=25CRESTOR
10 mg
N=23CRESTOR
20 mg
N=27CRESTOR
40 mg
N=25Triglycerides1 (-40, 72)-21 (-58, 38)-37 (-65, 5)-37 (-72, 11)-43 (-80, -7)NonHDL-C2 (-13, 19)-29 (-43, -8)-49 (-59, -20)-43 (-74, 12)-51 (-62, -6)VLDL-C2 (-36, 53)-25 (-62, 49)-48 (-72, 14)-49 (-83, 20)-56 (-83, 10)Total-C1 (-13, 17)-24 (-40, -4)-40 (-51, -14)-34 (-61, -11)-40 (-51, -4)LDL-C5 (-30, 52)-28 (-71, 2)-45 (-59, 7)-31 (-66, 34)-43 (-61, -3)HDL-C-3 (-25, 18)3 (-38, 33)8 (-8, 24)22 (-5, 50)17 (-14, 63)Homozygous Familial Hypercholesterolemia
In an open-label, forced-titration study, homozygous FH patients (n=40, 8-63 years) were evaluated for their response to CRESTOR 20 to 40 mg titrated at a 6-week interval. In the overall population, the mean LDL-C reduction from baseline was 22%. About one-third of the patients benefited from increasing their dose from 20 mg to 40 mg with further LDL lowering of greater than 6%. In the 27 patients with at least a 15% reduction in LDL-C, the mean LDL-C reduction was 30% (median 28% reduction). Among 13 patients with an LDL-C reduction of <15%, 3 had no change or an increase in LDL-C. Reductions in LDL-C of 15% or greater were observed in 3 of 5 patients with known receptor negative status.
INDICATIONS AND USAGE
CRESTOR is indicated:
- as an adjunct to diet to reduce elevated total-C, LDL-C, ApoB, nonHDL-C, and TG levels and to increase HDL-C in patients with primary hypercholesterolemia (heterozygous familial and nonfamilial) and mixed dyslipidemia (Fredrickson Type IIa and IIb);
- as an adjunct to diet for the treatment of patients with elevated serum TG levels (Fredrickson Type IV);
- to reduce LDL-C, total-C, and ApoB in patients with homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable.
According to NCEP-ATPIII guidelines, therapy with lipid-altering agents should be a component of multiple-risk-factor intervention in individuals at increased risk for coronary heart disease due to hypercholesterolemia. The two major modalities of LDL-lowering therapy are therapeutic lifestyle changes (TLC) and drug therapy. The TLC Diet stresses reductions in saturated fat and cholesterol intake. Table 5 defines LDL-C goals and cutpoints for initiation of TLC and for drug consideration.
Table 5.
NCEP Treatment Guidelines: LDL-C Goals and Cutpoints for Therapeutic Lifestyle
Changes and Drug Therapy in Different Risk CategoriesRisk Category LDL Goal LDL level at which
to initiate TLCLDL level at which to
consider drug therapyCHD a or CHD Risk Equivalent (10-year risk > 20%) <100 mg/dL >/=100 mg/dL >/=130 mg/dL
(100-129 mg/dL: drug optional) b2+ Risk Factors
(10-year risk </= 20%)<130
mg/dL>/=130 mg/dL >/=130 mg/dL
10-year risk 10-20%>/=160 mg/dL
10-year risk <10%0-1 Risk Factor c <160
mg/dL>/=160 mg/dL >/=190 mg/dL (160-189 mg/dL
(LDL-lowering drug optional)a CHD = coronary heart disease. b Some authorities recommend use of LDL-lowering drugs in this category if an LDL-C <100 mg/dL cannot be achieved by TLC. Others prefer use of drugs that primarily modify triglycerides and HDL-C, e.g., nicotinic acid or fibrate. Clinical judgment also may call for deferring drug therapy in this subcategory. c Almost all people with 0-1 risk factor have 10-year risk <10%; thus, 10-year risk assessment in people with 0-1 risk factor is not necessary. After the LDL-C goal has been achieved, if the TG is still >/= 200 mg/dL, nonHDL-C (total-C minus HDL-C) becomes a secondary target of therapy. NonHDL-C goals are set 30 mg/dL higher than LDL-C goals for each risk category.
At the time of hospitalization for a coronary event, consideration can be given to initiating drug therapy at discharge if the LDL-C is >/= 130 mg/dL (see NCEP Treatment Guidelines, above).
Patients >20 years of age should be screened for elevated cholesterol levels every 5 years.
Prior to initiating therapy with CRESTOR, secondary causes for hypercholesterolemia (e.g., poorly-controlled diabetes mellitus, hypothyroidism, nephrotic syndrome, dyslipoproteinemias, obstructive liver disease, other drug therapy, and alcoholism) should be excluded, and a lipid profile performed to measure total-C, LDL-C, HDL-C, and TG. For patients with TG <400 mg/dL (<4.5 mmol/L), LDL-C can be estimated using the following equation: LDL-C = total-C - (0.20 × [TG] + HDL-C). For TG levels >400 mg/dL (>4.5 mmol/L), this equation is less accurate and LDL-C concentrations should be determined by ultracentrifugation.
CRESTOR has not been studied in Fredrickson Type I, III, and V dyslipidemias.
CONTRAINDICATIONS
CRESTOR is contraindicated in patients with a known hypersensitivity to any component of this product.
Rosuvastatin is contraindicated in patients with active liver disease or with unexplained persistent elevations of serum transaminases (see WARNINGS , Liver Enzymes ).
Pregnancy and Lactation
Atherosclerosis is a chronic process and discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia. Cholesterol and other products of cholesterol biosynthesis are essential components for fetal development (including synthesis of steroids and cell membranes). Since HMG-CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, they may cause fetal harm when administered to pregnant women. Therefore, HMG-CoA reductase inhibitors are contraindicated during pregnancy and in nursing mothers. ROSUVASTATIN SHOULD BE ADMINISTERED TO WOMEN OF CHILDBEARING AGE ONLY WHEN SUCH PATIENTS ARE HIGHLY UNLIKELY TO CONCEIVE AND HAVE BEEN INFORMED OF THE POTENTIAL HAZARDS. If the patient becomes pregnant while taking this drug, therapy should be discontinued immediately and the patient apprised of the potential hazard to the fetus.
WARNINGS
Liver Enzymes
HMG-CoA reductase inhibitors, like some other lipid-lowering therapies, have been associated with biochemical abnormalities of liver function. The incidence of persistent elevations (>3 times the upper limit of normal [ULN] occurring on 2 or more consecutive occasions) in serum transaminases in fixed dose studies was 0.4, 0, 0, and 0.1% in patients who received rosuvastatin 5, 10, 20, and 40 mg, respectively. In most cases, the elevations were transient and resolved or improved on continued therapy or after a brief interruption in therapy. There were two cases of jaundice, for which a relationship to rosuvastatin therapy could not be determined, which resolved after discontinuation of therapy. There were no cases of liver failure or irreversible liver disease in these trials.
It is recommended that liver function tests be performed before and at 12 weeks following both the initiation of therapy and any elevation of dose, and periodically (e.g., semiannually) thereafter. Liver enzyme changes generally occur in the first 3 months of treatment with rosuvastatin. Patients who develop increased transaminase levels should be monitored until the abnormalities have resolved. Should an increase in ALT or AST of >3 times ULN persist, reduction of dose or withdrawal of rosuvastatin is recommended.
Rosuvastatin should be used with caution in patients who consume substantial quantities of alcohol and/or have a history of liver disease (see CLINICAL PHARMACOLOGY , Special Populations , Hepatic Insufficiency ). Active liver disease or unexplained persistent transaminase eleva-tions are contraindications to the use of rosuvastatin (see CONTRAINDICATIONS ).
Myopathy/Rhabdomyolysis
Rare cases of rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with rosuvastatin and with other drugs in this class.
Uncomplicated myalgia has been reported in rosuvastatin-treated patients (see ADVERSE REACTIONS ). Creatine kinase (CK) elevations (>10 times upper limit of normal) occurred in 0.2% to 0.4% of patients taking rosuvastatin at doses up to 40 mg in clinical studies. Treatment-related myopathy, defined as muscle aches or muscle weakness in conjunction with increases in CK values >10 times upper limit of normal, was reported in up to 0.1% of patients taking rosuvastatin doses of up to 40 mg in clinical studies. In clinical trials, the incidence of myopathy and rhabdomyolysis increased at doses of rosuvastatin above the recommended dosage range (5 to 40 mg). In postmarketing experience, effects on skeletal muscle, e.g. uncomplicated myalgia, myopathy and, rarely, rhabdomyolysis have been reported in patients treated with HMG-CoA reductase inhibitors including rosuvastatin. As with other HMG-CoA reductase inhibitors, reports of rhabdomyolysis with rosuvastatin are rare, but higher at the highest marketed dose (40 mg). Factors that may predispose patients to myopathy with HMG-CoA reductase inhibitors include advanced age (>/=65 years), hypothyroidism, and renal insufficiency.
Consequently:
- Rosuvastatin should be prescribed with caution in patients with predisposing factors for myopathy, such as, renal impairment (see DOSAGE AND ADMINISTRATION ), advanced age, and inadequately treated hypothyroidism.
- Patients should be advised to promptly report unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever. Rosuvastatin therapy should be discontinued if markedly elevated CK levels occur or myopathy is diagnosed or suspected.
- The 40 mg dose of rosuvastatin is reserved only for those patients who have not achieved their LDL-C goal utilizing the 20 mg dose of rosuvastatin once daily (see DOSAGE AND ADMINISTRATION ).
- The risk of myopathy during treatment with rosuvastatin may be increased with concurrent administration of other lipid-lowering therapies or cyclosporine, (see CLINICAL PHARMACOLOGY , Drug-Drug Interactions , PRECAUTIONS , Drug Interactions , and DOSAGE AND ADMINISTRATION ). The benefit of further alterations in lipid levels by the combined use of rosuvastatin with fibrates or niacin should be carefully weighed against the potential risks of this combination. Combination therapy with rosuvastatin and gemfibrozil should generally be avoided. (See DOSAGE AND ADMINISTRATION and PRECAUTIONS , Drug Interactions ).
- The risk of myopathy during treatment with rosuvastatin may be increased in circumstances which increase rosuvastatin drug levels (see CLINICAL PHARMACOLOGY , Special Populations , Race and Renal Insufficiency , and PRECAUTIONS , General ).
- Rosuvastatin therapy should also be temporarily withheld in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g., sepsis, hypotension, major surgery, trauma, severe metabolic, endocrine, and electrolyte disorders, or uncontrolled seizures).
PRECAUTIONS
General
Before instituting therapy with rosuvastatin, an attempt should be made to control hypercholesterolemia with appropriate diet and exercise, weight reduction in obese patients, and treatment of underlying medical problems (see INDICATIONS AND USAGE ).
Administration of rosuvastatin 20 mg to patients with severe renal impairment (CL cr <30 mL/min/1.73 m 2 ) resulted in a 3-fold increase in plasma concentrations of rosuva-statin compared with healthy volunteers (see WARNINGS , Myopathy/Rhabdomyolysis and DOSAGE AND ADMINISTRATION ).
The result of a large pharmacokinetic study conducted in the US demonstrated an approximate 2-fold elevation in median exposure in Asian subjects (having either Filipino, Chinese, Japanese, Korean, Vietnamese or Asian-Indian origin) compared with a Caucasian control group. This increase should be considered when making rosuvastatin dosing decisions for Asian patients (see WARNINGS Myopathy/Rhabdomyolysis ; CLINICAL PHARMACOLOGY , Special Populations , Race , and DOSAGE AND ADMINISTRATION ).
Information for Patients
Patients should be advised to report promptly unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever.
When taking rosuvastatin with an aluminum and magnesium hydroxide combination antacid, the antacid should be taken at least 2 hours after rosuvastatin administration (see CLINICAL PHARMACOLOGY , Drug-Drug Interactions ).
Laboratory Tests
In the rosuvastatin clinical trial program, dipstick-positive proteinuria and microscopic hematuria were observed among rosuvastatin treated patients, predominantly in patients dosed above the recommended dose range (i.e., 80 mg). However, this finding was more frequent in patients taking rosuvastatin 40 mg, when compared to lower doses of rosuvastatin or comparator statins, though it was generally transient and was not associated with worsening renal function. Although the clinical significance of this finding is unknown, a dose reduction should be considered for patients on rosuvastatin 40 mg therapy with unexplained persistent proteinuria during routine urinalysis testing.
Drug Interactions
Cyclosporine: When rosuvastatin 10 mg was coadministered with cyclosporine in cardiac transplant patients, rosuvastatin mean C max and mean AUC were increased 11-fold and 7-fold, respectively, compared with healthy volunteers. These increases are considered to be clinically significant and require special consideration in the dosing of rosuvastatin to patients taking concomitant cyclosporine (see WARNINGS , Myopathy/Rhabdomyolysis , and DOSAGE AND ADMINISTRATION ).
Warfarin: Coadministration of rosuvastatin to patients on stable warfarin therapy resulted in clinically significant rises in INR (>4, baseline 2-3). In patients taking coumarin anticoagulants and rosuvastatin concomitantly, INR should be determined before starting rosuvastatin and frequently enough during early therapy to ensure that no significant alteration of INR occurs. Once a stable INR time has been documented, INR can be monitored at the intervals usually recommended for patients on coumarin anticoagulants. If the dose of rosuvastatin is changed, the same procedure should be repeated. Rosuvastatin therapy has not been associated with bleeding or with changes in INR in patients not taking anticoagulants.
Gemfibrozil: Coadministration of a single rosuvastatin dose to healthy volunteers on gemfibrozil (600 mg twice daily) resulted in 2.2- and 1.9-fold, respectively, increase in mean C max and mean AUC of rosuvastatin (see DOSAGE AND ADMINISTRATION ).
Endocrine Function
Although clinical studies have shown that rosuvastatin alone does not reduce basal plasma cortisol concentration or impair adrenal reserve, caution should be exercised if any HMG-CoA reductase inhibitor or other agent used to lower cholesterol levels is administered concomitantly with drugs that may decrease the levels or activity of endogenous steroid hormones such as ketoconazole, spironolactone, and cimetidine.
CNS Toxicity
CNS vascular lesions, characterized by perivascular hemorrhages, edema, and mononuclear cell infiltration of perivascular spaces, have been observed in dogs treated with several other members of this drug class. A chemically similar drug in this class produced dose-dependent optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in dogs, at a dose that produced plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose. Edema, hemorrhage, and partial necrosis in the interstitium of the choroid plexus was observed in a female dog sacrificed moribund at day 24 at 90 mg/kg/day by oral gavage (systemic exposures 100 times the human exposure at 40 mg/day based on AUC comparisons). Corneal opacity was seen in dogs treated for 52 weeks at 6 mg/kg/day by oral gavage (systemic exposures 20 times the human exposure at 40 mg/day based on AUC comparisons). Cataracts were seen in dogs treated for 12 weeks by oral gavage at 30 mg/kg/day (systemic exposures 60 times the human exposure at 40 mg/day based on AUC comparisons). Retinal dysplasia and retinal loss were seen in dogs treated for 4 weeks by oral gavage at 90 mg/kg/day (systemic exposures 100 times the human exposure at 40 mg/day based on AUC). Doses </=30 mg/kg/day (systemic exposures </=60 times the human exposure at 40 mg/day based on AUC comparisons) following treatment up to one year, did not reveal retinal findings.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104-week carcinogenicity study in rats at dose levels of 2, 20, 60, or 80 mg/kg/day by oral gavage, the incidence of uterine stromal polyps was significantly increased in females at 80 mg/kg/day at systemic exposure 20 times the human exposure at 40 mg/day based on AUC. Increased incidence of polyps was not seen at lower doses.
In a 107-week carcinogenicity study in mice given 10, 60, 200 mg/kg/day by oral gavage, an increased incidence of hepatocellular adenoma/carcinoma was observed at 200 mg/kg/day at systemic exposures 20 times human exposure at 40 mg/day based on AUC. An increased incidence of hepatocellular tumors was not seen at lower doses.
Rosuvastatin was not mutagenic or clastogenic with or without metabolic activation in the Ames test with Salmonella typhimurium and Escherichia coli , the mouse lymphoma assay, and the chromosomal aberration assay in Chinese hamster lung cells. Rosuvastatin was negative in the in vivo mouse micronucleus test.
In rat fertility studies with oral gavage doses of 5, 15, 50 mg/kg/day, males were treated for 9 weeks prior to and throughout mating and females were treated 2 weeks prior to mating and throughout mating until gestation day 7. No adverse effect on fertility was observed at 50 mg/kg/day (systemic exposures up to 10 times human exposure at 40 mg/day based on AUC comparisons). In testicles of dogs treated with rosuvastatin at 30 mg/kg/day for one month, spermatidic giant cells were seen. Spermatidic giant cells were observed in monkeys after 6-month treatment at 30 mg/kg/day in addition to vacuolation of seminiferous tubular epithelium. Exposures in the dog were 20 times and in the monkey 10 times human exposure at 40 mg/day based on body surface area comparisons. Similar findings have been seen with other drugs in this class.
Pregnancy
Pregnancy Category X
See CONTRAINDICATIONS .
Rosuvastatin may cause fetal harm when administered to a pregnant woman. Rosuvastatin is contraindicated in women who are or may become pregnant. Safety in pregnant women has not been established. There are no adequate and well-controlled studies of rosuvastatin in pregnant women. Rosuvastatin crosses the placenta and is found in fetal tissue and amniotic fluid at 3% and 20%, respectively, of the maternal plasma concentration following a single 25 mg/kg oral gavage dose on gestation day 16 in rats. A higher fetal tissue distribution (25% maternal plasma concentration) was observed in rabbits after a single oral gavage dose of 1 mg/kg on gestation day 18. If this drug is administered to a woman with reproductive potential, the patient should be apprised of the potential hazard to a fetus.
In female rats given oral gavage doses of 5, 15, 50 mg/kg/day rosuvastatin before mating and continuing through day 7 postcoitus results in decreased fetal body weight (female pups) and delayed ossification at the high dose (systemic exposures 10 times human exposure at 40 mg/day based on AUC comparisons).
In pregnant rats given oral gavage doses of 2, 10, 50 mg/kg/day from gestation day 7 through lactation day 21 (weaning), decreased pup survival occurred in groups given 50 mg/kg/day, systemic exposures >/=12 times human exposure at 40 mg/day based on body surface area comparisons.
In pregnant rabbits given oral gavage doses of 0.3, 1, 3 mg/kg/day from gestation day 6 to lactation day 18 (weaning), exposures equivalent to human exposure at 40 mg/day based on body surface area comparisons, decreased fetal viability and maternal mortality was observed.
Rosuvastatin was not teratogenic in rats at </=25 mg/kg/day or in rabbits </=3 mg/kg/day (systemic exposures equivalent to human exposure at 40 mg/day based on AUC or body surface comparison, respectively).
Nursing Mothers
It is not known whether rosuvastatin is excreted in human milk. Studies in lactating rats have demonstrated that rosuvastatin is secreted into breast milk at levels 3 times higher than that obtained in the plasma following oral gavage dosing. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from rosuvastatin, a decision should be made whether to discontinue nursing or administration of rosuvastatin taking into account the importance of the drug to the lactating woman.
Pediatric Use
The safety and effectiveness in pediatric patients have not been established. Treatment experience with rosuvastatin in a pediatric population is limited to 8 patients with homozygous FH. None of these patients was below 8 years of age.
Geriatric Use
Of the 10,275 patients in clinical studies with rosuvastatin, 3,159 (31%) were 65 years and older, and 698 (6.8%) were 75 years and older. The overall frequency of adverse events and types of adverse events were similar in patients above and below 65 years of age. (See WARNINGS , Myopathy/Rhabdomyolysis .)
The efficacy of rosuvastatin in the geriatric population (>/=65 years of age) was comparable to the efficacy observed in the non-elderly.
ADVERSE REACTIONS
Rosuvastatin is generally well tolerated. Adverse reactions have usually been mild and transient. In clinical studies of 10,275 patients, 3.7% were discontinued due to adverse experiences attributable to rosuvastatin. The most frequent adverse events thought to be related to rosuvastatin were myalgia, constipation, asthenia, abdominal pain, and nausea.
Clinical Adverse Experiences
Adverse experiences, regardless of causality assessment, reported in >/=2% of patients in placebo-controlled clinical studies of rosuvastatin are shown in Table 6; discontinuations due to adverse events in these studies of up to 12 weeks duration occurred in 3% of patients on rosuvastatin and 5% on placebo.
Table 6. Adverse Events in Placebo-Controlled Studies Adverse EventRosuvastatin
N=744Placebo
N=382Pharyngitis9.0 7.6 Headache5.5 5.0 Diarrhea3.4 2.9 Dyspepsia3.4 3.1 Nausea3.4 3.1 Myalgia2.8 1.3 Asthenia2.7 2.6 Back Pain2.6 2.4 Flu syndrome2.3 1.8 Urinary tract infection2.3 1.6 Rhinitis2.2 2.1 Sinusitis2.0 1.8 In addition, the following adverse events were reported, regardless of causality assessment, in >/=1% of 10,275 patients treated with rosuvastatin in clinical studies. The events in italics occurred in >/=2% of these patients.
Body as a Whole: Abdominal pain, accidental injury, chest pain, infection, pain , pelvic pain, and neck pain.
Cardiovascular System: Hypertension , angina pectoris, vasodilatation, and palpitation.
Digestive System: Constipation, gastroenteritis , vomiting, flatulence, periodontal abscess, and gastritis.
Endocrine: Diabetes mellitus.
Hemic and Lymphatic System: Anemia and ecchymosis.
Metabolic and Nutritional Disorders: Peripheral edema .
Musculoskeletal System: Arthritis, arthralgia , and pathological fracture.
Nervous System: Dizziness, insomnia, hypertonia, paresthesia, depression , anxiety, vertigo and neuralgia.
Respiratory System: Bronchitis, cough increased , dyspnea, pneumonia, and asthma.
Skin and Appendages: Rash and pruritus.
Laboratory Abnormalities: In the rosuvastatin clinical trial program, dipstick-positive proteinuria and microscopic hematuria were observed among rosuvastatin-treated patients, predominantly in patients dosed above the recommended dose range (i.e., 80 mg). However, this finding was more frequent in patients taking rosuvastatin 40 mg, when compared to lower doses of rosuvastatin or comparator statins, though it was generally transient and was not associated with worsening renal function. (See PRECAUTIONS , Laboratory Tests .)
Other abnormal laboratory values reported were elevated creatine phosphokinase, transaminases, hyperglycemia, glutamyl transpeptidase, alkaline phosphatase, bilirubin, and thyroid function abnormalities.
Other adverse events reported less frequently than 1% in the rosuvastatin clinical study program, regardless of causality assessment, included arrhythmia, hepatitis, hypersensitivity reactions (i.e., face edema, thrombocytopenia, leukopenia, vesiculobullous rash, urticaria, and angioedema), kidney failure, syncope, myasthenia, myositis, pancreatitis, photosensitivity reaction, myopathy, and rhabdomyolysis.
Postmarketing Experience
In addition to the events reported above, as with other drugs in this class, the following event has been reported during postmarketing experience with CRESTOR, regardless of causality assessment: very rare cases of jaundice.
OVERDOSAGE
There is no specific treatment in the event of overdose. In the event of overdose, the patient should be treated symptomatically and supportive measures instituted as required. Hemodialysis does not significantly enhance clearance of rosuvastatin.
DOSAGE AND ADMINISTRATION
The patient should be placed on a standard cholesterol-lowering diet before receiving CRESTOR and should continue on this diet during treatment. CRESTOR can be administered as a single dose at any time of day, with or without food.
Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia (Fredrickson Type IIa and IIb)
The dose range for CRESTOR is 5 to 40 mg once daily. Therapy with CRESTOR should be individualized according to goal of therapy and response. The usual recommended starting dose of CRESTOR is 10 mg once daily. However, initiation of therapy with 5 mg once daily should be considered for patients requiring less aggressive LDL-C reductions, who have predisposing factors for myopathy, and as noted below for special populations such as patients taking cyclosporine, Asian patients, and patients with severe renal insufficiency (see CLINICAL PHARMACOLOGY , Race , and Renal Insufficiency , and Drug-Drug Interactions ). For patients with marked hypercholesterolemia (LDL-C > 190 mg/dL) and aggressive lipid targets, a 20-mg starting dose may be considered. After initiation and/or upon titration of CRESTOR, lipid levels should be analyzed within 2 to 4 weeks and dosage adjusted accordingly.
The 40-mg dose of CRESTOR is reserved only for those patients who have not achieved their LDL-C goal utilizing the 20 mg dose of CRESTOR once daily (see WARNINGS , Myopathy/Rhabdomyolysis). When initiating statin therapy or switching from another statin therapy, the appropriate CRESTOR starting dose should first be utilized, and only then titrated according to the patient's individualized goal of therapy.
Homozygous Familial Hypercholesterolemia
The recommended starting dose of CRESTOR is 20 mg once daily in patients with homozygous FH. The maximum recommended daily dose is 40 mg. CRESTOR should be used in these patients as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable. Response to therapy should be estimated from pre-apheresis LDL-C levels.
Dosage in Asian Patients
Initiation of CRESTOR therapy with 5 mg once daily should be considered for Asian patients. The potential for increased systemic exposures relative to Caucasians is relevant when considering escalation of dose in cases where hypercholesterolemia is not adequately controlled at doses of 5, 10, or 20 mg once daily (see WARNINGS , Myopathy/Rhabdomyolysis , CLINICAL PHARMACOLOGY , Special Populations , Race , and PRECAUTIONS , General ).
Dosage in Patients Taking Cyclosporine
In patients taking cyclosporine, therapy should be limited to CRESTOR 5 mg once daily (see WARNINGS Myopathy/Rhabdomyolysis , and PRECAUTIONS , Drug Interactions ).
Concomitant Lipid-Lowering Therapy
The effect of CRESTOR on LDL-C and total-C may be enhanced when used in combination with a bile acid binding resin. If CRESTOR is used in combination with gemfibrozil, the dose of CRESTOR should be limited to 10 mg once daily (see WARNINGS , Myopathy/Rhabdomyolysis , and PRECAUTIONS , Drug Interactions ).
Dosage in Patients With Renal Insufficiency
No modification of dosage is necessary for patients with mild to moderate renal insufficiency. For patients with severe renal impairment (CL cr <30 mL/min/1.73 m 2 ) not on hemodialysis, dosing of CRESTOR should be started at 5 mg once daily and not to exceed 10 mg once daily (see PRECAUTIONS , General , and CLINICAL PHARMACOLOGY , Special Populations , Renal Insufficiency ).
HOW SUPPLIED
CRESTOR ® (rosuvastatin calcium) Tablets are supplied as:
5 mg tablets: Yellow, round, biconvex, coated tablets identified as "CRESTOR" and "5" debossed on one side and plain on the other side of the tablet.
(NDC 0310-0755-90) bottles of 90
10 mg tablets: Pink, round, biconvex, coated tablets identified as "CRESTOR" and "10" debossed on one side and plain on the other side of the tablet.
(NDC 0310-0751-90) bottles of 90
(NDC 0310-0751-39) unit dose packages of 100
20 mg tablets: Pink, round, biconvex, coated tablets identified as "CRESTOR" and "20" debossed on one side and plain on the other side of the tablet.
(NDC 0310-0752-90) bottles of 90
(NDC 0310-0752-39) unit dose packages of 100
40 mg tablets: Pink, oval, biconvex, coated tablets identified as "CRESTOR" debossed on one side and "40" debossed on the other side of the tablet.
(NDC 0310-0754-30) bottles of 30
(NDC 0310-0754-39) unit dose packages of 100
Storage
Store at controlled room temperature, 20-25°C (68-77°F) [see USP]. Protect from moisture.
Rx only
CRESTOR is a trademark of the Astrazeneca group of companies.
© Astrazeneca 2003, 2005
Licensed from SHIONOGI & CO., LTD., Osaka, Japan
Manufactured for:
Astrazeneca Pharmaceuticals LP
Wilmington, DE 19850
By: IPR Pharmaceuticals, Inc.
Carolina, PR 00984
630301
Rev. 03/05
Subscribe to the "News" RSS Feed 
TOP ۞
