-
Imodium Capsules (McNeil Consumer)
DESCRIPTION
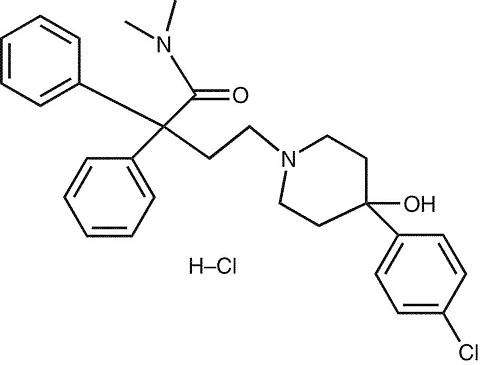
IMODIUM® (loperamide hydrochloride), 4-(p-chlorophenyl)-4-hydroxy-N, N-dimethyl-(alpha),(alpha)-diphenyl-1-piperidinebutyramide monohydrochloride, is a synthetic antidiarrheal for oral use.
IMODIUM® is available in 2 mg capsules.
The inactive ingredients are: Lactose, cornstarch, talc, and magnesium stearate. IMODIUM® capsules contain FD&C Yellow No. 6.
CLINICAL PHARMACOLOGY
In vitro and animal studies show that IMODIUM® (loperamide hydrochloride) acts by slowing intestinal motility and by affecting water and electrolyte movement through the bowel. IMODIUM® inhibits peristaltic activity by a direct effect on the circular and longitudinal muscles of the intestinal wall.
In man, IMODIUM® prolongs the transit time of the intestinal contents. It reduces the daily fecal volume, increases the viscosity and bulk density, and diminishes the loss of fluid and electrolytes. Tolerance to the antidiarrheal effect has not been observed.
Clinical studies have indicated that the apparent elimination half-life of loperamide in man is 10.8 hours with a range of 9.1-14.4 hours. Plasma levels of unchanged drug remain below 2 nanograms per ml after the intake of a 2 mg capsule of IMODIUM®. Plasma levels are highest approximately five hours after administration of the capsule and 2.5 hours after the liquid. The peak plasma levels of loperamide were similar for both formulations. Of the total excreted in urine and feces, most of the administered drug was excreted in feces.
In those patients in whom biochemical and hematological parameters were monitored during clinical trials, no trends toward abnormality during IMODIUM® therapy were noted. Similarly, urinalyses, EKG and clinical ophthalmological examinations did not show trends toward abnormality.
INDICATIONS AND USAGE
IMODIUM® (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease. IMODIUM® is also indicated for reducing the volume of discharge from ileostomies.
CONTRAINDICATIONS
IMODIUM® (loperamide hydrochloride) is contraindicated in patients with known hypersensitivity to the drug and in those in whom constipation must be avoided.
WARNINGS
IMODIUM® (loperamide hydrochloride) should not be used in the case of acute dysentery, which is characterized by blood in stools and high fever.
Fluid and electrolyte depletion often occur in patients who have diarrhea. In such cases, administration of appropriate fluid and electrolytes is very important. The use of IMODIUM® does not preclude the need for appropriate fluid and electrolyte therapy.
In some patients with acute ulcerative colitis, and in pseudomembranous colitis associated with broad-spectrum antibiotics, agents which inhibit intestinal motility or delay intestinal transit time have been reported to induce toxic megacolon.
IMODIUM® therapy should be discontinued promptly if abdominal distention, constipation, or ileus occurs.
IMODIUM® should be used with special caution in young children because of the greater variability of response in this age group. Dehydration, particularly in younger children, may further influence the variability of response to IMODIUM®.
PRECAUTIONS
General
Extremely rare allergic reactions including anaphylaxis and anaphylactic shock have been reported.
In acute diarrhea, if clinical improvement is not observed in 48 hours, the administration of IMODIUM® (loperamide hydrochloride) should be discontinued. Patients with hepatic dysfunction should be monitored closely for signs of CNS toxicity because of the apparent large first pass biotransformation.
Information for Patients
Patients should be advised to check with their physician if their diarrhea does not improve after a couple of days or if they note blood in their stools or develop a fever.
Drug Interactions
There was no evidence in clinical trials of drug interactions with concurrent medications.
Carcinogenesis, mutagenesis, impairment of fertility
In an 18-month rat study with doses up to 133 times the maximum human dose (on a mg/kg basis), there was no evidence of carcinogenesis. Mutagenicity studies were not conducted. Reproduction studies in rats indicated that high doses (150-200 times the human dose) could cause marked female infertility and reduced male fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies in rats and rabbits have revealed no evidence of impaired fertility or harm to the fetus at doses up to 30 times the human dose. Higher doses impaired the survival of mothers and nursing young. The studies offered no evidence of teratogenic activity. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when IMODIUM® is administered to a nursing woman.
Pediatric Use
See the "Warnings" Section for information on the greater variability of response in this age group.
In case of accidental overdosage of IMODIUM® by children, see "Overdosage" Section for suggested treatment.
ADVERSE REACTIONS
The adverse effects reported during clinical investigations of IMODIUM® (loperamide hydrochloride) are difficult to distinguish from symptoms associated with the diarrheal syndrome. Adverse experiences recorded during clinical studies with IMODIUM® were generally of a minor and self-limiting nature. They were more commonly observed during the treatment of chronic diarrhea.
The following adverse events have been reported: hypersensitivity reactions such as skin rash and urticaria, and extremely rare cases of anaphylactic shock and bullous eruption including Toxic Epidermal Necrolysis. In the majority of these cases, the patients were on other medications which may have caused or contributed to the events.
The following patient complaints have also been reported: abdominal pain, distention or discomfort, nausea, vomiting, constipation, tiredness, drowsiness or dizziness and dry mouth.
There have been rare reports of paralytic ileus associated with abdominal distention. Most of these reports occurred in the setting of acute dysentery, overdose, and with very young children of less than two years of age.
DRUG ABUSE AND DEPENDENCE
Abuse
A specific clinical study designed to assess the abuse potential of loperamide at high doses resulted in a finding of extremely low abuse potential.
Dependence
Studies in morphine-dependent monkeys demonstrated that loperamide hydrochloride at doses above those recommended for humans prevented signs of morphine withdrawal. However, in humans, the naloxone challenge pupil test, which when positive indicates opiate-like effects, performed after a single high dose, or after more than two years of therapeutic use of IMODIUM® (loperamide hydrochloride), was negative. Orally administered IMODIUM® (loperamide formulated with magnesium stearate) is both highly insoluble and penetrates the CNS poorly.
OVERDOSAGE
In cases of overdosage, paralytic ileus and CNS depression may occur. Children may be more sensitive to CNS effects than adults. Clinical trials have demonstrated that a slurry of activated charcoal administered promptly after ingestion of loperamide hydrochloride can reduce the amount of drug which is absorbed into the systemic circulation by as much as ninefold. If vomiting occurs spontaneously upon ingestion, a slurry of 100 gms of activated charcoal should be administered orally as soon as fluids can be retained.
If vomiting has not occurred, gastric lavage should be performed followed by administration of 100 gms of the activated charcoal slurry through the gastric tube. In the event of overdosage, patients should be monitored for signs of CNS depression for at least 24 hours. Children may be more sensitive to central nervous system effects than adults. If CNS depression is observed, naloxone may be administered. If responsive to naloxone, vital signs must be monitored carefully for recurrence of symptoms of drug overdose for at least 24 hours after the last dose of naloxone.
In view of the prolonged action of loperamide and the short duration (one to three hours) of naloxone, the patient must be monitored closely and treated repeatedly with naloxone as indicated. Since relatively little drug is excreted in the urine, forced diuresis is not expected to be effective for IMODIUM® (loperamide hydrochloride) overdosage.
In clinical trials an adult who took three 20 mg doses within a 24 hour period was nauseated after the second dose and vomited after the third dose. In studies designed to examine the potential for side effects, intentional ingestion of up to 60 mg of loperamide hydrochloride in a single dose to healthy subjects resulted in no significant adverse effects.
DOSAGE AND ADMINISTRATION
(1 capsule = 2 mg)
Patients should receive appropriate fluid and electrolyte replacement as needed.
Acute Diarrhea
Adults: The recommended initial dose is 4 mg (two capsules) followed by 2 mg (one capsule) after each unformed stool. Daily dosage should not exceed 16 mg (eight capsules). Clinical improvement is usually observed within 48 hours.
Children: IMODIUM® (loperamide hydrochloride) use is not recommended for children under 2 years of age. In children 2 to 5 years of age (20 kg or less), the non-prescription liquid formulation (IMODIUM A-D 1 mg/5 ml) should be used; for ages 6 to 12, either IMODIUM® Capsules of IMODIUM® A-D liquid may be used. For children 2 to 12 years of age, the following schedule for capsules or liquid will usually fulfill initial dosage requirements:
Recommended First Day Dosage Schedule
Two to five years: 1 mg t.i.d. (3 mg daily dose) (13 to 20 kg)
Six to eight years: 2 mg b.i.d. (4 mg daily dose) (20 to 30 kg)
Eight to twelve years: 2 mg t.i.d. (6 mg daily dose) (greater than 30 kg)
Recommended Subsequent Daily Dosage
Following the first treatment day, it is recommended that subsequent IMODIUM® doses (1 mg/10 kg body weight) be administered only after a loose stool. Total daily dosage should not exceed recommended dosages for the first day.
Chronic Diarrhea
Children: Although IMODIUM® has been studied in a limited number of children with chronic diarrhea, the therapeutic dose for the treatment of chronic diarrhea in a pediatric population has not been established.
Adults: The recommended initial dose is 4 mg (two capsules) followed by 2 mg (one capsule) after each unformed stool until diarrhea is controlled, after which the dosage of IMODIUM® should be reduced to meet individual requirements. When the optimal daily dosage has been established, this amount may then be administered as a single dose or in divided doses.
The average daily maintenance dosage in clinical trials was 4 to 8 mg (two to four capsules). A dosage of 16 mg (eight capsules) was rarely exceeded. If clinical improvement is not observed after treatment with 16 mg per day for at least 10 days, symptoms are unlikely to be controlled by further administration. IMODIUM® administration may be continued if diarrhea cannot be adequately controlled with diet or specific treatment.
HOW SUPPLIED
Capsules--each capsule contains 2 mg of loperamide hydrochloride. The capsules have a light green body and a dark green cap with "Janssen" imprinted on one segment and "IMODIUM" on the other segment. IMODIUM® capsules are supplied in bottles of 100.
NDC 50458-400-10
(100 capsules)
Store at 15°-25°C (59°-77°F).
Revised September 1996, July 1998
©Janssen Pharmaceutica Inc. 1998
CAUTION: FEDERAL LAW PROHIBITS DISPENSING WITHOUT A PRESCRIPTION
U.S. Patent 3,714,1597502205
Distributed by
McNeil Consumer & Specialty Pharmaceuticals
Subscribe to the "News" RSS Feed 
TOP ۞
