-
Kadian Capsules (Alpharma Branded Products)
DESCRIPTION
KADIAN® capsules 20, 30, 50, 60 and 100 mg contain identical polymer coated sustained release pellets of morphine sulfate for oral administration.
Chemically, morphine sulfate is 7,8-didehydro-4,5 (alpha)- epoxy-17-methyl-morphinan-3,6 (alpha)- diol sulfate (2:1) (salt) pentahydrate and has the following structural formula:
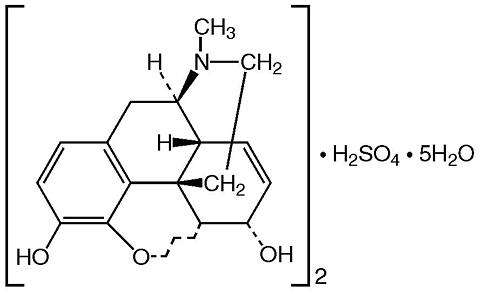
Morphine sulfate is an odorless, white, crystalline powder with a bitter taste and a molecular weight of 758 (as the sulfate). It has a solubility of 1 in 21 parts of water and 1 in 1000 parts of alcohol, but is practically insoluble in chloroform or ether. The octanol: water partition coefficient of morphine is 1.42 at physiologic pH and the pK b is 7.9 for the tertiary nitrogen (mostly ionized at pH 7.4).
Each KADIAN® sustained release capsule contains either 20, 30, 50, 60, or 100 mg of Morphine Sulfate USP and the following inactive ingredients common to all strengths: hypromellose, ethylcellulose, methacrylic acid copolymer, polyethylene glycol, diethyl phthalate, talc, corn starch, and sucrose. The 20 mg capsule shell contains gelatin, silicon dioxide, sodium lauryl sulfate, D&C yellow #10, titanium dioxide, and black ink SW-9009. The 30 mg capsule shell contains gelatin, silicon dioxide, sodium lauryl sulfate, FD&C red #3, FD&C blue #1, titanium dioxide and black ink S-1-8114 or S-1-8115. The 50 mg capsule shell contains gelatin, silicon dioxide, sodium lauryl sulfate, D&C red #28, FD&C red #40, FD&C blue #1, titanium dioxide, and black ink SW-9009. The 60 mg capsule shell contains gelatin, silicon dioxide, sodium lauryl sulfate, D&C red #28, FD&C red #40, FD&C blue #1, titanium dioxide and black ink S-1-8114 or S-1-8115. The 100 mg capsule shell contains gelatin, silicon dioxide, sodium lauryl sulfate, D&C yellow #10, FD&C blue #1, titanium dioxide, and black ink SW-9009.
CLINICAL PHARMACOLOGY
Morphine is a natural product that is the prototype for the class of natural and synthetic opioid analgesics. Opioids produce a wide spectrum of pharmacologic effects including analgesia, dysphoria, euphoria, somnolence, respiratory depression, diminished gastrointestinal motility, altered circulatory dynamics, histamine release and physical dependence.
Morphine produces both its therapeutic and its adverse effects by interaction with one or more classes of specific opioid receptors located throughout the body. Morphine acts as a pure agonist, binding with and activating opioid receptors at sites in the peri-aqueductal and peri-ventricular grey matter, the ventro-medial medulla and the spinal cord to produce analgesia.
Effects on the Central Nervous System
The principal therapeutic actions of morphine are analgesia, sedation and alterations of mood. Opioids of this class do not usually eliminate pain, but they do reduce the perception of pain by the central nervous system.
Morphine produces respiratory depression by reducing the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension (or to direct electrical stimulation).
Morphine depresses the cough reflex by direct effect on the cough center in the medulla. Antitussive effects may occur with doses lower than those usually required for analgesia.
Morphine causes miosis, even in total darkness, and little tolerance develops to this effect. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g. pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen due to severe hypoxia in overdose situations.
Effects on the Gastrointestinal Tract
Gastric, biliary and pancreatic secretions are decreased by morphine. Morphine causes a reduction in motility associated with an increase in tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone is increased to the point of spasm. The end result is constipation. Morphine can cause a marked increase in biliary tract pressure as a result of spasm of the sphincter of Oddi.
Effects on the Cardiovascular System
Morphine produces peripheral vasodilation which may result in orthostatic hypotension or syncope. Release of histamine may be induced by morphine and can contribute to opioid-induced hypotension. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes and sweating.
Pharmacodynamics
The relationship between the blood level of morphine and the analgesic response will depend on the patient's age, state of health, medical condition, and the extent of previous opioid treatment.
A minimum effective concentration (MEC) of morphine for pain relief has been reported as 27.2 ± 14.5 ng/mL (mean ± SD) in cancer patients treated with morphine solution. These results compare with the MEC for plasma morphine reported as 14.7 ± 4.8 ng/mL (mean ± SD) in patients with postoperative pain. The high degree of variation is of clinical significance as it may result in either under-dosing or over-dosing if the dosage is not adjusted to the patient's clinical status and analgesic response (see PRECAUTIONS and DOSAGE AND ADMINISTRATION ).
For opioid-tolerant patients the situation is much more complex. Some patients will become rapidly tolerant to the analgesic effects of morphine, and will require high daily oral morphine doses for adequate pain control. Since the development of tolerance to both the therapeutic and adverse effects of opioids is highly individualized, the dose of morphine should be individualized to the patient's condition and should not be based on an arbitrary choice of a dose or blood level to be achieved.
Pharmacokinetics
KADIAN® capsules contain polymer coated sustained release pellets of morphine sulfate that release morphine significantly more slowly than from morphine sulfate tablets and shorter-acting controlled-release oral morphine sulfate preparations. KADIAN® activity is primarily due to morphine. One metabolite, morphine-6-glucuronide, has been shown to have analgesic activity, but poorly crosses the blood-brain barrier.
Following oral administration, the extent of absorption is essentially the same for immediate or sustained release formulations, although the time to peak blood level (T max ) will be longer and the C max will be lower for formulations that delay the release of morphine in the gastrointestinal tract.
Elimination of morphine is primarily via hepatic metabolism to glucuronide metabolites (55 to 65%) which are then renally excreted. The terminal half-life of morphine is 2 to 4 hours, however, a longer term half-life of about 15 hours has been reported in studies where blood has been sampled up to 48 hours.
The single-dose pharmacokinetics of KADIAN® are linear over the dosage range of 30 to 100 mg. The single dose and multiple dose pharmacokinetic parameters of KADIAN® in normal volunteers are summarized in Table 1.
Table 1:Mean pharmacokinetic parameters (% coefficient variation) resulting from a fasting single dose study in normal volunteers and a multiple dose study in patients with cancer pain.Regimen/
Dosage FormAUC #, +
(ng.h/mL)C max +
(ng/mL)T max
(h)C min +
(ng/mL)Fluctuation * Single Dose (n=24)KADIAN® Capsule271.0 (19.4) 15.6 (24.4) 8.6 (41.1) na ^ na Controlled-Release Tablet304.3 (19.1) 30.5 (32.1) 2.5 (52.6) na na Morphine Solution362.4 (42.6) 64.4 (38.2) 0.9 (55.8) na na Multiple Dose (n=24)KADIAN® Capsule q24h500.9 (38.6) 37.3 (37.7) 10.3 (32.2) 9.9 (52.3) 3.0 (45.5) Controlled-Release Tablet q12h457.3 (40.2) 36.9 (42.0) 4.4 (53.0) 7.6 (60.3) 4.1 (51.5) # For single dose AUC = AUC 0-48h , for multiple dose AUC = AUC 0-24h at steady state+ For single dose parameter normalized to 100 mg, for multiple dose parameter normalized to 100 mg per 24 hours* Steady-state fluctuation in plasma concentrations = C max -C min /C min^ Not applicableAbsorption
Following the administration of oral morphine solution, approximately 50% of the morphine absorbed reaches the systemic circulation within 30 minutes. However, following the administration of an equal amount of KADIAN® to healthy volunteers, this occurs, on average, after 8 hours. As with most forms of oral morphine, because of pre-systemic elimination, only about 20 to 40% of the administered dose reaches the systemic circulation.
Food Effects: While concurrent administration of food slows the rate of absorption of KADIAN®, the extent of absorption is not affected and KADIAN® can be administered without regard to meals.
Steady State: When KADIAN® is given on a fixed dosing regimen to patients with chronic pain due to malignancy, steady state is achieved in about two days. At steady state, KADIAN® will have a significantly lower C max and a higher C min than equivalent doses of oral morphine solution and some other controlled-release preparations (see Graph 1).
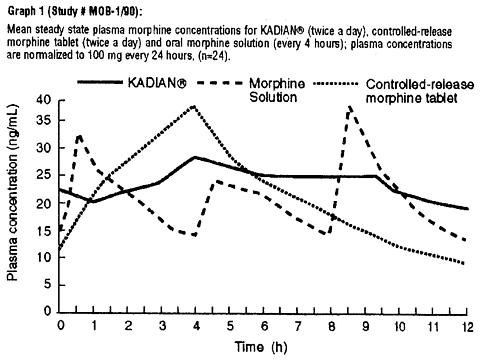
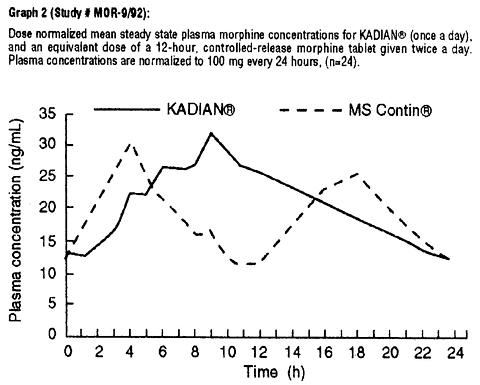
When given once-daily (every 24 hours) to 24 patients with malignancy, KADIAN® had a similar C max and higher C min at steady state in clinical usage, when compared to twice-daily (every 12 hours) controlled-release morphine tablets (MS Contin®), given at an equivalent total daily dosage (see Graph 2 and Table 1). Drug-disease interactions are frequently seen in the older and more gravely ill patients, and may result in both altered absorption and reduced clearance as compared to normal volunteers (see Geriatric, Hepatic Failure, and Renal Insufficiency sections).
Distribution
Once absorbed, morphine is distributed to skeletal muscle, kidneys, liver, intestinal tract, lungs, spleen and brain.
The volume of distribution of morphine is approximately 3 to 4 L/kg. Morphine is 30 to 35% reversibly bound to plasma proteins.
Although the primary site of action of morphine is in the CNS, only small quantities pass the blood-brain barrier.
Morphine also crosses the placental membranes (see PRECAUTIONS - Pregnancy ) and has been found in breast milk (see PRECAUTIONS - Nursing Mothers ).
Metabolism
The major pathway of the detoxification of morphine is conjugation, either with D-glucuronic acid in the liver to produce glucuronides or with sulfuric acid to give morphine-3-etheral sulfate. Although a small fraction (less than 5%) of morphine is demethylated, for all practical purposes, virtually all morphine is converted to glucuronide metabolites including morphine-3-glucuronide, M3G (about 50%) and morphine-6-glucuronide, M6G (about 5 to 15%). Studies in healthy subjects and cancer patients have shown that the glucuronide metabolite to morphine mean molar ratios (based on AUC) are similar after both single doses and at steady state for KADIAN®, 12-hour controlled-release morphine sulfate tablets and morphine sulfate solution.
M3G has no significant analgesic activity. M6G has been shown to have opioid agonist and analgesic activity in humans.
ExcretionApproximately 10% of morphine dose is excreted unchanged in the urine. Most of the dose is excreted in the urine as M3G and M6G. A small amount of the glucuronide metabolites is excreted in the bile and there is some minor enterohepatic cycling. Seven to 10% of administered morphine is excreted in the feces.
The mean adult plasma clearance is about 20-30 mL/minute/kg. The effective terminal half-life of morphine after IV administration is reported to be approximately 2.0 hours. Longer plasma sampling in some studies suggests a longer terminal half-life of morphine of about 15 hours.
Special Populations
Geriatric: The elderly may have increased sensitivity to morphine and may achieve higher and more variable serum levels than younger patients. In adults, the duration of analgesia increases progressively with age, though the degree of analgesia remains unchanged. KADIAN® pharmacokinetics have not been investigated in elderly patients (>65 years) although such patients were included in the clinical studies.
Nursing Mothers: Morphine is excreted in the maternal milk, and the milk to plasma morphine AUC ratio is about 2.5:1. The amount of morphine received by the infant depends on the maternal plasma concentration, amount of milk ingested by the infant, and the extent of first pass metabolism.
Pediatric: Infants under 1 month of age have a prolonged elimination half-life and decreased clearance relative to older infants and pediatric patients. The clearance of morphine and its elimination half-life begin to approach adult values by the second month of life. Pediatric patients old enough to take capsules should have pharmacokinetic parameters similar to adults, dosed on a per kilogram basis (see PRECAUTIONS - Pediatric Use ).
Gender: No meaningful differences between male and female patients were demonstrated in the analysis of the pharmacokinetic data from clinical studies.
Race: Pharmacokinetic differences due to race may exist. Chinese subjects given intravenous morphine in one study had a higher clearance when compared to caucasian subjects (1852 ± 116 mL/min versus 1495 ± 80 mL/min).
Hepatic Failure: The pharmacokinetics of morphine were found to be significantly altered in individuals with alcoholic cirrhosis. The clearance was found to decrease with a corresponding increase in half-life. The M3G and M6G to morphine plasma AUC ratios also decreased in these patients indicating a decrease in metabolic activity.
Renal Insufficiency: The pharmacokinetics of morphine are altered in renal failure patients. AUC is increased and clearance is decreased. The metabolites, M3G and M6G accumulate several fold in renal failure patients compared with healthy subjects.
Drug-Drug Interactions: The known drug interactions involving morphine are pharmacodynamic, not pharmacokinetic (see PRECAUTIONS - Drug Interactions ).
Clinical Studies
A total of 177 healthy subjects and 337 patients with cancer pain participated in a total of 15 studies (10 pharmacokinetic and 6 clinical; one study reported both pharmacokinetic and clinical data). Of these individuals, 158 healthy subjects and 268 patients received KADIAN®. In the controlled clinical studies patients were followed for a median duration of 7 days and in the open label studies patients were followed for up to 12-24 months. KADIAN® was compared to oral morphine solution and to either MS Contin® or to a 12-hour controlled-release morphine tablet bioequivalent to MS Contin® using trial designs that followed the clinical and pharmacokinetic performance of each treatment in cancer patients receiving chronic opioid therapy.
In two controlled studies, patients with moderate to severe cancer pain were titrated with immediate-release morphine (IRM) solution or tablets to a stable total daily dose of morphine for at least three consecutive days, then randomized to KADIAN® or 12-hour controlled-release morphine for seven days of observation. KADIAN® given once a day proved similar to the same total dose of morphine given in divided doses in a 12-hour dosage form, with respect to pain relief, use of rescue medication, patient and investigator global assessment, and quality of sleep. Individual patient differences in the pattern of pain control emphasize the need to individualize both dose and dosing interval (see DOSAGE AND ADMINISTRATION ).
INDICATIONS AND USAGE
KADIAN® is indicated for the management of moderate to severe pain where treatment with an opioid analgesic is indicated for more than a few days (see CLINICAL PHARMACOLOGY; Clinical Studies ).
KADIAN® was developed for use in patients with chronic pain who require repeated dosing with a potent opioid analgesic, and has been tested in patients with pain due to malignant conditions. KADIAN® has not been tested as an analgesic for the treatment of acute pain or in the postoperative setting and is not recommended for such use.
CONTRAINDICATIONS
KADIAN® is contraindicated in patients with a known hypersensitivity to morphine, morphine salts or any of the capsule components.
KADIAN® is contraindicated in patients with respiratory depression in the absence of resuscitative equipment, and in patients with acute or severe bronchial asthma.
KADIAN® is contraindicated in any patient who has or is suspected of having paralytic ileus.
WARNINGS
(See also CLINICAL PHARMACOLOGY )
Impaired Respiration
Respiratory depression is the chief hazard of all morphine preparations. Respiratory depression occurs more frequently in elderly and debilitated patients, and those suffering from conditions accompanied by hypoxia, hypercapnia, or upper airway obstruction (when even moderate therapeutic doses may significantly decrease pulmonary ventilation).
Morphine should be used with extreme caution in patients with chronic obstructive pulmonary disease or cor pulmonale, and in patients having a substantially decreased respiratory reserve (e.g. severe kyphoscoliosis), hypoxia, hypercapnia, or pre-existing respiratory depression. In such patients, even usual therapeutic doses of morphine may increase airway resistance and decrease respiratory drive to the point of apnea.
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of morphine with carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions, or a pre-existing increase in intracranial pressure. Morphine produces effects which may obscure neurologic signs of further increases in pressure in patients with head injuries. Morphine should only be administered under such circumstances when considered essential and then with extreme care.
Hypotensive Effect
KADIAN®, like all opioid analgesics, may cause severe hypotension in an individual whose ability to maintain blood pressure has already been compromised by a reduced blood volume, or a concurrent administration of drugs such as phenothiazines or general anesthetics. (see also PRECAUTIONS - Drug Interactions ). KADIAN® may produce orthostatic hypotension and syncope in ambulatory patients.
KADIAN®, like all opioid analgesics, should be administered with caution to patients in circulatory shock, as vasodilation produced by the drug may further reduce cardiac output and blood pressure.
Gastrointestinal Obstruction
KADIAN® should not be given to patients with gastrointestinal obstruction, particularly paralytic ileus, as there is a risk of the product remaining in the stomach for an extended period and the subsequent release of a bolus of morphine when normal gut motility is restored. As with other solid morphine formulations diarrhea may reduce morphine absorption.
PRECAUTIONS (See also CLINICAL PHARMACOLOGY )
General
KADIAN® is intended for use in patients who require continuous treatment with a potent opioid analgesic. As with any potent opioid, it is critical to adjust the dosing regimen for KADIAN® for each patient, taking into account the patient's prior analgesic treatment experience. Although it is clearly impossible to enumerate every consideration that is important to the selection of the initial dose of KADIAN®, attention should be given to the points under DOSAGE AND ADMINISTRATION.
Cordotomy
Patients taking KADIAN® who are scheduled for cordotomy or other interruption of pain transmission pathways should have KADIAN® ceased 24 hours prior to the procedure and the pain controlled by parenteral short-acting opioids. In addition, the post-procedure titration of analgesics for such patients should be individualized to avoid either oversedation or withdrawal syndromes.
Use in Pancreatic/Biliary Tract Disease
KADIAN® may cause spasm of the sphincter of Oddi and should be used with caution in patients with biliary tract disease, including acute pancreatitis. Opioids may cause increases in the serum amylase level.
Special risk groups
KADIAN® should be administered with caution, and in reduced dosages in elderly or debilitated patients; patients with severe renal or hepatic insufficiency; patients with Addison's disease; myxedema; hypothyroidism; prostatic hypertrophy or urethral stricture.
Caution should also be exercised in the administration of KADIAN® to patients with CNS depression, toxic psychosis, acute alcoholism and delirium tremens, and convulsive disorders.
Driving and operating machinery
Morphine may impair the mental and/or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Patients must be cautioned accordingly. Patients should also be warned about the potential combined effects of morphine with other CNS depressants, including other opioids, phenothiazines, sedative/hypnotics and alcohol (see Drug Interactions ).
Information for Patients
If clinically advisable, patients receiving KADIAN® should be given the following instructions by the physician:
- KADIAN® capsules should be swallowed whole (not chewed, crushed, or dissolved). Alternatively, KADIAN® capsules may be opened and the entire contents sprinkled on a small amount of applesauce immediately prior to ingestion. The pellets should NOT be chewed, crushed, or dissolved due to risk of overdose. When prescribing KADIAN® by the sprinkle method, details of proper technique should be explained to the patient. KADIAN® capsules may also be opened and the entire contents sprinkled over about 10 mL of water in a beaker then flushed with swirling through a pre-wetted 16-French gastrostomy tube fitted with a plastic funnel at the port end. The beaker is rinsed with additional aliquots of water as necessary to transfer all of the pellets to flush the tube. NASOGASTRIC TUBES SHOULD NOT BE USED. (also see DOSAGE AND ADMINISTRATION )
- The dose of KADIAN® should not be adjusted without consulting the physician.
- Morphine may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g. driving, operating machinery). Patients started on KADIAN® or whose dose has been changed should refrain from dangerous activity until it is established that they are not adversely affected.
- Morphine should not be taken with alcohol or other CNS depressants (sleeping medication, tranquilizers) because additive effects including CNS depression may occur. A physician should be consulted if other medications are currently being used or are prescribed for future use.
- Women of childbearing potential who become or are planning to become pregnant, should consult a physician.
- Upon completion of therapy, it may be appropriate to taper the morphine dose, rather than abruptly discontinuing it.
- While psychological dependence ("addiction") to morphine used in the treatment of pain is very rare, morphine is one of a class of drugs known to be abused and should be handled accordingly.
- As with other opioids, patients taking KADIAN® should be advised that severe constipation could occur and appropriate laxatives, stool softeners and other appropriate treatments should be initiated from the beginning of opioid therapy.
Drug Interactions
CNS Depressants: Morphine should be used with great caution and in reduced dosage in patients who are concurrently receiving other central nervous system (CNS) depressants including sedatives, hypnotics, general anesthetics, antiemetics, phenothiazines, other tranquilizers and alcohol because of the risk of respiratory depression, hypotension and profound sedation or coma. When such combined therapy is contemplated, the initial dose of one or both agents should be reduced by at least 50%.
Muscle Relaxants: Morphine may enhance the neuromuscular blocking action of skeletal relaxants and produce an increased degree of respiratory depression.
Mixed Agonist/Antagonist Opioid Analgesics: From a theoretical perspective, mixed agonist/antagonist analgesics (i.e. pentazocine, nalbuphine and butorphanol) should NOT be administered to patients who have received or are receiving a course of therapy with a pure opioid agonist analgesic. In these patients, mixed agonist/antagonist analgesics may reduce the analgesic effect and/or may precipitate withdrawal symptoms.
Monoamine Oxidase Inhibitors (MAOIs): MAOIs have been reported to intensify the effects of at least one opioid drug causing anxiety, confusion and significant depression of respiration or coma. We do not recommend the use of KADIAN® in patients taking MAOIs or within 14 days of stopping such treatment.
Cimetidine: There is an isolated report of confusion and severe respiratory depression when a hemodialysis patient was concurrently administered morphine and cimetidine.
Diuretics: Morphine can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. Morphine may also lead to acute retention of urine by causing spasm of the sphincter of the bladder, particularly in men with prostatism.
Food: KADIAN® capsules should be swallowed whole (not chewed, crushed, or dissolved). Alternatively, KADIAN® capsules may be opened and the entire contents sprinkled on a small amount of applesauce immediately prior to ingestion. The pellets in KADIAN® should NOT be chewed, crushed, or dissolved due to risk of overdose. (see DOSAGE AND ADMINISTRATION, and INFORMATION FOR PATIENTS )
Carcinogenicity/Mutagenicity/Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of morphine have not been conducted. There are no reports of carcinogenic effects in humans.
In vitro studies have reported that morphine is non-mutagenic in the Ames test with Salmonella , and induces chromosomal aberrations in human leukocytes and lethal mutation induction in Drosophila . Morphine was found to be mutagenic in vitro in human T-cells, increasing the DNA fragmentation. In vivo , morphine was mutagenic in the mouse micronucleus test and induced chromosomal aberrations in spermatids and murine lymphocytes.
Chronic opioid abusers (e.g., heroin abusers) and their offspring display higher rates of chromosomal damage. However, the rates of chromosomal abnormalities were similar in nonexposed individuals and in heroin users enrolled in long term opioid maintenance programs.
Pregnancy
Teratogenic effects (Pregnancy Category C)
Teratogenic effects of morphine have been reported in the animal literature. High parental doses during the second trimester were teratogenic in neurological, soft and skeletal tissue. The abnormalities included encephalopathy and axial skeletal fusions. These doses were often maternally toxic and were 0.3 to 3-fold the maximum recommended human dose (MRHD) on a mg/m 2 basis. The relative contribution of morphine-induced maternal hypoxia and malnutrition, each of which can be teratogenic, has not been clearly defined. Treatment of male rats with approximately 3-fold the MRHD for 10 days prior to mating decreased litter size and viability.
Nonteratogenic effects
Morphine given subcutaneously, at non-maternally toxic doses, to rats during the third trimester with approximately 0.15-fold the MRHD caused reversible reductions in brain and spinal cord volume, and testes size and body weight in the offspring, and decreased fertility in female offspring. The offspring of rats and hamsters treated orally or intraperitoneally throughout pregnancy with 0.04- to 0.3-fold the MRHD of morphine have demonstrated delayed growth, motor and sexual maturation and decreased male fertility. Chronic morphine exposure of fetal animals resulted in mild withdrawal, altered reflex and motor skill development, and altered responsiveness to morphine that persisted into adulthood.
There are no well-controlled studies of chronic in utero exposure to morphine sulfate in human subjects. However, uncontrolled retrospective studies of human neonates chronically exposed to other opioids in utero , demonstrated reduced brain volume which normalized over the first month of life. Infants born to opioid-abusing mothers are more often small for gestational age, have a decreased ventilatory response to CO 2 and increased risk of sudden infant death syndrome.
Morphine should only be used during pregnancy if the need for strong opioid analgesia justifies the potential risk to the fetus.
Labor and Delivery
KADIAN® is not recommended for use in women during and immediately prior to labor, where shorter acting analgesics or other analgesic techniques are more appropriate. Occasionally, opioid analgesics may prolong labor through actions which temporarily reduce the strength, duration and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilatation which tends to shorten labor.
Neonates whose mothers received opioid analgesics during labor should be observed closely for signs of respiratory depression. A specific opioid antagonist, such as naloxone or nalmefene, should be available for reversal of opioid-induced respiratory depression in the neonate.
Neonatal Withdrawal Syndrome
Chronic maternal use of opiates or opioids during pregnancy coexposes the fetus. The newborn may experience subsequent neonatal withdrawal syndrome (NWS). Manifestations of NWS include irritability, hyperactivity, abnormal sleep pattern, high-pitched cry, tremor, vomiting, diarrhea, weight loss, and failure to gain weight. The onset, duration, and severity of the disorder differ based on such factors as the addictive drug used, time and amount of mother's last dose, and rate of elimination of the drug from the newborn. Approaches to the treatment of this syndrome have included supportive care and, when indicated, drugs such as paragoric or phenobarbital.
Nursing Mothers
Low levels of morphine sulfate have been detected in human milk. Withdrawal symptoms can occur in breast-feeding infants when maternal administration of morphine sulfate is stopped. Because of the potential for adverse reactions in nursing infants from KADIAN®, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There are studies from the literature reporting the safe and effective use of both immediate and sustained release oral morphine preparations for analgesia in pediatric patients who were dosed on a per kilogram basis. However, the safety of KADIAN®, both the entire capsule and the pellets sprinkled on applesauce, have not been directly investigated in pediatric patients below the age of 18 years. The range of doses available is not suitable for the treatment of very young pediatric patients or those who are not old enough to take capsules safely. The applesauce sprinkling method is not an appropriate alternative for these patients.
ADVERSE REACTIONS
Serious adverse reactions that may be associated with KADIAN® therapy in clinical use are those observed with other opioid analgesics and include: respiratory depression, respiratory arrest, circulatory depression, cardiac arrest, hypotension, and/or shock (see OVERDOSAGE, WARNINGS ).
The less severe adverse events seen on initiation of therapy with KADIAN® are also typical opioid side effects. These events are dose dependent, and their frequency depends on the clinical setting, the patient's level of opioid tolerance, and host factors specific to the individual. They should be expected and managed as a part of opioid analgesia. The most frequent of these include drowsiness, dizziness, constipation and nausea. In many cases, the frequency of these events during initiation of therapy may be minimized by careful individualization of starting dosage, slow titration, and the avoidance of large rapid swings in plasma concentrations of the opioid. Many of these adverse events, will cease or decrease as KADIAN® therapy is continued and some degree of tolerance is developed, but others may be expected to remain troublesome throughout therapy.
Management of Excessive Drowsiness
Most patients receiving morphine will experience initial drowsiness. This usually disappears within 3-5 days and is not a cause of concern unless it is excessive, or accompanied by unsteadiness or confusion. Dizziness and unsteadiness may be associated with postural hypotension, particularly in elderly or debilitated patients, and has been associated with syncope and falls in non-tolerant patients started on opioids.
Excessive or persistent sedation should be investigated. Factors to be considered should include: concurrent sedative medications, the presence of hepatic or renal insufficiency, hypoxia or hypercapnia due to exacerbated respiratory failure, intolerance to the dose used (especially in older patients), disease severity and the patient's general condition.
The dosage should be adjusted according to individual needs, but additional care should be used in the selection of initial doses for the elderly patient, the cachectic or gravely ill patient, or in patients not already familiar with opioid analgesic medications to prevent excessive sedation at the onset of treatment.
Management of Nausea and Vomiting
Nausea and vomiting are common after single doses of morphine or as an early undesirable effect of chronic opioid therapy. The prescription of a suitable antiemetic should be considered, with the awareness that sedation may result (see Drug Interactions ). The frequency of nausea and vomiting usually decreases within a week or so but may persist due to opioid-induced gastric stasis. Metoclopramide is often useful in such patients.
Management of Constipation
Virtually all patients suffer from constipation while taking opioids on a chronic basis. Some patients, particularly elderly, debilitated or bedridden patients may become impacted. Tolerance does not usually develop for the constipating effects of opioids. Patients must be cautioned accordingly and laxatives, softeners and other appropriate treatments should be used prophylactically from the beginning of opioid therapy.
Adverse Events Probably Related to KADIAN® Administration
In controlled clinical trials in patients with chronic cancer pain the most common adverse events reported by patients at least once during therapy were drowsiness (9%), constipation (9%), nausea (7%), dizziness (6%), and anxiety (6%). Other less common side effects expected from morphine or seen in less than 3% of patients in the clinical trials were:
Body as a Whole: Asthenia, accidental injury, fever, pain, chest pain, headache, diaphoresis, chills, flu syndrome, back pain, malaise, withdrawal syndrome
Cardiovascular: Tachycardia, atrial fibrillation, hypotension, hypertension, pallor, facial flushing, palpitations, bradycardia, syncope
Central Nervous System: Confusion, dry mouth, anxiety, abnormal thinking, abnormal dreams, lethargy, depression, tremor, loss of concentration, insomnia, amnesia, paresthesia, agitation, vertigo, foot drop, ataxia, hypesthesia, slurred speech, hallucinations, vasodilation, euphoria, apathy, seizures, myoclonus
Endocrine: Hyponatremia due to inappropriate ADH secretion, gynecomastia
Gastrointestinal: Vomiting, anorexia, dysphagia, dyspepsia, diarrhea, abdominal pain, stomach atony disorder, gastro-esophageal reflux, delayed gastric emptying, biliary colic
Hemic & Lymphatic: Anemia, leukopenia, thrombocytopenia
Metabolic & Nutritional: Peripheral edema, hyponatremia, edema
Musculoskeletal: Back pain, bone pain, arthralgia
Respiratory: Hiccup, rhinitis, atelectasis, asthma, hypoxia, dyspnea, respiratory insufficiency, voice alteration, depressed cough reflex, non-cardiogenic pulmonary edema
Skin and Appendages: Rash, decubitus ulcer, pruritus, skin flush
Special Senses: Amblyopia, conjunctivitis, miosis, blurred vision, nystagmus, diplopia
Urogenital: Urinary abnormality, amenorrhea, urinary retention, urinary hesitancy, reduced libido, reduced potency, prolonged labor
DRUG ABUSE AND DEPENDENCE
Morphine is the prototype of opioid agonist drugs, and may be subject to misuse, abuse and addiction. Addiction to opioids prescribed for pain management is rare, but requests for opioids from patients addicted to opioids are common and physicians should take appropriate care in prescribing this controlled substance.
Opioid analgesics may cause physical dependence. Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug. Withdrawal also may be precipitated through the administration of drugs with opioid antagonist activity, e.g. naloxone, nalmefene, or mixed agonist/antagonist analgesics (pentazocine, butorphanol, nalbuphine), (see also OVERDOSAGE ).
Physical dependence usually does not occur to a clinically significant degree until after several weeks of continued opioid usage. Tolerance, in which increasingly large doses are required in order to produce the same degree of analgesia, is initially manifested by a shortened duration of analgesic effect, and subsequently, by decreases in the intensity of analgesia.
In chronic pain patients, and in opioid-tolerant cancer patients, the administration of KADIAN® should be guided by the degree of tolerance manifested. Physical dependence, per se, is not ordinarily a concern when one is dealing with a patient in pain, and fear of tolerance should not deter using adequate doses to adequately relieve pain.
If morphine is abruptly discontinued an abstinence syndrome may occur. This is usually mild and is characterized by rhinitis, myalgia, abdominal cramping and occasional diarrhea. Most observable symptoms disappear in 5-14 days without treatment; however, there may be a phase of secondary or chronic abstinence which may last for 2-6 months characterized by insomnia, irritability and muscular aches.
If treatment of physical dependence of patients taking morphine is necessary, the patient may be detoxified by gradual reduction of the dose. Gastrointestinal disturbances or dehydration should be treated with supportive care.
KADIAN® has no role in the management of opioid addiction.
OVERDOSAGE
Symptoms
Acute overdosage with morphine is manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and, sometimes, pulmonary edema, bradycardia, hypotension and death. Marked mydriasis rather than miosis may be seen due to severe hypoxia in overdose situations.
Treatment
Primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Gastric contents may need to be emptied to remove unabsorbed drug when a sustained release formulation such as KADIAN® has been taken. Care should be taken to secure the airway before attempting treatment by gastric emptying or activated charcoal.
The pure opioid antagonists, naloxone or nalmefene, are specific antidotes to respiratory depression which results from opioid overdose. Since the duration of reversal would be expected to be less than the duration of action of KADIAN®, the patient must be carefully monitored until spontaneous respiration is reliably re-established. KADIAN® will continue to release and add to the morphine load for up to 24 hours after administration and the management of an overdose should be monitored accordingly. If the response to opioid antagonists is suboptimal or not sustained, additional antagonist should be given as directed by the manufacturer of the product.
Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to morphine overdose. Such agents should be administered cautiously to persons who are known, or suspected to be physically dependent on KADIAN®. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome.
Opioid Tolerant Individuals: In an individual physically dependent on opioids, administration of the usual dose of the antagonist will precipitate an acute withdrawal. The severity of the withdrawal produced will depend on the degree of physical dependence and the dose of the antagonist administered. Use of an opioid antagonist should be reserved for cases where such treatment is clearly needed. If it is necessary to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses.
Supportive measures (including oxygen, vasopressors) should be employed in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
DOSAGE AND ADMINISTRATION
KADIAN® CAPSULES SHOULD BE SWALLOWED WHOLE (NOT CHEWED, CRUSHED, OR DISSOLVED).
ALTERNATIVELY, KADIAN® CAPSULES MAY BE OPENED AND THE ENTIRE CONTENTS SPRINKLED ON A SMALL AMOUNT OF APPLESAUCE IMMEDIATELY PRIOR TO INGESTION. THE PELLETS IN KADIAN® CAPSULES SHOULD NOT BE CHEWED, CRUSHED, OR DISSOLVED DUE TO RISK OF OVERDOSE.
TAKING CHEWED OR CRUSHED KADIAN® CAPSULES OR PELLETS WILL LEAD TO THE RAPID RELEASE AND ABSORPTION OF A POTENTIALLY TOXIC DOSE OF MORPHINE.
KADIAN® CAPSULES MAY BE OPENED AND THE ENTIRE CONTENTS SPRINKLED OVER ABOUT 10 ML OF WATER AND FLUSHED WITH SWIRLING THROUGH A PRE-WETTED 16 FRENCH GASTROSTOMY TUBE FITTED WITH FUNNEL AT THE PORT END. ADDITIONAL ALIQUOTS OF WATER ARE USED TO TRANSFER ALL PELLETS AND TO FLUSH THE TUBE. THE ADMINISTRATION OF KADIAN® PELLETS THROUGH A NASOGASTRIC TUBE SHOULD NOT BE ATTEMPTED.
The sustained release nature of KADIAN® allows it to be administered on either a once-a-day or twice-a-day schedule. KADIAN® produces analgesia similar to that produced by conventional immediate-release and controlled-release formulations for the same total daily dose of morphine. However, peak and trough blood levels depend on the release characteristics of each specific formulation, and other oral morphines may not be therapeutically equivalent to KADIAN® for an individual patient.
KADIAN® capsules have the same extent of absorption (AUC) as immediate-release oral formulations and controlled-release oral formulations of morphine sulfate. However, key pharmacokinetic parameters (e.g. C max , T max ) for KADIAN® are significantly different from other controlled-release oral formulations.
As with any potent opioid drug product, it is critical to adjust the dosing regimen for each patient individually, taking into account the patient's prior analgesic treatment experience. In the selection of the initial dose of KADIAN®, attention should be given to:
- the total daily dose, potency and kind of opioid the patient has been taking previously;
- the reliability of the relative potency estimate used to calculate the equivalent dose of morphine needed;
- the patient's degree of opioid tolerance;
- the general condition and medical status of the patient;
- concurrent medication;
- the type and severity of the patient's pain.
The following dosing recommendations, therefore, can only be considered suggested approaches to what is actually a series of clinical decisions over time in the management of the pain of an individual patient.
Conversion from Other Oral Morphine Formulations to KADIAN®
Patients on other oral morphine formulations may be converted to KADIAN® by administering one-half of the patient's total daily oral morphine dose as KADIAN® capsules every 12 hours (twice-a-day) or by administering the total daily oral morphine dose as KADIAN® capsules every 24 hours (once-a-day). KADIAN® should not be given more frequently than every 12 hours.
Conversion from Parenteral Morphine or Other Parenteral or Oral Opioids to KADIAN®
KADIAN® can be administered to patients previously receiving treatment with parenteral morphine or other opioids. While there are useful tables of oral and parenteral equivalents in cancer analgesia, there is substantial interpatient variation in the relative potency of different opioid drugs and formulations. For these reasons, it is better to underestimate the patient's 24 hour oral morphine requirement and provide rescue medication, than to overestimate and manage an adverse event. The following general points should be considered:
Parenteral to oral morphine ratio: It may take anywhere from 2-6 mg of oral morphine to provide analgesia equivalent to 1 mg of parenteral morphine. A dose of oral morphine three times the daily parenteral morphine requirement may be sufficient in chronic use settings.
Other parenteral or oral opioids to oral morphine sulfate: Physicians are advised to refer to published relative potency data, keeping in mind that such ratios are only approximate. In general, it is safest to give half of the estimated daily morphine demand as the initial dose, and to manage inadequate analgesia by supplementation with immediate-release morphine. (See discussion which follows.)
The first dose of KADIAN® may be taken with the last dose of any immediate-release (short-acting) opioid medication due to the long delay until the peak effect after administration of KADIAN®.
Use of KADIAN® as the First Opioid Analgesic
There has been no evaluation of KADIAN® as an initial opioid analgesic in the management of pain. Because it may be more difficult to titrate a patient to adequate analgesia using a sustained release morphine, it is ordinarily advisable to begin treatment using an immediate-release morphine formulation.
Individualization of Dosage
The best use of opioid analgesics in the management of chronic malignant and non-malignant pain is challenging, and is well described in materials published by the World Health Organization and the Agency for Health Care Policy and Research which are available from Alpharma upon request. KADIAN® is a third step drug which is most useful when the patient requires a constant level of opioid analgesia as a "floor" or "platform" from which to manage breakthrough pain. When a patient has reached the point where comfort cannot be provided with a combination of non-opioid medications (NSAIDs and acetaminophen) and intermittent use of moderate or strong opioids, the patient's total opioid therapy should be converted into a 24 hour oral morphine equivalent.
KADIAN® should be started by administering one-half of the estimated total daily oral morphine dose every 12 hours (twice-a-day) or by administering the total daily oral morphine dose every 24 hours (once-a-day). The dose should be titrated no more frequently than every-other-day to allow the patients to stabilize before escalating the dose. If breakthrough pain occurs, the dose may be supplemented with a small dose (less than 20% of the total daily dose) of a short-acting analgesic. Patients who are excessively sedated after a once-a-day dose or who regularly experience inadequate analgesia before the next dose should be switched to twice-a-day dosing.
Patients who do not have a proven tolerance to opioids should be started only on the 20 mg strength, and usually should be increased at a rate not greater than 20 mg every-other-day. Most patients will rapidly develop some degree of tolerance, requiring dosage adjustment until they have achieved their individual best balance between baseline analgesia and opioid side effects such as confusion, sedation and constipation. No guidance can be given as to the recommended maximal dose, especially in patients with chronic pain of malignancy. In such cases the total dose of KADIAN® should be advanced until the desired therapeutic endpoint is reached or clinically significant opioid-related adverse reactions intervene.
Alternative Methods of Administration
In a study of healthy volunteers, KADIAN® pellets sprinkled over applesauce were found to be bioequivalent to KADIAN® capsules swallowed whole with applesauce under fasting conditions. Other foods have not been tested. Patients who have difficulty swallowing whole capsules or tablets may benefit from this alternative method of administration.
- Sprinkle the pellets onto a small amount of applesauce. Applesauce should be room temperature or cooler.
- Use immediately.
- Rinse mouth to ensure all pellets have been swallowed.
- Patients should consume entire portion and should not divide applesauce into separate doses.
The entire capsule contents may be administered through a 16 French gastrostomy tube.
- Flush the gastrostomy tube with water to ensure that it is wet.
- Sprinkle the KADIAN® Pellets into 10 mL of water.
- Use a swirling motion to pour the pellets and water into the gastrostomy tube through a funnel.
- Rinse the beaker with a further 10 mL of water and pour this into the funnel.
- Repeat rinsing until no pellets remain in the beaker.
THE ADMINISTRATION OF KADIAN® PELLETS THROUGH A NASOGASTRIC TUBE SHOULD NOT BE ATTEMPTED.
Considerations in the Adjustment of Dosing Regimens
If signs of excessive opioid effects are observed early in the dosing interval, the next dose should be reduced. If this adjustment leads to inadequate analgesia, that is, if breakthrough pain occurs when KADIAN® is administered on an every 24 hours dosing regimen, consideration should be given to dosing every 12 hours. If breakthough pain occurs on a 12 hour dosing regimen a supplemental dose of short-acting analgesic may be given. As experience is gained, adjustments in both dose and dosing interval can be made to obtain an appropriate balance between pain relief and opioid side effects. To avoid accumulation the dosing interval of KADIAN® should not be reduced below 12 hours.
Conversion from KADIAN® to Other Controlled-Release Oral Morphine Formulations
KADIAN® is not bioequivalent to other controlled-release morphine preparations. Although for a given dose the same total amount of morphine is available from KADIAN® as from morphine solution or controlled-release morphine tablets, the slower release of morphine from KADIAN® results in reduced maximum and increased minimum plasma morphine concentrations than with shorter acting morphine products. Conversion from KADIAN® to the same total daily dose of controlled-release morphine preparations may lead to either excessive sedation at peak or inadequate analgesia at trough and close observation and appropriate dosage adjustments are recommended.
Conversion from KADIAN® to Parenteral Opioids
When converting a patient from KADIAN® to parenteral opioids, it is best to calculate an equivalent parenteral dose, and then initiate treatment at half of this calculated value. For example, to estimate the required 24 hour dose of parenteral morphine for a patient taking KADIAN®, one would take the 24 hour KADIAN® dose, divide by an oral to parenteral conversion ratio of 3, divide the estimated 24 hour parenteral dose into six divided doses (for a four hour dosing interval), then halve this dose as an initial trial.
For example, to estimate the required parenteral morphine dose for a patient taking 360 mg of KADIAN® a day, divide the 360 mg daily oral morphine dose by a conversion ratio of 1 mg of parenteral morphine for every 3 mg of oral morphine. The estimated 120 mg daily parenteral requirement is then divided into six 20 mg doses, and half of this, or 10 mg, is then given every 4 hours as an initial trial dose.
This approach is likely to require a dosage increase in the first 24 hours for many patients, but is recommended because it is less likely to cause overdose than trying to establish an equivalent dose without titration.
Opioid analgesic agents may not effectively relieve dysesthetic pain, post-herpetic neuralgia, stabbing pains, activity-related pain, and some forms of headache. This does not mean that patients suffering from these types of pain should not be given an adequate trial of opioid analgesics. However, such patients may need to be promptly evaluated for other types of pain therapy.
Safety and Handling
KADIAN® consists of closed hard gelatin capsules containing polymer coated morphine sulfate pellets that pose no known handling risk to health care workers. Oral morphine products are not known to be associated with a high risk of diversion, but all strong opioids are liable to diversion and misuse both by the general public and health care workers, and should be handled accordingly.
HOW SUPPLIED
KADIAN® capsules contain white to off-white or tan colored polymer coated sustained release pellets of morphine sulfate and are available in five dose strengths:
20 mg size 4 capsule, yellow opaque cap imprinted KADIAN and yellow opaque body imprinted with 20 mg. Capsules are supplied in bottles of 30 (NDC 63857-322-03), 60 (NDC 63857-322-06), and 100 (NDC 63857-322-11).
30 mg size 4 capsule, blue violet opaque cap imprinted KADIAN and blue violet opaque body imprinted with 30 mg. Capsules are supplied in bottles of 30 (NDC 63857-325-03), 60 (NDC 63857-325-06), and 100 (NDC 63857-325-11).
50 mg size 2 capsule, blue opaque cap imprinted KADIAN and blue opaque body imprinted with 50 mg. Capsules are supplied in bottles of 30 (NDC 63857-323-03), 60 (NDC 63857-323-06), and 100 (NDC 63857-323-11).
60 mg size 1 capsule, pink opaque cap imprinted KADIAN and pink opaque body imprinted with 60 mg. Capsules are supplied in bottles of 30 (NDC 63857-326-03), 60 (NDC 63857-326-06), and 100 (NDC 63857-326-11).
100 mg size 0 capsule, green opaque cap imprinted KADIAN and green opaque body imprinted with 100 mg. Capsules are supplied in bottles of 30 (NDC 63857-324-03), 60 (NDC 63857-324-06), and 100 (NDC 63857-324-11).
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). Protect from light and moisture.
Dispense in a sealed, tamper-evident, childproof, light-resistant container.
CAUTION
DEA Order Form Required.
Rx only
KADIAN® is a licensed trademark of Alpharma Branded Products Division Inc.
MS Contin® is a registered trademark of The Purdue Frederick Company
Manufactured for: Alpharma Branded Products Division Inc.
One New England Avenue
Piscataway, NJ 08854
by: Purepac Pharmaceutical Co.
Elizabeth, NJ 07207 USA
40-8984Revised--November 2004
Subscribe to the "News" RSS Feed 
TOP ۞
