-
Lipitor Tablets (Parke-Davis)
DESCRIPTION
Lipitor ® (atorvastatin calcium) is a synthetic lipid-lowering agent. Atorvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, an early and rate-limiting step in cholesterol biosynthesis.
Atorvastatin calcium is [R-(R*, R*)]-2-(4-fluorophenyl)-(beta), [dgr ]-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid, calcium salt (2:1) trihydrate. The empirical formula of atorvastatin calcium is (C 33 H 34 FN 2 O 5 ) 2 Ca·3H 2 O and its molecular weight is 1209.42. Its structural formula is:

Atorvastatin calcium is a white to off-white crystalline powder that is insoluble in aqueous solutions of pH 4 and below. Atorvastatin calcium is very slightly soluble in distilled water, pH 7.4 phosphate buffer, and acetonitrile, slightly soluble in ethanol, and freely soluble in methanol.
Lipitor tablets for oral administration contain 10, 20, 40 or 80 mg atorvastatin and the following inactive ingredients: calcium carbonate, USP; candelilla wax, FCC; croscarmellose sodium, NF; hydroxypropyl cellulose, NF; lactose monohydrate, NF; magnesium stearate, NF; microcrystalline cellulose, NF; Opadry White YS-1-7040 (hypromellose, polyethylene glycol, talc, titanium dioxide); polysorbate 80, NF; simethicone emulsion.
CLINICAL PHARMACOLOGY
Mechanism of Action
Atorvastatin is a selective, competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl-coenzyme A to mevalonate, a precursor of sterols, including cholesterol. Cholesterol and triglycerides circulate in the bloodstream as part of lipoprotein complexes. With ultracentrifugation, these complexes separate into HDL (high-density lipoprotein), IDL (intermediate-density lipoprotein), LDL (low-density lipoprotein), and VLDL (very-low-density lipoprotein) fractions. Triglycerides (TG) and cholesterol in the liver are incorporated into VLDL and released into the plasma for delivery to peripheral tissues. LDL is formed from VLDL and is catabolized primarily through the high-affinity LDL receptor. Clinical and pathologic studies show that elevated plasma levels of total cholesterol (total-C), LDL-cholesterol (LDL-C), and apolipoprotein B (apo B) promote human atherosclerosis and are risk factors for developing cardiovascular disease, while increased levels of HDL-C are associated with a decreased cardiovascular risk.
In animal models, Lipitor lowers plasma cholesterol and lipoprotein levels by inhibiting HMG-CoA reductase and cholesterol synthesis in the liver and by increasing the number of hepatic LDL receptors on the cell-surface to enhance uptake and catabolism of LDL; Lipitor also reduces LDL production and the number of LDL particles. Lipitor reduces LDL-C in some patients with homozygous familial hypercholesterolemia (FH), a population that rarely responds to other lipid-lowering medication(s).
A variety of clinical studies have demonstrated that elevated levels of total-C, LDL-C, and apo B (a membrane complex for LDL-C) promote human atherosclerosis. Similarly, decreased levels of HDL-C (and its transport complex, apo A) are associated with the development of atherosclerosis. Epidemiologic investigations have established that cardiovascular morbidity and mortality vary directly with the level of total-C and LDL-C, and inversely with the level of HDL-C.
Lipitor reduces total-C, LDL-C, and apo B in patients with homozygous and heterozygous FH, nonfamilial forms of hypercholesterolemia, and mixed dyslipidemia. Lipitor also reduces VLDL-C and TG and produces variable increases in HDL-C and apolipoprotein A-1. Lipitor reduces total-C, LDL-C, VLDL-C, apo B, TG, and non-HDL-C, and increases HDL-C in patients with isolated hypertriglyceridemia. Lipitor reduces intermediate density lipoprotein cholesterol (IDL-C) in patients with dysbetalipoproteinemia.
Like LDL, cholesterol-enriched triglyceride-rich lipoproteins, including VLDL, intermediate density lipoprotein (IDL), and remnants, can also promote atherosclerosis. Elevated plasma triglycerides are frequently found in a triad with low HDL-C levels and small LDL particles, as well as in association with non-lipid metabolic risk factors for coronary heart disease. As such, total plasma TG has not consistently been shown to be an independent risk factor for CHD. Furthermore, the independent effect of raising HDL or lowering TG on the risk of coronary and cardiovascular morbidity and mortality has not been determined.
Pharmacodynamics
Atorvastatin as well as some of its metabolites are pharmacologically active in humans. The liver is the primary site of action and the principal site of cholesterol synthesis and LDL clearance. Drug dosage rather than systemic drug concentration correlates better with LDL-C reduction. Individualization of drug dosage should be based on therapeutic response (see DOSAGE AND ADMINISTRATION ).
Pharmacokinetics and Drug Metabolism
Absorption: Atorvastatin is rapidly absorbed after oral administration; maximum plasma concentrations occur within 1 to 2 hours. Extent of absorption increases in proportion to atorvastatin dose. The absolute bioavailability of atorvastatin (parent drug) is approximately 14% and the systemic availability of HMG-CoA reductase inhibitory activity is approximately 30%. The low systemic availability is attributed to presystemic clearance in gastrointestinal mucosa and/or hepatic first-pass metabolism. Although food decreases the rate and extent of drug absorption by approximately 25% and 9%, respectively, as assessed by Cmax and AUC, LDL-C reduction is similar whether atorvastatin is given with or without food. Plasma atorvastatin concentrations are lower (approximately 30% for Cmax and AUC) following evening drug administration compared with morning. However, LDL-C reduction is the same regardless of the time of day of drug administration (see DOSAGE AND ADMINISTRATION ).
Distribution: Mean volume of distribution of atorvastatin is approximately 381 liters. Atorvastatin is >/=98% bound to plasma proteins. A blood/plasma ratio of approximately 0.25 indicates poor drug penetration into red blood cells. Based on observations in rats, atorvastatin is likely to be secreted in human milk (see CONTRAINDICATIONS , Pregnancy and Lactation , and PRECAUTIONS , Nursing Mothers ).
Metabolism: Atorvastatin is extensively metabolized to ortho- and parahydroxylated derivatives and various beta-oxidation products. In vitro inhibition of HMG-CoA reductase by ortho- and parahydroxylated metabolites is equivalent to that of atorvastatin. Approximately 70% of circulating inhibitory activity for HMG-CoA reductase is attributed to active metabolites. In vitro studies suggest the importance of atorvastatin metabolism by cytochrome P450 3A4, consistent with increased plasma concentrations of atorvastatin in humans following coadministration with erythromycin, a known inhibitor of this isozyme (see PRECAUTIONS , Drug Interactions ). In animals, the ortho-hydroxy metabolite undergoes further glucuronidation.
Excretion: Atorvastatin and its metabolites are eliminated primarily in bile following hepatic and/or extra-hepatic metabolism; however, the drug does not appear to undergo enterohepatic recirculation. Mean plasma elimination half-life of atorvastatin in humans is approximately 14 hours, but the half-life of inhibitory activity for HMG-CoA reductase is 20 to 30 hours due to the contribution of active metabolites. Less than 2% of a dose of atorvastatin is recovered in urine following oral administration.
Special Populations
Geriatric: Plasma concentrations of atorvastatin are higher (approximately 40% for Cmax and 30% for AUC) in healthy elderly subjects (age >/=65 years) than in young adults. Clinical data suggest a greater degree of LDL-lowering at any dose of drug in the elderly patient population compared to younger adults (see PRECAUTIONS section; Geriatric Use subsection).
Pediatric: Pharmacokinetic data in the pediatric population are not available.
Gender: Plasma concentrations of atorvastatin in women differ from those in men (approximately 20% higher for Cmax and 10% lower for AUC); however, there is no clinically significant difference in LDL-C reduction with Lipitor between men and women.
Renal Insufficiency: Renal disease has no influence on the plasma concentrations or LDL-C reduction of atorvastatin; thus, dose adjustment in patients with renal dysfunction is not necessary (see DOSAGE AND ADMINISTRATION ).
Hemodialysis: While studies have not been conducted in patients with end-stage renal disease, hemodialysis is not expected to significantly enhance clearance of atorvastatin since the drug is extensively bound to plasma proteins.
Hepatic Insufficiency: In patients with chronic alcoholic liver disease, plasma concentrations of atorvastatin are markedly increased. Cmax and AUC are each 4-fold greater in patients with Childs-Pugh A disease. Cmax and AUC are approximately 16-fold and 11-fold increased, respectively, in patients with Childs-Pugh B disease (see CONTRAINDICATIONS ).
Clinical Studies
Prevention of Cardiovascular Disease
In the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), the effect of LIPITOR (atorvastatin calcium) on fatal and non-fatal coronary heart disease was assessed in 10,305 hypertensive patients 40-80 years of age (mean of 63 years), without a previous myocardial infarction and with TC levels </=251 mg/dl (6.5 mmol/l). Additionally all patients had at least 3 of the following cardiovascular risk factors: male gender (81.1%), age >55 years (84.5%), smoking (33.2%), diabetes (24.3%), history of CHD in a first-degree relative (26%), TC:HDL >6 (14.3%), peripheral vascular disease (5.1%), left ventricular hypertrophy (14.4%), prior cer-ebrovascular event (9.8%), specific ECG abnormality (14.3%), proteinuria/albuminuria (62.4%)]. In this double-blind, placebo-controlled study patients were treated with anti-hypertensive therapy (Goal BP <140/90 mm Hg for non-diabetic patients, <130/80 mm Hg for diabetic patients) and allocated to either LIPITOR 10 mg daily (n=5168) or placebo (n=5137), using a covariate adaptive method which took into account the distribution of fourteen baseline characteristics of patients already enrolled and minimized the imbalance of those characteristics across the groups. Patients were followed for a median duration of 3.3 years.
The effect of 10 mg/day of LIPITOR on lipid levels was similar to that seen in previous clinical trials.
LIPITOR significantly reduced the rate of coronary events [either fatal coronary heart disease (46 events in the placebo group vs 40 events in the LIPITOR group) or nonfatal MI (108 events in the placebo group vs 60 events in the LIPITOR group)] with a relative risk reduction of 36% [(based on incidences of 1.9% for LIPITOR vs 3.0% for placebo), p=0.0005 (see Figure 1)]. The risk reduction was consistent regardless of age, smoking status, obesity or presence of renal dysfunction. The effect of LIPITOR was seen regardless of baseline LDL levels. Due to the small number of events, results for women were inconclusive.
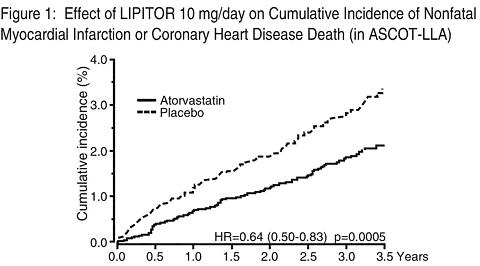
LIPITOR also significantly decreased the relative risk for revascularization procedures by 42%. Although the reduction of fatal and non-fatal strokes did not reach a pre-defined significance level (p = 0.01), a favorable trend was observed with a 26% relative risk reduction (incidences of 1.7% for LIPITOR and 2.3% for placebo). There was no significant difference between the treatment groups for death due to cardiovascular causes (p=0.51) or noncardiovascular causes (p=0.17).
Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia ( Fredrickson Types IIa and IIb)
Lipitor reduces total-C, LDL-C, VLDL-C, apo B, and TG, and increases HDL-C in patients with hypercholesterolemia and mixed dyslipidemia. Therapeutic response is seen within 2 weeks, and maximum response is usually achieved within 4 weeks and maintained during chronic therapy.
Lipitor is effective in a wide variety of patient populations with hypercholesterolemia, with and without hypertriglyceridemia, in men and women, and in the elderly. Experience in pediatric patients has been limited to patients with homozygous FH. In two multicenter, placebo-controlled, dose-response studies in patients with hypercholesterolemia, Lipitor given as a single dose over 6 weeks significantly reduced total-C, LDL-C, apo B, and TG (Pooled results are provided in Table 1).
TABLE 1. Dose-Response in Patients With Primary Hypercholesterolemia
(Adjusted Mean % Change From Baseline) aDose N TC LDL-C Apo B TG HDL-C Non-HDL-C/
HDL-CPlacebo 21 4 4 3 10 -3 7 10 22 -29 -39 -32 -19 6 -34 20 20 -33 -43 -35 -26 9 -41 40 21 -37 -50 -42 -29 6 -45 80 23 -45 -60 -50 -37 5 -53 a Results are pooled from 2 dose-response studies.
In patients with Fredrickson Types IIa and IIb hyperlipoproteinemia pooled from 24 controlled trials, the median (25 th and 75 th percentile) percent changes from baseline in HDL-C for atorvastatin 10, 20, 40, and 80 mg were 6.4 (-1.4, 14), 8.7 (0, 17), 7.8 (0, 16), and 5.1 (-2.7, 15), respectively. Additionally, analysis of the pooled data demonstrated consistent and significant decreases in total-C, LDL-C, TG, total-C/HDL-C, and LDL-C/HDL-C.
In three multicenter, double-blind studies in patients with hypercholesterolemia, Lipitor was compared to other HMG-CoA reductase inhibitors. After randomization, patients were treated for 16 weeks with either Lipitor 10 mg per day or a fixed dose of the comparative agent (Table 2).
TABLE 2. Mean Percent Change From Baseline at End Point
(Double-Blind, Randomized, Active-Controlled Trials)Treatment
(Daily Dose)N Total-C LDL-C Apo B TG HDL-C Non-HDL-C/
HDL-CStudy 1Atorvastatin 10 mg707 -27 a -36 a -28 a -17 a +7 -37 a Lovastatin 20 mg191 -19 -27 -20 -6 +7 -28 95% CI for Diff 1-9.2, -6.5 -10.7, -7.1 -10.0, -6.5 -15.2, -7.1 -1.7, 2.0 -11.1, -7.1 Study 2Atorvastatin 10 mg222 -25 b -35 b -27 b -17 b +6 -36 b Pravastatin 20 mg77 -17 -23 -17 -9 +8 -28 95% CI for Diff 1-10.8, -6.1 -14.5, -8.2 -13.4, -7.4 -14.1, -0.7 -4.9, 1.6 -11.5, -4.1 Study 3Atorvastatin 10 mg132 -29 c -37 c -34 c -23 c +7 -39 c Simvastatin 10 mg45 -24 -30 -30 -15 +7 -33 95% CI for Diff 1-8.7, -2.7 -10.1, -2.6 -8.0, -1.1 -15.1, -0.7 -4.3, 3.9 -9.6, -1.9 1 A negative value for the 95% CI for the difference between treatments favors atorvastatin for all except HDL-C, for which a positive value favors atorvastatin. If the range does not include 0, this indicates a statistically significant difference.a Significantly different from lovastatin, ANCOVA, p </=0.05b Significantly different from pravastatin, ANCOVA, p </=0.05c Significantly different from simvastatin, ANCOVA, p </=0.05The impact on clinical outcomes of the differences in lipid-altering effects between treatments shown in Table 2 is not known. Table 2 does not contain data comparing the effects of atorvastatin 10 mg and higher doses of lovastatin, pravastatin, and simvastatin. The drugs compared in the studies summarized in the table are not necessarily interchangeable.
Hypertriglyceridemia ( Fredrickson Type IV)
The response to Lipitor in 64 patients with isolated hypertriglyceridemia treated across several clinical trials is shown in the table below. For the atorvastatin-treated patients, median (min, max) baseline TG level was 565 (267-1502).
TABLE 3. Combined Patients With Isolated Elevated TG:
Median (min, max) Percent Changes From BaselinePlacebo
(N=12)Atorvastatin 10 mg
(N=37)Atorvastatin 20 mg
(N=13)Atorvastatin 80 mg
(N=14)Triglycerides-12.4 (-36.6, 82.7) -41.0 (-76.2, 49.4) -38.7 (-62.7, 29.5) -51.8 (-82.8, 41.3) Total-C-2.3 (-15.5, 24.4) -28.2 (-44.9, -6.8) -34.9 (-49.6, -15.2) -44.4 (-63.5, -3.8) LDL-C3.6 (-31.3, 31.6) -26.5 (-57.7, 9.8) -30.4 (-53.9, 0.3) -40.5 (-60.6, -13.8) HDL-C3.8 (-18.6, 13.4) 13.8 (-9.7, 61.5) 11.0 (-3.2, 25.2) 7.5 (-10.8, 37.2) VLDL-C-1.0 (-31.9, 53.2) -48.8 (-85.8, 57.3) -44.6 (-62.2, -10.8) -62.0 (-88.2, 37.6) non-HDL-C-2.8 (-17.6, 30.0) -33.0 (-52.1, -13.3) -42.7 (-53.7, -17.4) -51.5 (-72.9, -4.3) Dysbetalipoproteinemia ( Fredrickson Type III)
The results of an open-label crossover study of 16 patients (genotypes: 14 apo E2/E2 and 2 apo E3/E2) with dysbeta-lipoproteinemia ( Fredrickson Type III) are shown in the table below.
TABLE 4. Open-Label Crossover Study of 16 Patients
With Dysbetalipoproteinemia ( Fredrickson Type III)Median % Change (min, max) Median (min, max) at
Baseline (mg/dL)Atorvastatin
10 mgAtorvastatin
80 mgTotal-C442 (225, 1320) -37 (-85, 17) -58 (-90, -31) Triglycerides678 (273, 5990) -39 (-92, -8) -53 (-95, -30) IDL-C + VLDL-C215 (111, 613) -32 (-76, 9) -63 (-90, -8) non-HDL-C411 (218, 1272) -43 (-87, -19) -64 (-92, -36) Homozygous Familial Hypercholesterolemia
In a study without a concurrent control group, 29 patients ages 6 to 37 years with homozygous FH received maximum daily doses of 20 to 80 mg of Lipitor. The mean LDL-C reduction in this study was 18%. Twenty-five patients with a reduction in LDL-C had a mean response of 20% (range of 7% to 53%, median of 24%); the remaining 4 patients had 7% to 24% increases in LDL-C. Five of the 29 patients had absent LDL-receptor function. Of these, 2 patients also had a portacaval shunt and had no significant reduction in LDL-C. The remaining 3 receptor-negative patients had a mean LDL-C reduction of 22%.
Heterozygous Familial Hypercholesterolemia in Pediatric Patients
In a double-blind, placebo-controlled study followed by an open-label phase, 187 boys and postmenarchal girls 10-17 years of age (mean age 14.1 years) with heterozygous familial hypercholesterolemia (FH) or severe hypercholesterolemia were randomized to Lipitor (n=140) or placebo (n=47) for 26 weeks and then all received Lipitor for 26 weeks. Inclusion in the study required 1) a baseline LDL-C level >/= 190 mg/dL or 2) a baseline LDL-C >/= 160 mg/dL and positive family history of FH or documented premature cardiovascular disease in a first- or second-degree relative. The mean baseline LDL-C value was 218.6 mg/dL (range: 138.5-385.0 mg/dL) in the Lipitor group compared to 230.0 mg/dL (range: 160.0-324.5 mg/dL) in the placebo group. The dosage of Lipitor (once daily) was 10 mg for the first 4 weeks and up-titrated to 20 mg if the LDL-C level was > 130 mg/dL. The number of Lipitor-treated patients who required up-titration to 20 mg after Week 4 during the double-blind phase was 80 (57.1%).
Lipitor significantly decreased plasma levels of total-C, LDL-C, triglycerides, and apolipoprotein B during the 26 week double-blind phase (see Table 5).
TABLE 5
Lipid-altering Effects of Lipitor in Adolescent Boys and Girls with Heterozygous Familial
Hypercholesterolemia or Severe Hypercholesterolemia
(Mean Percent Change from Baseline at Endpoint in Intention-to-Treat Population)DOSAGE N Total-C LDL-C HDL-C TG Apolipoprotein B Placebo47 -1.5 -0.4 -1.9 1.0 0.7 Lipitor140 -31.4 -39.6 2.8 -12.0 -34.0
The mean achieved LDL-C value was 130.7 mg/dL (range: 70.0-242.0 mg/dL) in the Lipitor group compared to 228.5 mg/dL (range: 152.0-385.0 mg/dL) in the placebo group during the 26 week double-blind phase.
The safety and efficacy of doses above 20 mg have not been studied in controlled trials in children. The long-term efficacy of Lipitor therapy in childhood to reduce morbidity and mortality in adulthood has not been established.
INDICATIONS AND USAGE
Prevention of Cardiovascular Disease
In adult patients without clinically evident coronary heart disease, but with multiple risk factors for coronary heart disease such as age >/= 55 years, smoking, hypertension, low HDL-C, or a family history of early coronary heart disease, Lipitor is indicated to:
- Reduce the risk of myocardial infarction
- Reduce the risk for revascularization procedures and angina
Hypercholesterolemia
Lipitor is indicated:
- as an adjunct to diet to reduce elevated total-C, LDL-C, apo B, and TG levels and to increase HDL-C in patients with primary hypercholesterolemia (heterozygous familial and nonfamilial) and mixed dyslipidemia ( Fredrickson Types IIa and IIb);
- as an adjunct to diet for the treatment of patients with elevated serum TG levels ( Fredrickson Type IV);
- for the treatment of patients with primary dysbetalipoproteinemia ( Fredrickson Type III) who do not respond adequately to diet;
- to reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering treatments (eg, LDL apheresis) or if such treatments are unavailable;
-
as an adjunct to diet to reduce total-C, LDL-C, and apo B levels in boys and postmenarchal girls, 10 to 17 years of age, with heterozygous familial hypercholesterolemia if after an adequate trial of diet therapy the following findings are present:
- LDL-C remains >/= 190 mg/dL or
-
LDL-C remains >/= 160 mg/dL and:
- there is a positive family history of premature cardiovascular disease or
- two or more other CVD risk factors are present in the pediatric patient
Therapy with lipid-altering agents should be a component of multiple-risk-factor intervention in individuals at increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Lipid-altering agents should be used in addition to a diet restricted in saturated fat and cholesterol only when the response to diet and other nonpharmacological measures has been inadequate (see National Cholesterol Education Program (NCEP) Guidelines , summarized in Table 6).
TABLE 6. NCEP Treatment Guidelines: LDL-C Goals and Cutpoints for Therapeutic Lifestyle Changes
and Drug Therapy in Different Risk CategoriesRisk Category LDL Goal
(mg/dL)LDL Level at Which to
Initiate Therapeutic
Lifestyle Changes
(mg/dL)LDL Level at Which to Consider
Drug
Therapy (mg/dL)CHD a or CHD risk
equivalents
(10-year risk >20%)<100 >/=100 >/=130
(100-129: drug optional) b2+ Risk Factors
(10-year risk </=20%)<130 >/=130 10-year risk 10%-20%: >/=130
10-year risk <10%: >/=1600-1 Risk factor c<160 >/=160 >/=190
(160-189: LDL-lowering drug optional)a CHD, coronary heart disease b Some authorities recommend use of LDL-lowering drugs in this category if an LDL-C level of <100 mg/dL cannot be achieved by therapeutic lifestyle changes. Others prefer use of drugs that primarily modify triglycerides and HDL-C, e.g., nicotinic acid or fibrate. Clinical judgement also may call for deferring drug therapy in this subcategory. c Almost all people with 0-1 risk factor have 10-year risk <10%; thus, 10-year risk assessment in people with 0-1 risk factor is not necessary.
After the LDL-C goal has been achieved, if the TG is still >/=200 mg/dL, non HDL-C (total-C minus HDL-C) becomes a secondary target of therapy. Non-HDL-C goals are set 30 mg/dL higher than LDL-C goals for each risk category.
Prior to initiating therapy with Lipitor, secondary causes for hypercholesterolemia (eg, poorly controlled diabetes mellitus, hypothyroidism, nephrotic syndrome, dysproteinemias, obstructive liver disease, other drug therapy, and alcoholism) should be excluded, and a lipid profile performed to measure total-C, LDL-C, HDL-C, and TG. For patients with TG <400 mg/dL (<4.5 mmol/L), LDL-C can be estimated using the following equation: LDL-C = total-C - (0.20 × [TG] + HDL-C). For TG levels >400 mg/dL (>4.5 mmol/L), this equation is less accurate and LDL-C concentrations should be determined by ultracentrifugation.
Lipitor has not been studied in conditions where the major lipoprotein abnormality is elevation of chylomicrons ( Fredrickson Types I and V).
The NCEP classification of cholesterol levels in pediatric patients with a familial history of hypercholesterolemia or premature cardiovascular disease is summarized below:
CategoryTotal-C (mg/dL) LDL-C (mg/dL) Acceptable<170 <110 Borderline170-199 110-129 High>/=200 >/=130 CONTRAINDICATIONS
Active liver disease or unexplained persistent elevations of serum transaminases.
Hypersensitivity to any component of this medication.
Pregnancy and Lactation
Atherosclerosis is a chronic process and discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia. Cholesterol and other products of cholesterol biosynthesis are essential components for fetal development (including synthesis of steroids and cell membranes). Since HMG-CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, they may cause fetal harm when administered to pregnant women. Therefore, HMG-CoA reductase inhibitors are contrain-dicated during pregnancy and in nursing mothers. ATORVASTATIN SHOULD BE ADMINISTERED TO WOMEN OF CHILDBEARING AGE ONLY WHEN SUCH PATIENTS ARE HIGHLY UNLIKELY TO CONCEIVE AND HAVE BEEN INFORMED OF THE POTENTIAL HAZARDS. If the patient becomes pregnant while taking this drug, therapy should be discontinued and the patient apprised of the potential hazard to the fetus.
WARNINGS
Liver Dysfunction
HMG-CoA reductase inhibitors, like some other lipid-lowering therapies, have been associated with biochemical abnormalities of liver function. Persistent elevations (>3 times the upper limit of normal [ULN] occurring on 2 or more occasions) in serum transaminases occurred in 0.7% of patients who received atorvastatin in clinical trials. The incidence of these abnormalities was 0.2%, 0.2%, 0.6%, and 2.3% for 10, 20, 40, and 80 mg, respectively.
One patient in clinical trials developed jaundice. Increases in liver function tests (LFT) in other patients were not associated with jaundice or other clinical signs or symptoms. Upon dose reduction, drug interruption, or discontinuation, transaminase levels returned to or near pretreatment levels without sequelae. Eighteen of 30 patients with persistent LFT elevations continued treatment with a reduced dose of atorvastatin.
It is recommended that liver function tests be performed prior to and at 12 weeks following both the initiation of therapy and any elevation of dose, and periodically (eg, semiannually) thereafter. Liver enzyme changes generally occur in the first 3 months of treatment with atorvastatin. Patients who develop increased transaminase levels should be monitored until the abnormalities resolve. Should an increase in ALT or AST of >3 times ULN persist, reduction of dose or withdrawal of atorvastatin is recommended.
Atorvastatin should be used with caution in patients who consume substantial quantities of alcohol and/or have a history of liver disease. Active liver disease or unexplained persistent transaminase elevations are contraindications to the use of atorvastatin (see CONTRAINDICATIONS ).
Skeletal Muscle
Rare cases of rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with atorvastatin and with other drugs in this class.
Uncomplicated myalgia has been reported in atorvastatin-treated patients (see ADVERSE REACTIONS ). Myopathy, defined as muscle aches or muscle weakness in conjunction with increases in creatine phosphokinase (CPK) values >10 times ULN, should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevation of CPK. Patients should be advised to report promptly unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever. Atorvastatin therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected.
The risk of myopathy during treatment with drugs in this class is increased with concurrent administration of cyclosporine, fibric acid derivatives, erythromycin, niacin, or azole antifungals. Physicians considering combined therapy with atorvastatin and fibric acid derivatives, erythromycin, immunosuppressive drugs, azole antifungals, or lipid-lowering doses of niacin should carefully weigh the potential benefits and risks and should carefully monitor patients for any signs or symptoms of muscle pain, tenderness, or weakness, particularly during the initial months of therapy and during any periods of upward dosage titration of either drug. Periodic creatine phosphokinase (CPK) determinations may be considered in such situations, but there is no assurance that such monitoring will prevent the occurrence of severe myopathy.
Atorvastatin therapy should be temporarily withheld or discontinued in any patient with an acute, serious condition suggestive of a myopathy or having a risk factor predisposing to the development of renal failure secondary to rhabdomyolysis (eg, severe acute infection, hypotension, major surgery, trauma, severe metabolic, endocrine and electrolyte disorders, and uncontrolled seizures).
PRECAUTIONS
General
Before instituting therapy with atorvastatin, an attempt should be made to control hypercholesterolemia with appropriate diet, exercise, and weight reduction in obese patients, and to treat other underlying medical problems (see INDICATIONS AND USAGE ).
Information for Patients
Patients should be advised to report promptly unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever.
Drug Interactions
The risk of myopathy during treatment with drugs of this class is increased with concurrent administration of cyclosporine, fibric acid derivatives, niacin (nicotinic acid), erythromycin, azole antifungals (see WARNINGS , Skeletal Muscle ).
Antacid: When atorvastatin and Maalox® TC suspension were coadministered, plasma concentrations of atorvastatin decreased approximately 35%. However, LDL-C reduction was not altered.
Antipyrine: Because atorvastatin does not affect the pharmacokinetics of antipyrine, interactions with other drugs metabolized via the same cytochrome isozymes are not expected.
Colestipol: Plasma concentrations of atorvastatin decreased approximately 25% when colestipol and atorvastatin were coadministered. However, LDL-C reduction was greater when atorvastatin and colestipol were coadministered than when either drug was given alone.
Cimetidine: Atorvastatin plasma concentrations and LDL-C reduction were not altered by coadministration of cimetidine.
Digoxin: When multiple doses of atorvastatin and digoxin were coadministered, steady-state plasma digoxin concentrations increased by approximately 20%. Patients taking digoxin should be monitored appropriately.
Erythromycin: In healthy individuals, plasma concentrations of atorvastatin increased approximately 40% with coadministration of atorvastatin and erythromycin, a known inhibitor of cytochrome P450 3A4 (see WARNINGS , Skeletal Muscle ).
Oral Contraceptives: Coadministration of atorvastatin and an oral contraceptive increased AUC values for norethindrone and ethinyl estradiol by approximately 30% and 20%. These increases should be considered when selecting an oral contraceptive for a woman taking atorvastatin.
Warfarin: Atorvastatin had no clinically significant effect on prothrombin time when administered to patients receiving chronic warfarin treatment.
Endocrine Function
HMG-CoA reductase inhibitors interfere with cholesterol synthesis and theoretically might blunt adrenal and/or gonadal steroid production. Clinical studies have shown that atorvastatin does not reduce basal plasma cortisol concentration or impair adrenal reserve. The effects of HMG-CoA reductase inhibitors on male fertility have not been studied in adequate numbers of patients. The effects, if any, on the pituitary-gonadal axis in premenopausal women are unknown. Caution should be exercised if an HMG-CoA reductase inhibitor is administered concomitantly with drugs that may decrease the levels or activity of endogenous steroid hormones, such as ketoconazole, spironolactone, and cimetidine.
CNS Toxicity
Brain hemorrhage was seen in a female dog treated for 3 months at 120 mg/kg/day. Brain hemorrhage and optic nerve vacuolation were seen in another female dog that was sacrificed in moribund condition after 11 weeks of escalating doses up to 280 mg/kg/day. The 120 mg/kg dose resulted in a systemic exposure approximately 16 times the human plasma area-under-the-curve (AUC, 0-24 hours) based on the maximum human dose of 80 mg/day. A single tonic convulsion was seen in each of 2 male dogs (one treated at 10 mg/kg/day and one at 120 mg/kg/day) in a 2-year study. No CNS lesions have been observed in mice after chronic treatment for up to 2 years at doses up to 400 mg/kg/day or in rats at doses up to 100 mg/kg/day. These doses were 6 to 11 times (mouse) and 8 to 16 times (rat) the human AUC (0-24) based on the maximum recommended human dose of 80 mg/day.
CNS vascular lesions, characterized by perivascular hemorrhages, edema, and mononuclear cell infiltration of perivascular spaces, have been observed in dogs treated with other members of this class. A chemically similar drug in this class produced optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in clinically normal dogs in a dose-dependent fashion at a dose that produced plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study in rats at dose levels of 10, 30, and 100 mg/kg/day, 2 rare tumors were found in muscle in high-dose females: in one, there was a rhabdomyosarcoma and, in another, there was a fibrosarcoma. This dose represents a plasma AUC (0-24) value of approximately 16 times the mean human plasma drug exposure after an 80 mg oral dose.
A 2-year carcinogenicity study in mice given 100, 200, or 400 mg/kg/day resulted in a significant increase in liver adenomas in high-dose males and liver carcinomas in high-dose females. These findings occurred at plasma AUC (0-24) values of approximately 6 times the mean human plasma drug exposure after an 80 mg oral dose.
In vitro, atorvastatin was not mutagenic or clastogenic in the following tests with and without metabolic activation: the Ames test with Salmonella typhimurium and Escherichia coli, the HGPRT forward mutation assay in Chinese hamster lung cells, and the chromosomal aberration assay in Chinese hamster lung cells. Atorvastatin was negative in the in vivo mouse micronucleus test.
Studies in rats performed at doses up to 175 mg/kg (15 times the human exposure) produced no changes in fertility. There was aplasia and aspermia in the epididymis of 2 of 10 rats treated with 100 mg/kg/day of atorvastatin for 3 months (16 times the human AUC at the 80 mg dose); testis weights were significantly lower at 30 and 100 mg/kg and epididymal weight was lower at 100 mg/kg. Male rats given 100 mg/kg/day for 11 weeks prior to mating had decreased sperm motility, spermatid head concentration, and increased abnormal sperm. Atorvastatin caused no adverse effects on semen parameters, or reproductive organ histopathology in dogs given doses of 10, 40, or 120 mg/kg for two years.
Pregnancy
Pregnancy Category X
See CONTRAINDICATIONS
Safety in pregnant women has not been established. Atorvastatin crosses the rat placenta and reaches a level in fetal liver equivalent to that of maternal plasma. Atorvastatin was not teratogenic in rats at doses up to 300 mg/kg/day or in rabbits at doses up to 100 mg/kg/day. These doses resulted in multiples of about 30 times (rat) or 20 times (rabbit) the human exposure based on surface area (mg/m 2 ).
In a study in rats given 20, 100, or 225 mg/kg/day, from gestation day 7 through to lactation day 21 (weaning), there was decreased pup survival at birth, neonate, weaning, and maturity in pups of mothers dosed with 225 mg/kg/day. Body weight was decreased on days 4 and 21 in pups of mothers dosed at 100 mg/kg/day; pup body weight was decreased at birth and at days 4, 21, and 91 at 225 mg/kg/day. Pup development was delayed (rotorod performance at 100 mg/kg/day and acoustic startle at 225 mg/kg/day; pinnae detachment and eye opening at 225 mg/kg/day). These doses correspond to 6 times (100 mg/kg) and 22 times (225 mg/kg) the human AUC at 80 mg/day. Rare reports of congenital anomalies have been received following intrauterine exposure to HMG-CoA reductase inhibitors. There has been one report of severe congenital bony deformity, tracheo-esophageal fistula, and anal atresia (VATER association) in a baby born to a woman who took lovastatin with dextroamphetamine sulfate during the first trimester of pregnancy. Lipitor should be administered to women of child-bearing potential only when such patients are highly unlikely to conceive and have been informed of the potential hazards. If the woman becomes pregnant while taking Lipitor, it should be discontinued and the patient advised again as to the potential hazards to the fetus.
Nursing Mothers
Nursing rat pups had plasma and liver drug levels of 50% and 40%, respectively, of that in their mother's milk. Because of the potential for adverse reactions in nursing infants, women taking Lipitor should not breast-feed (see CONTRAINDICATIONS ).
Pediatric Use
Safety and effectiveness in patients 10-17 years of age with heterozygous familial hypercholesterolemia have been evaluated in a controlled clinical trial of 6 months duration in adolescent boys and postmenarchal girls. Patients treated with Lipitor had an adverse experience profile generally similar to that of patients treated with placebo, the most common adverse experiences observed in both groups, regardless of causality assessment, were infections. Doses greater than 20 mg have not been studied in this patient population. In this limited controlled study, there was no detectable effect on growth or sexual maturation in boys or on menstrual cycle length in girls (see CLINICAL PHARMACOLOGY , Clinical Studies section; ADVERSE REACTIONS , Pediatric Patients (ages 10-17 years); and DOSAGE AND ADMINISTRATION , Heterozygous Familial Hypercholesterolemia in Pediatric Patients (10-17 years of age). Adolescent females should be counseled on appropriate contraceptive methods while on Lipitor therapy (see CONTRAINDICATIONS and PRECAUTIONS , Pregnancy ). Lipitor has not been studied in controlled clinical trials involving pre-pubertal patients or patients younger than 10 years of age.
Clinical efficacy with doses up to 80 mg/day for 1 year have been evaluated in an uncontrolled study of patients with homozygous FH including 8 pediatric patients (see CLINICAL PHARMACOLOGY , Clinical Studies : Homozygous Familial Hypercholesterolemia).
Geriatric Use
The safety and efficacy of atorvastatin (10-80 mg) in the geriatric population (>/=65 years of age) was evaluated in the ACCESS study. In this 54-week open-label trial 1,958 patients initiated therapy with atorvastatin 10 mg. Of these, 835 were elderly (>/=65 years) and 1,123 were non-elderly. The mean change in LDL-C from baseline after 6 weeks of treatment with atorvastatin 10 mg was -38.2% in the elderly patients versus -34.6% in the non-elderly group.
The rates of discontinuation due to adverse events were similar between the two age groups. There were no differences in clinically relevant laboratory abnormalities between the age groups.
ADVERSE REACTIONS
Lipitor is generally well-tolerated. Adverse reactions have usually been mild and transient. In controlled clinical studies of 2502 patients, <2% of patients were discontinued due to adverse experiences attributable to atorvastatin. The most frequent adverse events thought to be related to atorvastatin were constipation, flatulence, dyspepsia, and abdominal pain.
Clinical Adverse Experiences
Adverse experiences reported in >/=2% of patients in placebo-controlled clinical studies of atorvastatin, regardless of causality assessment, are shown in Table 7.
TABLE 7. Adverse Events in Placebo-Controlled Studies
(% of Patients)BODY SYSTEM/
Adverse EventPlacebo
N = 270Atorvastatin
10 mg
N = 863Atorvastatin
20 mg
N = 36Atorvastatin
40 mg
N = 79Atorvastatin
80 mg
N = 94BODY AS A WHOLEInfection10.0 10.3 2.8 10.1 7.4 Headache7.0 5.4 16.7 2.5 6.4 Accidental Injury3.7 4.2 0.0 1.3 3.2 Flu Syndrome1.9 2.2 0.0 2.5 3.2 Abdominal Pain0.7 2.8 0.0 3.8 2.1 Back Pain3.0 2.8 0.0 3.8 1.1 Allergic Reaction2.6 0.9 2.8 1.3 0.0 Asthenia1.9 2.2 0.0 3.8 0.0 DIGESTIVE SYSTEMConstipation1.8 2.1 0.0 2.5 1.1 Diarrhea1.5 2.7 0.0 3.8 5.3 Dyspepsia4.1 2.3 2.8 1.3 2.1 Flatulence3.3 2.1 2.8 1.3 1.1 RESPIRATORY SYSTEMSinusitis2.6 2.8 0.0 2.5 6.4 Pharyngitis1.5 2.5 0.0 1.3 2.1 SKIN AND APPENDAGESRash0.7 3.9 2.8 3.8 1.1 MUSCULOSKELETAL SYSTEMArthralgia1.5 2.0 0.0 5.1 0.0 Myalgia1.1 3.2 5.6 1.3 0.0 Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT)
In ASCOT (see CLINICAL PHARMACOLOGY , Clinical Studies ) involving 10,305 participants treated with Lipitor 10 mg daily (n=5,168) or placebo (n=5,137), the safety and tolerability profile of the group treated with Lipitor was comparable to that of the group treated with placebo during a median of 3.3 years of follow-up.
The following adverse events were reported, regardless of causality assessment in patients treated with atorvastatin in clinical trials. The events in italics occurred in >/=2% of patients and the events in plain type occurred in <2% of patients.
Body as a Whole: Chest pain, face edema, fever, neck rigidity, malaise, photosensitivity reaction, generalized edema.
Digestive System: Nausea, gastroenteritis, liver function tests abnormal, colitis, vomiting, gastritis, dry mouth, rectal hemorrhage, esophagitis, eructation, glossitis, mouth ulceration, anorexia, increased appetite, stomatitis, biliary pain, cheilitis, duodenal ulcer, dysphagia, enteritis, melena, gum hemorrhage, stomach ulcer, tenesmus, ulcerative stomatitis, hepatitis, pancreatitis, cholestatic jaundice.
Respiratory System: Bronchitis, rhinitis, pneumonia, dyspnea, asthma, epistaxis.
Nervous System: Insomnia, dizziness, paresthesia, somnolence, amnesia, abnormal dreams, libido decreased, emotional lability, incoordination, peripheral neuropathy, torticollis, facial paralysis, hyperkinesia, depression, hypesthesia, hypertonia.
Musculoskeletal System: Arthritis, leg cramps, bursitis, tenosynovitis, myasthenia, tendinous contracture, myositis.
Skin and Appendages: Pruritus, contact dermatitis, alopecia, dry skin, sweating, acne, urticaria, eczema, seborrhea, skin ulcer.
Urogenital System: Urinary tract infection, urinary frequency, cystitis, hematuria, impotence, dysuria, kidney calculus, nocturia, epididymitis, fibrocystic breast, vaginal hemorrhage, albuminuria, breast enlargement, metrorrhagia, nephritis, urinary incontinence, urinary retention, urinary urgency, abnormal ejaculation, uterine hemorrhage.
Special Senses: Amblyopia, tinnitus, dry eyes, refraction disorder, eye hemorrhage, deafness, glaucoma, parosmia, taste loss, taste perversion.
Cardiovascular System: Palpitation, vasodilatation, syncope, migraine, postural hypotension, phlebitis, arrhythmia, angina pectoris, hypertension.
Metabolic and Nutritional Disorders: Peripheral edema, hyperglycemia, creatine phosphokinase increased, gout, weight gain, hypoglycemia.
Hemic and Lymphatic System: Ecchymosis, anemia, lymphadenopathy, thrombocytopenia, petechia.
Postintroduction Reports
Adverse events associated with Lipitor therapy reported since market introduction, that are not listed above, regardless of causality assessment, include the following: anaphylaxis, angioneurotic edema, bullous rashes (including erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis), and rhabdomyolysis.
Pediatric Patients (ages 10-17 years)
In a 26-week controlled study in boys and postmenarchal girls (n=140), the safety and tolerability profile of Lipitor 10 to 20 mg daily was generally similar to that of placebo (see CLINICAL PHARMACOLOGY , Clinical Studies section and PRECAUTIONS , Pediatric Use ).
OVERDOSAGE
There is no specific treatment for atorvastatin overdosage. In the event of an overdose, the patient should be treated symptomatically, and supportive measures instituted as required. Due to extensive drug binding to plasma proteins, hemodialysis is not expected to significantly enhance atorvastatin clearance.
DOSAGE AND ADMINISTRATION
The patient should be placed on a standard cholesterol-lowering diet before receiving Lipitor and should continue on this diet during treatment with Lipitor.
Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia ( Fredrickson Types IIa and IIb)
The recommended starting dose of Lipitor is 10 or 20 mg once daily. Patients who require a large reduction in LDL-C (more than 45%) may be started at 40 mg once daily. The dosage range of Lipitor is 10 to 80 mg once daily. Lipitor can be administered as a single dose at any time of the day, with or without food. The starting dose and maintenance doses of Lipitor should be individualized according to patient characteristics such as goal of therapy and response (see NCEP Guidelines, summarized in Table 5). After initiation and/or upon titration of Lipitor, lipid levels should be analyzed within 2 to 4 weeks and dosage adjusted accordingly.
Since the goal of treatment is to lower LDL-C, the NCEP recommends that LDL-C levels be used to initiate and assess treatment response. Only if LDL-C levels are not available, should total-C be used to monitor therapy.
Heterozygous Familial Hypercholesterolemia in Pediatric Patients (10-17 years of age)
The recommended starting dose of Lipitor is 10 mg/day; the maximum recommended dose is 20 mg/day (doses greater than 20 mg have not been studied in this patient population). Doses should be individualized according to the recommended goal of therapy (see NCEP Pediatric Panel Guidelines 1 , CLINICAL PHARMACOLOGY , and INDICATIONS AND USAGE ). Adjustments should be made at intervals of 4 weeks or more.
1 National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children Adolescents, Pediatrics . 89(3):495-501. 1992.
Homozygous Familial Hypercholesterolemia
The dosage of Lipitor in patients with homozygous FH is 10 to 80 mg daily. Lipitor should be used as an adjunct to other lipid-lowering treatments (eg, LDL apheresis) in these patients or if such treatments are unavailable.
Concomitant Therapy
Atorvastatin may be used in combination with a bile acid binding resin for additive effect. The combination of HMG-CoA reductase inhibitors and fibrates should generally be avoided (see WARNINGS , Skeletal Muscle , and PRECAUTIONS , Drug Interactions for other drug-drug interactions).
Dosage in Patients With Renal Insufficiency
Renal disease does not affect the plasma concentrations nor LDL-C reduction of atorvastatin; thus, dosage adjustment in patients with renal dysfunction is not necessary (see CLINICAL PHARMACOLOGY , Pharmacokinetics ).
HOW SUPPLIED
Lipitor is supplied as white, elliptical, film-coated tablets of atorvastatin calcium containing 10, 20, 40 and 80 mg atorvastatin.
10 mg tablets: coded "PD 155" on one side and "10" on the other.
NDC 0071-0155-23 bottles of 90
NDC 0071-0155-34 bottles of 5000
NDC 0071-0155-40 10 × 10 unit dose blisters
20 mg tablets: coded "PD 156" on one side and "20" on the other.
NDC 0071-0156-23 bottles of 90
NDC 0071-0156-40 10 × 10 unit dose blisters
NDC 0071-0156-94 bottles of 5000
40 mg tablets: coded "PD 157" on one side and "40" on the other.
NDC 0071-0157-23 bottles of 90
NDC 0071-0157-73 bottles of 500
80 mg tablets: coded "PD 158" on one side and "80" on the other.
NDC 0071-0158-23 bottles of 90
NDC 0071-0158-73 bottles of 500
Storage
Store at controlled room temperature 20-25°C (68-77°F) [see USP].
Rx Only
©2004 Pfizer Ireland Pharmaceuticals
Manufactured by:
Pfizer Ireland Pharmaceuticals
Dublin, Ireland
Distributed by:
Division of Pfizer Inc, NY, NY 10017
LAB-0021-7.0 Revised July 2004
Subscribe to the "News" RSS Feed 
TOP ۞
