-
Nimotop Capsules (Bayer)
DESCRIPTION
Nimotop® (nimodipine) belongs to the class of pharmacological agents known as calcium channel blockers. Nimodipine is isopropyl 2 - methoxyethyl 1, 4 - dihydro - 2, 6 - dimethyl - 4 - (m - nitrophenyl) - 3, 5 - pyridinedicarboxylate It has a molecular weight of 418.5 and a molecular formula of C 21 H 26 N 2 O 7 . The structural formula is:
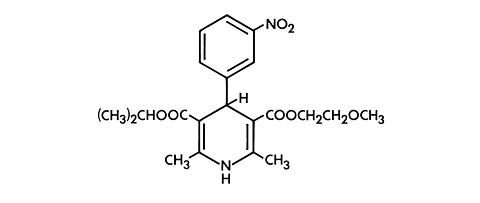
Nimodipine is a yellow crystalline substance, practically insoluble in water.
NIMOTOP® capsules are formulated as soft gelatin capsules for oral administration. Each liquid filled capsule contains 30 mg of nimodipine in a vehicle of glycerin, peppermint oil, purified water and polyethylene glycol 400. The soft gelatin capsule shell contains gelatin, glycerin, purified water and titanium dioxide.
CLINICAL PHARMACOLOGY
Mechanism of Action: Nimodipine is a calcium channel blocker. The contractile processes of smooth muscle cells are dependent upon calcium ions, which enter these cells during depolarization as slow ionic transmembrane currents. Nimodipine inhibits calcium ion transfer into these cells and thus inhibits contractions of vascular smooth muscle. In animal experiments, nimodipine had a greater effect on cerebral arteries than on arteries elsewhere in the body perhaps because it is highly lipophilic, allowing it to cross the blood-brain barrier; concentrations of nimodipine as high as 12.5 ng/mL have been detected in the cerebrospinal fluid of nimodipine-treated subarachnoid hemorrhage (SAH) patients.
The precise mechanism of action of nimodipine in humans is unknown. Although the clinical studies described below demonstrate a favorable effect of nimodipine on the severity of neurological deficits caused by cerebral vasospasm following SAH, there is no arteriographic evidence that the drug either prevents or relieves the spasm of these arteries. However, whether or not the arteriographic methodology utilized was adequate to detect a clinically meaningful effect, if any, on vasospasm is unknown.
Pharmacokinetics and Metabolism: In man, nimodipine is rapidly absorbed after oral administration, and peak concentrations are generally attained within one hour. The terminal elimination half-life is approximately 8 to 9 hours but earlier elimination rates are much more rapid, equivalent to a half-life of 1-2 hours; a consequence is the need for frequent (every 4 hours) dosing. There were no signs of accumulation when nimodipine was given three times a day for seven days. Nimodipine is over 95% bound to plasma proteins. The binding was concentration independent over the range of 10 ng/mL to 10 µg/mL. Nimodipine is eliminated almost exclusively in the form of metabolites and less than 1% is recovered in the urine as unchanged drug. Numerous metabolites, all of which are either inactive or considerably less active than the parent compound, have been identified. Because of a high first-pass metabolism, the bioavailability of nimodipine averages 13% after oral administration. The bioavailability is significantly increased in patients with hepatic cirrhosis, with C max approximately double that in normals which necessitates lowering the dose in this group of patients (see Dosage and Administration ). In a study of 24 healthy male volunteers, administration of nimodipine capsules following a standard breakfast resulted in a 68% lower peak plasma concentration and 38% lower bioavailability relative to dosing under fasted conditions.
In a single parallel-group study involving 24 elderly subjects (aged 59-79) and 24 younger subjects (aged 22-40), the observed AUC and C max of nimodipine was approximately 2-fold higher in the elderly population compared to the younger study subjects following oral administration (given as a single dose of 30 mg and dosed to steady-state with 30 mg t.i.d. for 6 days). The clinical response to these age-related pharmacokinetic differences, however, was not considered significant. (See PRECAUTIONS: Geriatric Use .)
Clinical Trials: Nimodipine has been shown, in 4 randomized, double-blind, placebo-controlled trials, to reduce the severity of neurological deficits resulting from vasospasm in patients who have had a recent subarachnoid hemorrhage (SAH). The trials used doses ranging from 20-30 mg to 90 mg every 4 hours, with drug given for 21 days in 3 studies, and for at least 18 days in the other. Three of the four trials followed patients for 3-6 months. Three of the trials studied relatively well patients, with all or most patients in Hunt and Hess Grades I - III (essentially free of focal deficits after the initial bleed) the fourth studied much sicker patients, Hunt and Hess Grades III - V. Two studies, one U.S., one French, were similar in design, with relatively unimpaired SAH patients randomized to nimodipine or placebo. In each, a judgment was made as to whether any late-developing deficit was due to spasm or other causes, and the deficits were graded. Both studies showed significantly fewer severe deficits due to spasm in the nimodipine group; the second (French) study showed fewer spasm-related deficits of all severities. No effect was seen on deficits not related to spasm.
StudyDose Grade * Patients Number
AnalyzedAny Deficit
Due to SpasmNumbers with
Severe DeficitU.S.20-30 mg I-III Nimodipine56 13 1 Placebo60 16 8 ** French60 mg I-III Nimodipine31 4 2 Placebo39 11 10 ** *Hunt and Hess Grade**p=0.03
A third, large, study was performed in the United Kingdom in SAH patients with all grades of severity (but 89% were in Grades I-III). Nimodipine was dosed 60 mg every 4 hours. Outcomes were not defined as spasm related or not but there was a significant reduction in the overall rate of infarction and severely disabling neurological outcome at 3 months:
Total patientsNimodipine Placebo 278 276 Good Recovery199 * 169 Moderate disability24 16 Severe disability12 ** 31 Death43 *** 60 * p = 0.0444 - good and moderate vs severe and dead** p = 0.001 - severe disability***p = 0.056 - death
A Canadian study entered much sicker patients, (Hunt and Hess Grades III-V), who had a high rate of death and disability, and used a dose of 90 mg every 4 hours, but was otherwise similar to the first two studies. Analysis of delayed ischemic deficits, many of which result from spasm, showed a significant reduction in spasm-related deficits. Among analyzed patients (72 nimodipine, 82 placebo), there were the following outcomes.
Delayed Ischemic
Deficits (DID)Permanent
DeficitsNimodipine
n (%)Placebo
n (%)Nimodipine
n (%)Placebo
n (%)DID Spasm Alone8 (11) * 25 (31) 5 (7) * 22 (27) DID Spasm
Contributing18 (25) 21 (26) 16 (22) 17 (21) DID Without Spasm7 (10) 8 (10) 6 (8) 7 (9) No DID39 (54) 28 (34) 45 (63) 36 (44) *p = 0.001, nimodipine vs placebo
When data were combined for the Canadian and the United Kingdom studies, the treatment difference on success rate (i.e. good recovery) on the Glasgow Outcome Scale was 25.3% (nimodipine) versus 10.9% (placebo) for Hunt and Hess Grades IV or V. The table below demonstrates that nimodipine tends to improve good recovery of SAH patients with poor neurological status post-ictus, while decreasing the numbers with severe disability and vegetative survival.
Glasgow Outcome *Nimodipine
(n=87)Placebo
(n=101)Good Recovery22 (25.3%) 11 (10.9%) Moderate Disability8 (9.2%) 12 (11.9%) Severe Disability6 (6.9%) 15 (14.9%) Vegetative Survival4 (4.6%) 9 (8.9%) Death47 (54.0%) 54 (53.5%) * p = 0.045, nimodipine vs placebo
A dose-ranging study comparing 30, 60 and 90 mg doses found a generally low rate of spasm-related neurological deficits but no dose response relationship.
INDICATIONS AND USAGE
Nimotop® (nimodipine) is indicated for the improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in patients with subarachnoid hemorrhage from ruptured intracranial berry aneurysms regardless of their post-ictus neurological condition (i.e., Hunt and Hess Grades I-V).
CONTRAINDICATIONS
None known.
PRECAUTIONS
General: Blood Pressure: Nimodipine has the hemodynamic effects expected of a calcium channel blocker, although they are generally not marked. However, intravenous administration of the contents of Nimotop Capsules has resulted in serious adverse consequences including hypotension, cardiovascular collapse, and cardiac arrest. In patients with subarachnoid hemorrhage given Nimotop® in clinical studies, about 5% were reported to have had lowering of the blood pressure and about 1% left the study because of this (not all could be attributed to nimodipine). Nevertheless, blood pressure should be carefully monitored during treatment with Nimotop® based on its known pharmacology and the known effects of calcium channel blockers.
Hepatic Disease: The metabolism of Nimotop® is decreased in patients with impaired hepatic function. Such patients should have their blood pressure and pulse rate monitored closely and should be given a lower dose (see Dosage and Administration ).
Intestinal pseudo-obstruction and ileus have been reported rarely in patients treated with nimodipine. A causal relationship has not been established. The condition has responded to conservative management.
Laboratory Test Interactions: None known.
Drug Interaction: It is possible that the cardiovascular action of other calcium channel blockers could be enhanced by the addition of Nimotop®.
In Europe, Nimotop® was observed to occasionally intensify the effect of antihypertensive compounds taken concomitantly by patients suffering from hypertension; this phenomenon was not observed in North American clinical trials.
A study in eight healthy volunteers has shown a 50% increase in mean peak nimodipine plasma concentrations and a 90% increase in mean area under the curve, after a one-week course of cimetidine at 1,000 mg/day and nimodipine at 90 mg/day. This effect may be mediated by the known inhibition of hepatic cytochrome P-450 by cimetidine, which could decrease first-pass metabolism of nimodipine.
Carcinogenesis, Mutagenesis, Impairment of Fertility: In a two-year study, higher incidences of adenocarcinoma of the uterus and Leydig-cell adenoma of the testes were observed in rats given a diet containing 1800 ppm nimodipine (equivalent to 91 to 121 mg/kg/day nimodipine) than in placebo controls. The differences were not statistically significant, however, and the higher rates were well within historical control range for these tumors in the Wistar strain. Nimodipine was found not to be carcinogenic in a 91-week mouse study but the high dose of 1800 ppm nimodipine-in-feed (546 to 774 mg/kg/day) shortened the life expectancy of the animals. Mutagenicity studies, including the Ames, micronucleus and dominant lethal tests were negative.
Nimodipine did not impair the fertility and general reproductive performance of male and female Wistar rats following oral doses of up to 30 mg/kg/day when administered daily for more than 10 weeks in the males and 3 weeks in the females prior to mating and continued to day 7 of pregnancy. This dose in a rat is about 4 times the equivalent clinical dose of 60 mg q4h in a 50 kg patient.
Pregnancy: Pregnancy Category C. Nimodipine has been shown to have a teratogenic effect in Himalayan rabbits. Incidences of malformations and stunted fetuses were increased at oral doses of 1 and 10 mg/kg/day administered (by gavage) from day 6 through day 18 of pregnancy but not at 3.0 mg/kg/day in one of two identical rabbit studies. In the second study an increased incidence of stunted fetuses was seen at 1.0 mg/kg/day but not at higher doses. Nimodipine was embryotoxic, causing resorption and stunted growth of fetuses, in Long Evans rats at 100 mg/kg/day administered by gavage from day 6 through day 15 of pregnancy. In two other rat studies, doses of 30 mg/kg/day nimodipine administered by gavage from day 16 of gestation and continued until sacrifice (day 20 of pregnancy or day 21 post partum) were associated with higher incidences of skeletal variation, stunted fetuses and stillbirths but no malformations. There are no adequate and well controlled studies in pregnant women to directly assess the effect on human fetuses. Nimodipine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: Nimodipine and/or its metabolites have been shown to appear in rat milk at concentrations much higher than in maternal plasma. It is not known whether the drug is excreted in human milk. Because many drugs are excreted in human milk, nursing mothers are advised not to breast feed their babies when taking the drug.
Pediatric Use: Safety and effectiveness in children have not been established.
Geriatric Use: Clinical studies of nimodipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dosing in elderly patients should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
Adverse experiences were reported by 92 of 823 patients with subarachnoid hemorrhage (11.2%) who were given nimodipine. The most frequently reported adverse experience was decreased blood pressure in 4.4% of these patients. Twenty-nine of 479 (6.1%) placebo treated patients also reported adverse experiences. The events reported with a frequency greater than 1% are displayed below by dose.
DOSE q4h Number of Patients (%) Nimodipine 0.35
mg/kg30
mg60
mg90
mg120
mgPlacebo Sign/Symptom(n=82) (n=71) (n=494) (n=172) (n=4) (n=479) Decreased
Blood Pressure1 (1.2) 0 19 (3.8) 14 (8.1) 2 (50.0) 6 (1.2) Abnormal Liver
Function Test1 (1.2) 0 2 (0.4) 1 (0.6) 0 7 (1.5) Edema0 0 2 (0.4) 2 (1.2) 0 3 (0.6) Diarrhea0 3 (4.2) 0 3 (1.7) 0 3 (0.6) Rash2 (2.4) 0 3 (0.6) 2 (1.2) 0 3 (0.6) Headache0 1 (1.4) 6 (1.2) 0 0 1 (0.2) Gastrointestinal
Symptoms2 (2.4) 0 0 2 (1.2) 0 0 Nausea1 (1.2) 1 (1.4) 6 (1.2) 1 (0.6) 0 0 Dyspnea1 (1.2) 0 0 0 0 0 EKG
Abnormalities
0
1 (1.4)
0
1 (0.6)
0
0Tachycardia0 1 (1.4) 0 0 0 0 Bradycardia0 0 5 (1.0) 1 (0.6) 0 0 Muscle Pain/
Cramp
0
1 (1.4)
1 (0.2)
1 (0.6)
0
0Acne0 1 (1.4) 0 0 0 0 Depression0 1 (1.4) 0 0 0 0 There were no other adverse experiences reported by the patients who were given 0.35 mg/kg q4h, 30 mg q4h or 120 mg q4h. Adverse experiences with an incidence rate of less than 1% in the 60 mg q4h dose group were: hepatitis; itching; gastrointestinal hemorrhage; thrombocytopenia; anemia; palpitations; vomiting; flushing; diaphoresis; wheezing; phenytoin toxicity; lightheadedness; dizziness; rebound vasospasm; jaundice; hypertension; hematoma.
Adverse experiences with an incidence rate less than 1% in the 90 mg q4h dose group were: itching; gastrointestinal hemorrhage; thrombocytopenia; neurological deterioration; vomiting; diaphoresis; congestive heart failure; hyponatremia; decreasing platelet count; disseminated intravascular coagulation; deep vein thrombosis.
As can be seen from the table, side effects that appear related to nimodipine use based on increased incidence with higher dose or a higher rate compared to placebo control, included decreased blood pressure, edema and headaches which are known pharmacologic actions of calcium channel blockers. It must be noted, however, that SAH is frequently accompanied by alterations in consciousness which lead to an under reporting of adverse experiences. Patients who received nimodipine in clinical trials for other indications reported flushing (2.1%), headache (4.1%) and fluid retention (0.3%), typical responses to calcium channel blockers. As a calcium channel blocker, nimodipine may have the potential to exacerbate heart failure in susceptible patients or to interfere with A-V conduction, but these events were not observed.
No clinically significant effects on hematologic factors, renal or hepatic function or carbohydrate metabolism have been causally associated with oral nimodipine. Isolated cases of non-fasting elevated serum glucose levels (0.8%), elevated LDH levels (0.4%), decreased platelet counts (0.3%), elevated alkaline phosphatase levels (0.2%) and elevated SGPT levels (0.2%) have been reported rarely.
DRUG ABUSE AND DEPENDENCE
There have been no reported instances of drug abuse or dependence with Nimotop®.
OVERDOSAGE
There have been no reports of overdosage from the oral administration of Nimotop®. Symptoms of overdosage would be expected to be related to cardiovascular effects such as excessive peripheral vasodilation with marked systemic hypotension. Clinically significant hypotension due to Nimotop® overdosage may require active cardiovascular support. Norepinephrine or dopamine may be helpful in restoring blood pressure. Since Nimotop® is highly protein-bound, dialysis is not likely to be of benefit.
DOSAGE AND ADMINISTRATION
Nimotop is given orally in the form of ivory colored, soft gelatin 30 mg capsules for subarachnoid hemorrhage.
The oral dose is 60 mg (two 30 mg capsules) every 4 hours for 21 consecutive days, preferably not less than one hour before or two hours after meals. Oral Nimotop® therapy should commence within 96 hours of the subarachnoid hemorrhage.
If the capsule cannot be swallowed, e.g., at the time of surgery, or if the patient is unconscious, a hole should be made in both ends of the capsule with an 18 gauge needle, and the contents of the capsule extracted into a syringe. The contents should then be emptied into the patient's in situ naso-gastric tube and washed down the tube with 30 mL of normal saline (0.9%).
The contents of Nimotop Capsules must not be administered by intravenous injection or other parenteral routes.
Patients with hepatic cirrhosis have substantially reduced clearance and approximately doubled C max . Dosage should be reduced to 30 mg every 4 hours, with close monitoring of blood pressure and heart rate.
HOW SUPPLIED
Each ivory colored, soft gelatin NIMOTOP® capsule is imprinted with the word Nimotop and contains 30 mg of nimodipine. The 30 mg capsules are packaged in unit dose foil pouches and supplied in cartons containing 100 capsules. The product is also available in child resistant unit dose safety pak foil pouches containing 30 capsules per carton. The capsules should be stored in the manufacturer's original foil package at 25°C (77°F), excursions permitted to 15-30°C (59-86°F) [See USP controlled Room Temperature.]
Capsules should be protected from light and freezing.
Strength NDC Code Capsule
IdentificationUnit DosePackage of 100:30 mg 0026-2855-48 Nimotop Unit DosePackage of 30:30 mg 0026-2855-70 Nimotop Distributed by:
Bayer Pharmaceuticals Corporation
400 Morgan Lane
West Haven, CT 06516
Manufactured by:
Cardinal Health
St. Petersburg, FL 33716
Rx Only
08897207 4/04 BAY e 97365202-7-A-U.S.-10
©2004 Bayer Pharmaceuticals Corporation 12324
Printed in USA
Subscribe to the "News" RSS Feed 
TOP ۞
