-
Noroxin Tablets (Merck)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of NOROXIN **/* and other antibacterial drugs, NOROXIN should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
NOROXIN (Norfloxacin) is a synthetic, broad-spectrum antibacterial agent for oral administration. Norfloxacin, a fluoroquinolone, is 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C 16 H 18 FN 3 O 3 and the structural formula is:
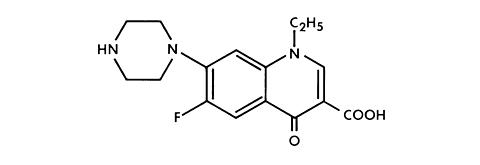
Norfloxacin is a white to pale yellow crystalline powder with a molecular weight of 319.34 and a melting point of about 221°C. It is freely soluble in glacial acetic acid, and very slightly soluble in ethanol, methanol and water.
NOROXIN is available in 400-mg tablets. Each tablet contains the following inactive ingredients: cellulose, croscarmellose sodium, hydroxypropyl cellulose, hydroxypropyl methylcellulose, iron oxide, magnesium stearate, and titanium dioxide.
Norfloxacin, a fluoroquinolone, differs from non-fluorinated quinolones by having a fluorine atom at the 6 position and a piperazine moiety at the 7 position.
**/* Registered trademark of Merck & CO., Inc.
COPYRIGHT © Merck & CO., Inc., 1986, 1989, 1999, 2001
All rights reserved
CLINICAL PHARMACOLOGY
In fasting healthy volunteers, at least 30-40% of an oral dose of NOROXIN is absorbed. Absorption is rapid following single doses of 200 mg, 400 mg and 800 mg. At the respective doses, mean peak serum and plasma concentrations of 0.8, 1.5 and 2.4 µg/mL are attained approximately one hour after dosing. The presence of food and/or dairy products may decrease absorption. The effective half-life of norfloxacin in serum and plasma is 3-4 hours. Steady-state concentrations of norfloxacin will be attained within two days of dosing.
In healthy elderly volunteers (65-75 years of age with normal renal function for their age), norfloxacin is eliminated more slowly because of their slightly decreased renal function. Following a single 400-mg dose of norfloxacin, the mean (± SD) AUC and C max of 9.8 (2.83) µg·hr/mL and 2.02 (0.77) µg/mL, respectively, were observed in healthy elderly volunteers. The extent of systemic exposure was slightly higher than that seen in younger adults (AUC 6.4 µg·hr/mL and C max 1.5 µg/mL). Drug absorption appears unaffected. However, the effective half-life of norfloxacin in these elderly subjects is 4 hours.
There is no information on accumulation of norfloxacin with repeated administration in elderly patients. However, no dosage adjustment is required based on age alone. In elderly patients with reduced renal function, the dosage should be adjusted as for other patients with renal impairment (see DOSAGE AND ADMINISTRATION , Renal Impairment ).
The disposition of norfloxacin in patients with creatinine clearance rates greater than 30 mL/min/1.73m 2 is similar to that in healthy volunteers. In patients with creatinine clearance rates equal to or less than 30 mL/min/1.73m 2 , the renal elimination of norfloxacin decreases so that the effective serum half-life is 6.5 hours. In these patients, alteration of dosage is necessary (see DOSAGE AND ADMINISTRATION ). Drug absorption appears unaffected by decreasing renal function.
Norfloxacin is eliminated through metabolism, biliary excretion, and renal excretion. After a single 400-mg dose of NOROXIN, mean antimicrobial activities equivalent to 278, 773, and 82 µg of norfloxacin/g of feces were obtained at 12, 24, and 48 hours, respectively. Renal excretion occurs by both glomerular filtration and tubular secretion as evidenced by the high rate of renal clearance (approximately 275 mL/min). Within 24 hours of drug administration, 26 to 32% of the administered dose is recovered in the urine as norfloxacin with an additional 5-8% being recovered in the urine as six active metabolites of lesser antimicrobial potency. Only a small percentage (less than 1%) of the dose is recovered thereafter. Fecal recovery accounts for another 30% of the administered dose. In elderly subjects (average creatinine clearance was 91 mL/min/1.73m 2 ) approximately 22% of the administered dose was recovered in urine and renal clearance averaged 154 mL/min.
Two to three hours after a single 400-mg dose, urinary concentrations of 200 µg/mL or more are attained in the urine. In healthy volunteers, mean urinary concentrations of norfloxacin remain above 30 µg/mL for at least 12 hours following a 400-mg dose. The urinary pH may affect the solubility of norfloxacin. Norfloxacin is least soluble at urinary pH of 7.5 with greater solubility occurring at pHs above and below this value. The serum protein binding of norfloxacin is between 10 and 15%.
The following are mean concentrations of norfloxacin in various fluids and tissues measured 1 to 4 hours post-dose after two 400-mg doses, unless otherwise indicated:
Renal Parenchyma7.3 µg/gProstate2.5 µg/gSeminal Fluid2.7 µg/mLTesticle1.6 µg/gUterus/Cervix3.0 µg/gVagina4.3 µg/gFallopian Tube1.9 µg/gBile6.9 µg/mL
(after two 200-mg doses)Microbiology
Norfloxacin has in vitro activity against a broad range of gram-positive and gram-negative aerobic bacteria. The fluorine atom at the 6 position provides increased potency against gram-negative organisms, and the piperazine moiety at the 7 position is responsible for antipseudomonal activity.
Norfloxacin inhibits bacterial deoxyribonucleic acid synthesis and is bactericidal. At the molecular level, three specific events are attributed to norfloxacin in E. coli cells:
- inhibition of the ATP-dependent DNA supercoiling reaction catalyzed by DNA gyrase,
- inhibition of the relaxation of supercoiled DNA,
- promotion of double-stranded DNA breakage.
Resistance to norfloxacin due to spontaneous mutation in vitro is a rare occurrence (range: 10 - 9 to 10 -12 cells). Resistant organisms have emerged during therapy with norfloxacin in less than 1% of patients treated. Organisms in which development of resistance is greatest are the following:
Pseudomonas aeruginosa
Klebsiella pneumoniae
Acinetobacter spp.
Enterococcus spp.
For this reason, when there is a lack of satisfactory clinical response, repeat culture and susceptibility testing should be done. Nalidixic acid-resistant organisms are generally susceptible to norfloxacin in vitro; however, these organisms may have higher minimum inhibitory concentrations (MIC's) to norfloxacin than nalidixic acid-susceptible strains. There is generally no cross-resistance between norfloxacin and other classes of antibacterial agents. Therefore, norfloxacin may demonstrate activity against indicated organisms resistant to some other antimicrobial agents including the aminoglycosides, penicillins, cephalosporins, tetracyclines, macrolides, and sulfonamides, including combinations of sulfamethoxazole and trimethoprim. Antagonism has been demonstrated in vitro between norfloxacin and nitrofurantoin.
Norfloxacin has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Gram-positive aerobes:
Enterococcus faecalis
Staphylococcus aureus
Staphylococcus epidermidis
Staphylococcus saprophyticus
Streptococcus agalactiae
Gram-negative aerobes:
Citrobacter freundii
Enterobacter aerogenes
Enterobacter cloacae
Escherichia coli
Klebsiella pneumoniae
Neisseria gonorrhoeae
Proteus mirabilis
Proteus vulgaris
Pseudomonas aeruginosa
Serratia marcescens
The following in vitro data are available, but their clinical significance is unknown .
Norfloxacin exhibits in vitro minimal inhibitory concentrations (MIC's) of </=4 µg/mL against most (>/=90%) strains of the following microorganisms; however, the safety and effectiveness of norfloxacin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Gram-negative aerobes:
Citrobacter diversus
Edwardsiella tarda
Enterobacter agglomerans
Haemophilus ducreyi
Klebsiella oxytoca
Morganella morganii
Providencia alcalifaciens
Providencia rettgeri
Providencia stuartii
Pseudomonas fluorescens
Pseudomonas stutzeri
Other:
Ureaplasma urealyticum
NOROXIN is not generally active against obligate anaerobes.
Norfloxacin has not been shown to be active against Treponema pallidum. (See WARNINGS .)
Susceptibility Tests
Dilution Techniques:
Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MIC's). These MIC's provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MIC's should be determined using a standardized procedure. Standardized procedures are based on a dilution method 1 (broth, agar, or microdilution) or equivalent with standardized inoculum concentrations and standardized concentrations of norfloxacin powder. The MIC values should be interpreted according to the following criteria * :
MIC (µg/mL) Interpretation </=4 Susceptible (S) 8 Intermediate (I) >/=16 Resistant (R)
A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
* These interpretative criteria apply only to isolates from urinary tract infections. There are no established norfloxacin interpretive criteria for Neisseria gonorrhoeae or organisms isolated from other infection sites.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard norfloxacin powder should provide the following MIC values:
OrganismMIC range (µg/mL) E. coli ATCC 259220.03-0.12 E. faecalis ATCC 292122-8 P. aeruginosa ATCC 278531-4 S. aureus ATCC 292130.5-2 Diffusion Techniques:
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure 2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 10-µg norfloxacin to test the susceptibility of microorganisms to norfloxacin. Reports from the laboratory providing results of the standard single-disk susceptibility test with a 10-µg norfloxacin disk should be interpreted according to the following criteria * :
Zone diameter (mm) Interpretation >/=17 Susceptible (S) 13-16 Intermediate (I) </=12 Resistant (R)
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for norfloxacin.
As with standard dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion techniques, the 10-µg norfloxacin disk should provide the following zone diameters in these laboratory test quality control strains:
Organism Zone Diameter (mm) E. coli ATCC 2592228-35 P. aeruginosa ATCC 2785322-29 S. aureus ATCC 2592317-28 INDICATIONS AND USAGE
NOROXIN is indicated for the treatment of adults with the following infections caused by susceptible strains of the designated microorganisms:
Urinary tract infections:
Uncomplicated urinary tract infections (including cystitis) due to Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus epidermidis, Staphylococcus saprophyticus, Citrobacter freundii ** , Enterobacter aerogenes ** , Enterobacter cloacae ** , Proteus vulgaris ** , Staphylococcus aureus ** , or Streptococcus agalactiae ** .
Complicated urinary tract infections due to Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pro-teus mirabilis, Pseudomonas aeruginosa, or Serratia marcescens ** .
Sexually transmitted diseases (see WARNINGS ):
Uncomplicated urethral and cervical gonorrhea due to Neisseria gonorrhoeae.
Prostatitis:
Prostatitis due to Escherichia coli.
(See DOSAGE AND ADMINISTRATION for appropriate dosing instructions.)
Penicillinase production should have no effect on norfloxacin activity.
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing the infection and to determine their susceptibility to norfloxacin. Therapy with norfloxacin may be initiated before results of these tests are known; once results become available, appropriate therapy should be given. Repeat culture and susceptibility testing performed periodically during therapy will provide information not only on the therapeutic effect of the antimicrobial agents but also on the possible emergence of bacterial resistance.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of NOROXIN and other antibacterial drugs, NOROXIN should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
** Efficacy for this organism in this organ system was studied in fewer than 10 infections.
CONTRAINDICATIONS
NOROXIN (norfloxacin) is contraindicated in persons with a history of hypersensitivity, tendinitis, or tendon rupture associated with the use of norfloxacin or any member of the quinolone group of antimicrobial agents.
WARNINGS
Safety in Children, Adolescents, Nursing mothers, and during Pregnancy: THE SAFETY AND EFFICACY OF ORAL NORFLOXACIN IN PEDIATRIC PATIENTS, ADOLESCENTS (UNDER THE AGE OF 18), PREGNANT WOMEN, AND NURSING MOTHERS HAVE NOT BEEN ESTABLISHED . (See PRECAUTIONS , Pediatric Use , Pregnancy , and Nursing Mothers subsections.) The oral administration of single doses of norfloxacin, 6 times *** the recommended human clinical dose (on a mg/kg basis), caused lameness in immature dogs. Histologic examination of the weight-bearing joints of these dogs revealed permanent lesions of the cartilage. Other quinolones also produced erosions of the cartilage in weight-bearing joints and other signs of arthropathy in immature animals of various species. (See ANIMAL PHARMACOLOGY .)
Seizures: Convulsions have been reported in patients receiving norfloxacin. Convulsions, increased intracranial pressure, and toxic psychoses have been reported in patients receiving drugs in this class. Quinolones may also cause central nervous system (CNS) stimulation which may lead to tremors, restlessness, lightheadedness, confusion, and hallucinations. If these reactions occur in patients receiving norfloxacin, the drug should be discontinued and appropriate measures instituted.
The effects of norfloxacin on brain function or on the electrical activity of the brain have not been tested. Therefore, until more information becomes available, norfloxacin, like all other quinolones, should be used with caution in patients with known or suspected CNS disorders, such as severe cerebral arteriosclerosis, epilepsy, and other factors which predispose to seizures. (See ADVERSE REACTIONS .)
Hypersensitivity/anaphylaxis: Serious and occasionally fatal hypersensitivity (anaphylactoid or anaphylactic) reactions, some following the first dose, have been reported in patients receiving quinolone therapy. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, tingling, pharyngeal or facial edema, dyspnea, urticaria and itching. Only a few patients had a history of hypersensitivity reactions. If an allergic reaction to norfloxacin occurs, discontinue the drug. Serious acute hypersensitivity reactions may require immediate emergency treatment with epinephrine. Oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines, and airway management, including intubation, should be administered as indicated.
Pseudomembranous colitis: Pseudomembranous colitis has been reported with nearly all antibacterial agents, including norfloxacin, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of "antibiotic-associated colitis".
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis.
***Based on a patient weight of 50 kg.
Peripheral neuropathy: Rare cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving quinolones, including norfloxacin. Norfloxacin should be discontinued if the patient experiences symptoms of neuropathy including pain, burning, tingling, numbness, and/or weakness, or is found to have deficits in light touch, pain, temperature, position sense, vibratory sensation, and/or motor strength in order to prevent the development of an irreversible condition.
Tendon effects: Ruptures of the shoulder, hand, Achilles tendons or other tendons that required surgical repair or resulted in prolonged disability have been reported in patients receiving quinolones, including norfloxacin. Post-marketing surveillance reports indicate that this risk may be increased in patients receiving concomitant corticosteroids, especially in the elderly. Norfloxacin should be discontinued if the patient experiences pain, inflammation, or rupture of a tendon. Patients should rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been excluded. Tendon rupture can occur during or after therapy with quinolones, including norfloxacin.
Syphilis treatment: Norfloxacin has not been shown to be effective in the treatment of syphilis. Antimicrobial agents used in high doses for short periods of time to treat gonorrhea may mask or delay the symptoms of incubating syphilis. All patients with gonorrhea should have a serologic test for syphilis at the time of diagnosis. Patients treated with norfloxacin should have a follow-up serologic test for syphilis after three months.
PRECAUTIONS
General
Needle-shaped crystals were found in the urine of some volunteers who received either placebo, 800 mg norfloxacin, or 1600 mg norfloxacin (at or twice the recommended daily dose, respectively) while participating in a double-blind, crossover study comparing single doses of norfloxacin with placebo. While crystalluria is not expected to occur under usual conditions with a dosage regimen of 400 mg b.i.d., as a precaution, the daily recommended dosage should not be exceeded and the patient should drink sufficient fluids to ensure a proper state of hydration and adequate urinary output.
Alteration in dosage regimen is necessary for patients with impaired renal function (see DOSAGE AND ADMINISTRATION ).
Moderate to severe phototoxicity reactions have been observed in patients who are exposed to excessive sunlight while receiving some members of this drug class. Excessive sunlight should be avoided. Therapy should be discontinued if phototoxicity occurs.
Rarely, hemolytic reactions have been reported in patients with latent or actual defects in glucose-6-phosphate dehydrogenase activity who take quinolone antibacterial agents, including norfloxacin. (See ADVERSE REACTIONS .)
Quinolones, including norfloxacin, may exacerbate the signs of myasthenia gravis and lead to life-threatening weakness of the respiratory muscles. Caution should be exercised when using quinolones, including NOROXIN, in patients with myasthenia gravis (see ADVERSE REACTIONS ).
Prescribing NOROXIN in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Patients should be advised:
- that norfloxacin may cause changes in the electrocardiogram (QTc interval prolongation).
- that norfloxacin should be avoided in patients receiving class IA (e.g., quinidine, procainamide) or class III (e.g., amiodarone, sotalol) antiarrhythmic agents.
- that norfloxacin should be used with caution in subjects receiving drugs that affect the QTc interval such as cisapride, erythromycin, antipsychotics, and tricyclic antidepressants.
- to inform their physicians of any personal or family history of QTc prolongation or proarrhythmic conditions such as hypokalemia, bradycardia or recent myocardial ischemia.
- that peripheral neuropathies have been associated with norfloxacin use. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness develop, they should discontinue treatment and contact their physicians.
- to drink fluids liberally.
- that norfloxacin should be taken at least one hour before or at least two hours after a meal or ingestion of milk and/or other dairy products.
- that multivitamins or other products containing iron or zinc, antacids or Videx® **/** (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution, should not be taken within the two-hour period before or within the two-hour period after taking norfloxacin. (See PRECAUTIONS , Drug Interactions .)
- that norfloxacin can cause dizziness and lightheadedness and, therefore, patients should know how they react to norfloxacin before they operate an automobile or machinery or engage in activities requiring mental alertness and coordination.
- to discontinue treatment and inform their physician if they experience pain, inflammation, or rupture of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been confidently excluded.
- that norfloxacin may be associated with hypersensitivity reactions, even following the first dose, and to discontinue the drug at the first sign of a skin rash or other allergic reaction.
- to avoid undue exposure to excessive sunlight while receiving norfloxacin and to discontinue therapy if phototoxicity occurs.
- that some quinolones may increase the effects of theophylline and/or caffeine. (See PRECAUTIONS , Drug Interactions . )
- that convulsions have been reported in patients taking quinolones, including norfloxacin, and to notify their physician before taking this drug if there is a history of this condition.
**/** Registered trademark of Bristol-Myers Squibb Company
Patients should be counseled that antibacterial drugs including NOROXIN should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When NOROXIN is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by NOROXIN or other antibacterial drugs in the future.
Laboratory Tests
As with any potent antibacterial agent, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, is advisable during prolonged therapy.
Drug Interactions
Elevated plasma levels of theophylline have been reported with concomitant quinolone use. There have been reports of theophylline-related side effects in patients on concomitant therapy with norfloxacin and theophylline. Therefore, monitoring of theophylline plasma levels should be considered and dosage of theophylline adjusted as required.
Elevated serum levels of cyclosporine have been reported with concomitant use of cyclosporine with norfloxacin. Therefore, cyclosporine serum levels should be monitored and appropriate cyclosporine dosage adjustments made when these drugs are used concomitantly.
Quinolones, including norfloxacin, may enhance the effects of oral anticoagulants, including warfarin or its derivatives or similar agents. When these products are administered concomitantly, prothrombin time or other suitable coagulation tests should be closely monitored.
The concomitant administration of quinolones including norfloxacin with glyburide (a sulfonylurea agent) has, on rare occasions, resulted in severe hypoglycemia. Therefore, monitoring of blood glucose is recommended when these agents are co-administered.
Diminished urinary excretion of norfloxacin has been reported during the concomitant administration of probenecid and norfloxacin.
The concomitant use of nitrofurantoin is not recommended since nitrofurantoin may antagonize the antibacterial effect of NOROXIN in the urinary tract.
Multivitamins, or other products containing iron or zinc, antacids or sucralfate should not be administered concomitantly with, or within 2 hours of, the administration of norfloxacin, because they may interfere with absorption resulting in lower serum and urine levels of norfloxacin.
Videx® (Didanosine) chewable/buffered tablets or the pediatric powder for oral solution should not be administered concomitantly with, or within 2 hours of, the administration of norfloxacin, because these products may interfere with absorption resulting in lower serum and urine levels of norfloxacin.
Some quinolones have also been shown to interfere with the metabolism of caffeine. This may lead to reduced clearance of caffeine and a prolongation of its plasma half-life.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No increase in neoplastic changes was observed with norfloxacin as compared to controls in a study in rats, lasting up to 96 weeks at doses 8-9 times *** the usual human dose (on a mg/kg basis).
Norfloxacin was tested for mutagenic activity in a number of in vivo and in vitro tests. Norfloxacin had no mutagenic effect in the dominant lethal test in mice and did not cause chromosomal aberrations in hamsters or rats at doses 30-60 times *** the usual human dose (on a mg/kg basis). Norfloxacin had no mutagenic activity in vitro in the Ames microbial mutagen test, Chinese hamster fibroblasts and V-79 mammalian cell assay. Although norfloxacin was weakly positive in the Rec-assay for DNA repair, all other mutagenic assays were negative including a more sensitive test (V-79).
Norfloxacin did not adversely affect the fertility of male and female mice at oral doses up to 30 times *** the usual human dose (on a mg/kg basis).
Pregnancy
Teratogenic Effects. Pregnancy Category C. Norfloxacin has been shown to produce embryonic loss in monkeys when given in doses 10 times *** the maximum daily total human dose (on a mg/kg basis). At this dose, peak plasma levels obtained in monkeys were approximately 2 times those obtained in humans. There has been no evidence of a teratogenic effect in any of the animal species tested (rat, rabbit, mouse, monkey) at 6-50 times *** the maximum daily human dose (on a mg/kg basis). There are, however, no adequate and well-controlled studies in pregnant women. Norfloxacin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether norfloxacin is excreted in human milk.
When a 200-mg dose of NOROXIN was administered to nursing mothers, norfloxacin was not detected in human milk. However, because the dose studied was low, because other drugs in this class are secreted in human milk, and because of the potential for serious adverse reactions from norfloxacin in nursing infants, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of oral norfloxacin in pediatric patients and adolescents below the age of 18 years have not been established. Norfloxacin causes arthropathy in juvenile animals of several animal species. (See WARNINGS and ANIMAL PHARMACOLOGY .)
Geriatric Use
Of the 340 subjects in one large clinical study of NOROXIN for treatment of urinary tract infections, 103 patients were 65 and older, 77 of whom were 70 and older; no overall differences in safety and effectiveness were evident between these subjects and younger subjects. In clinical practice, no difference in the type of reported adverse experiences have been observed between the elderly and younger patients except for a possible increased risk of tendon rupture in elderly patients receiving concomitant corticosteroids (see WARNINGS). In addition, increased risk for other adverse experiences in some older individuals cannot be ruled out (see ADVERSE REACTIONS ).
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see DOSAGE AND ADMINISTRATION ).
A pharmacokinetic study of NOROXIN in elderly volunteers (65 to 75 years of age with normal renal function for their age) was carried out (see CLINICAL PHARMACOLOGY ).
ADVERSE REACTIONS
Single-Dose Studies
In clinical trials involving 82 healthy subjects and 228 patients with gonorrhea, treated with a single dose of norfloxacin, 6.5% reported drug-related adverse experiences. However, the following incidence figures were calculated without reference to drug relationship.
The most common adverse experiences (>1.0%) were: dizziness (2.6%), nausea (2.6%), headache (2.0%), and abdominal cramping (1.6%).
Additional reactions (0.3%-1.0%) were: anorexia, diarrhea, hyperhidrosis, asthenia, anal/rectal pain, constipation, dyspepsia, flatulence, tingling of the fingers, and vomiting.
Laboratory adverse changes considered drug-related were reported in 4.5% of patients/subjects. These laboratory changes were: increased AST (SGOT) (1.6%), decreased WBC (1.3%), decreased platelet count (1.0%), increased urine protein (1.0%), decreased hematocrit and hemoglobin (0.6%), and increased eosinophils (0.6%).
Multiple-Dose Studies
In clinical trials involving 52 healthy subjects and 1980 patients with urinary tract infections or prostatitis treated with multiple doses of norfloxacin, 3.6% reported drug-related adverse experiences. However, the incidence figures below were calculated without reference to drug relationship.
The most common adverse experiences (>1.0%) were: nausea (4.2%), headache (2.8%), dizziness (1.7%), and asthenia (1.3%).
Additional reactions (0.3%-1.0%) were: abdominal pain, back pain, constipation, diarrhea, dry mouth, dyspepsia/heartburn, fever, flatulence, hyperhidrosis, loose stools, pruritus, rash, somnolence, and vomiting.
Less frequent reactions (0.1%-0.2%) included: abdominal swelling, allergies, anorexia, anxiety, bitter taste, blurred vision, bursitis, chest pain, chills, depression, dysmenorrhea, edema, erythema, foot or hand swelling, insomnia, mouth ulcer, myocardial infarction, palpitation, pruritus ani, renal colic, sleep disturbances, and urticaria.
Abnormal laboratory values observed in these patients/subjects were: eosinophilia (1.5%), elevation of ALT (SGPT) (1.4%), decreased WBC and/or neutrophil count (1.4%), elevation of AST (SGOT) (1.4%), and increased alkaline phosphatase (1.1%). Those occurring less frequently included increased BUN, increased LDH, increased serum creatinine, decreased hematocrit, and glycosuria.
Post Marketing
The most frequently reported adverse reaction in post-marketing experience is rash.
CNS effects characterized as generalized seizures, myoclonus and tremors have been reported with NOROXIN (see WARNINGS ). Visual disturbances have been reported with drugs in this class.
The following additional adverse reactions have been reported since the drug was marketed:
Hypersensitivity Reactions
Hypersensitivity reactions have been reported including anaphylactoid reactions, angioedema, dyspnea, vasculitis, urticaria, arthritis, arthralgia and myalgia (see WARNINGS ).
Skin
Toxic epidermal necrolysis, Stevens-Johnson syn-drome and erythema multiforme, exfoliative dermatitis, photosensitivity.
Gastrointestinal
Pseudomembranous colitis, hepatitis, jaundice including cholestatic jaundice and elevated liver function tests, pancreatitis (rare), stomatitis. The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment (see WARNINGS ).
Cardiovascular
On rare occasions, prolonged QTc interval and ventricular arrhythmia including torsades de pointes.
Renal
Interstitial nephritis, renal failure.
Nervous System/Psychiatric
Peripheral neuropathy, Guillain-Barré syndrome, ataxia, paresthesia; psychic disturbances including psychotic reactions and confusion.
Musculoskeletal
Tendinitis, tendon rupture; exacerbation of myasthenia gravis (see PRECAUTIONS ); elevated creatine kinase (CK).
Hematologic
Neutropenia; leukopenia; agranulocytosis; hemolytic anemia, sometimes associated with glucose-6-phosphate dehydrogenase deficiency; thrombocytopenia.
Special Senses
Hearing loss, tinnitus, diplopia, dysgeusia.
Other adverse events reported with quinolones include: agranulocytosis, albuminuria, candiduria, crystalluria, cylindruria, dysphagia, elevation of blood glucose, elevation of serum cholesterol, elevation of serum potassium, elevation of serum triglycerides, hematuria, hepatic necrosis, symptomatic hypoglycemia, nystagmus, postural hypotension, prolongation of prothrombin time, and vaginal candidiasis.
OVERDOSAGE
No significant lethality was observed in male and female mice and rats at single oral doses up to 4 g/kg.
In the event of acute overdosage, the stomach should be emptied by inducing vomiting or by gastric lavage, and the patient carefully observed and given symptomatic and supportive treatment. Adequate hydration must be maintained.
DOSAGE AND ADMINISTRATION
Tablets NOROXIN should be taken at least one hour before or at least two hours after a meal or ingestion of milk and/or other dairy products. Multivitamins, other products containing iron or zinc, antacids containing magnesium and aluminum, sucralfate, or Videx® (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution, should not be taken within 2 hours of administration of norfloxacin. Tablets NOROXIN should be taken with a glass of water. Patients receiving NOROXIN should be well hydrated (see PRECAUTIONS ).
Normal Renal Function
The recommended daily dose of NOROXIN is as described in the following chart:
Infection DescriptionUnit Dose Frequency Duration Daily Dose Urinary Tract Uncomplicated UTI's
(crystitis) due to E. coli,
K. pneumoniae, or P. mirabilis400 mg q12h 3 days 800 mg Uncomplicated UTI's due to
other indicated organisms400 mg q12h 7-10 days 800 mg Complicated UTI's400 mg q12h 10-21 days 800 mg Sexually
Transmitted
DiseasesUncomplicated Gonorrhea800 mg single dose 1 day 800 mg ProstatitisAcute or Chronic400 mg q12h 28 days 800 mg Renal Impairment
NOROXIN may be used for the treatment of urinary tract infections in patients with renal insufficiency. In patients with a creatinine clearance rate of 30 mL/min/1.73m 2 or less, the recommended dosage is one 400-mg tablet once daily for the duration given above. At this dosage, the urinary concentration exceeds the MICs for most urinary pathogens susceptible to norfloxacin, even when the creatinine clearance is less than 10 mL/min/1.73m 2 .
When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to convert this value into creatinine clearance. The serum creatinine should represent a steady state of renal function.
Males:(weight in kg) × (140 - age)
(72) × serum creatinine (mg/100 mL)
Females:(0.85) × (above value) Elderly
Elderly patients being treated for urinary tract infections who have a creatinine clearance of greater than 30 mL/min/1.73m 2 should receive the dosages recommended under Normal Renal Function.
Elderly patients being treated for urinary tract infections who have a creatinine clearance of 30 mL/min/1.73m 2 or less should receive 400 mg once daily as recommended under Renal Impairment.
HOW SUPPLIED
No. 3522--Tablets NOROXIN 400 mg are dark pink, oval shaped, film-coated tablets, coded MSD 705 on one side and NOROXIN on the other. They are supplied as follows:
NDC 0006-0705-68 bottles of 100
NDC 0006-0705-20 unit of use bottles of 20.
Storage
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Keep container tightly closed.
ANIMAL PHARMACOLOGY
Norfloxacin and related drugs have been shown to cause arthropathy in immature animals of most species tested (see WARNINGS ).
Crystalluria has occurred in laboratory animals tested with norfloxacin. In dogs, needle-shaped drug crystals were seen in the urine at doses of 50 mg/kg/day. In rats, crystals were reported following doses of 200 mg/kg/day.
Embryo lethality and slight maternotoxicity (vomiting and anorexia) were observed in cynomolgus monkeys at doses of 150 mg/kg/day or higher.
Ocular toxicity, seen with some related drugs, was not observed in any norfloxacin-treated animals.
REFERENCES
- National Committee for Clinical Laboratory Standards, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically-3rd ed., Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25, NCCLS, Villanova, PA, 1993.
- National Committee for Clinical Laboratory Standards, Performance standards for antimicrobial disk susceptibility tests-5th ed., Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24, NCCLS, Villanova, PA, 1993.
Merck & CO., INC., Whitehouse Station, NJ 08889, USA
7898535
Issued July 2004
Printed in USA
Subscribe to the "News" RSS Feed 
TOP ۞
