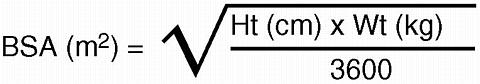
-
Norvir Oral Solution, Norvir Soft Gelatin Capsules (Abbott)
Close- Warnings
- Description
- Chemical Structure
- Clinical Pharmacology
- Indications and Usage
- Contraindications
- Warnings (2)
- Precautions
- Drug Interactions
- Adverse Reactions
- Overdosage
- Dosage and Administration
- Chemical Structure
- How Supplied
- Chemical Structure
- References
- How Supplied (2)
- Patient Package Insert
WARNING
CO-ADMINISTRATION OF NORVIR WITH CERTAIN NONSEDATING ANTIHISTAMINES, SEDATIVE HYPNOTICS, ANTIARRHYTHMICS, OR ERGOT ALKALOID PREPARATIONS MAY RESULT IN POTENTIALLY SERIOUS AND/OR LIFE-THREATENING ADVERSE EVENTS DUE TO POSSIBLE EFFECTS OF NORVIR ON THE HEPATIC METABOLISM OF CERTAIN DRUGS. SEE CONTRAINDICATIONS AND PRECAUTIONS SECTIONS.
DESCRIPTION
NORVIR (ritonavir) is an inhibitor of HIV protease with activity against the Human Immunodeficiency Virus (HIV).
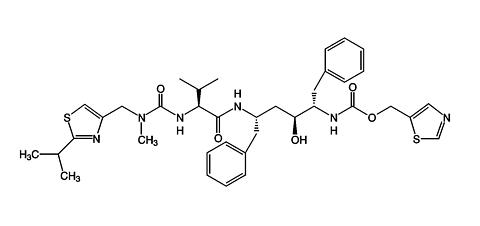
Ritonavir is chemically designated as 10-Hydroxy-2-methyl-5-(1-methylethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-2,4,7,12-tetraazatridecan-13-oic acid, 5-thiazolylmethyl ester, [5S-(5R*,8R*,10R*,11R*)]. Its molecular formula is C 37 H 48 N 6 O 5 S 2 , and its molecular weight is 720.95. Ritonavir has the following structural formula:
Ritonavir is a white-to-light-tan powder. Ritonavir has a bitter metallic taste. It is freely soluble in methanol and ethanol, soluble in isopropanol and practically insoluble in water.
NORVIR soft gelatin capsules are available for oral administration in a strength of 100 mg ritonavir with the following inactive ingredients: Butylated hydroxytoluene, ethanol, gelatin, iron oxide, oleic acid, polyoxyl 35 castor oil, and titanium dioxide.
NORVIR oral solution is available for oral administration as 80 mg/mL of ritonavir in a peppermint and caramel flavored vehicle. Each 8-ounce bottle contains 19.2 grams of ritonavir. NORVIR oral solution also contains ethanol, water, polyoxyl 35 castor oil, propylene glycol, anhydrous citric acid to adjust pH, saccharin sodium, peppermint oil, creamy caramel flavoring, and FD&C Yellow No. 6.
CLINICAL PHARMACOLOGY
Microbiology
Mechanism of action : Ritonavir is a peptidomimetic inhibitor of both the HIV-1 and HIV-2 proteases. Inhibition of HIV protease renders the enzyme incapable of processing the gag-pol polyprotein precursor which leads to production of non-infectious immature HIV particles.
Antiviral activity in vitro : The activity of ritonavir was assessed in vitro in acutely infected lymphoblastoid cell lines and in peripheral blood lymphocytes. The concentration of drug that inhibits 50% (EC 50 ) of viral replication ranged from 3.8 to 153 nM depending upon the HIV-1 isolate and the cells employed. The average EC 50 for low passage clinical isolates was 22 nM (n=13). In MT 4 cells, ritonavir demonstrated additive effects against HIV-1 in combination with either zidovudine (ZDV) or didanosine (ddI). Studies which measured cytotoxicity of ritonavir on several cell lines showed that >20 µM was required to inhibit cellular growth by 50% resulting in an in vitro therapeutic index of at least 1000.
Resistance : HIV-1 isolates with reduced susceptibility to ritonavir have been selected in vitro . Genotypic analysis of these isolates showed mutations in the HIV protease gene at amino acid positions 84 (Ile to Val), 82 (Val to Phe), 71 (Ala to Val), and 46 (Met to Ile). Phenotypic (n=18) and genotypic (n=44) changes in HIV isolates from selected patients treated with ritonavir were monitored in phase I/II trials over a period of 3 to 32 weeks. Mutations associated with the HIV viral protease in isolates obtained from 41 patients appeared to occur in a stepwise and ordered fashion; in sequence, these mutations were position 82 (Val to Ala/Phe), 54 (Ile to Val), 71 (Ala to Val/Thr), and 36 (Ile to Leu), followed by combinations of mutations at an additional 5 specific amino acid positions. Of 18 patients for which both phenotypic and genotypic analysis were performed on free virus isolated from plasma, 12 showed reduced susceptibility to ritonavir in vitro . All 18 patients possessed one or more mutations in the viral protease gene. The 82 mutation appeared to be necessary but not sufficient to confer phenotypic resistance. Phenotypic resistance was defined as a >/=5-fold decrease in viral sensitivity in vitro from baseline. The clinical relevance of phenotypic and genotypic changes associated with ritonavir therapy has not been established.
Cross-resistance to other antiretrovirals : Among protease inhibitors variable cross-resistance has been recognized. Serial HIV isolates obtained from six patients during ritonavir therapy showed a decrease in ritonavir susceptibility in vitro but did not demonstrate a concordant decrease in susceptibility to saquinavir in vitro when compared to matched baseline isolates. However, isolates from two of these patients demonstrated decreased susceptibility to indinavir in vitro (8-fold). Isolates from 5 patients were also tested for cross-resistance to amprenavir and nelfinavir; isolates from 2 patients had a decrease in susceptibility to nelfinavir (12- to 14-fold), and none to amprenavir. Cross-resistance between ritonavir and reverse transcriptase inhibitors is unlikely because of the different enzyme targets involved. One ZDV-resistant HIV isolate tested in vitro retained full susceptibility to ritonavir.
Pharmacokinetics
The pharmacokinetics of ritonavir have been studied in healthy volunteers and HIV-infected patients (CD 4 >/= 50 cells/µL). See Table 1 for ritonavir pharmacokinetic characteristics.
Absorption: The absolute bioavailability of ritonavir has not been determined. After a 600 mg dose of oral solution, peak concentrations of ritonavir were achieved approximately 2 hours and 4 hours after dosing under fasting and non-fasting (514 KCal; 9% fat, 12% protein, and 79% carbohydrate) conditions, respectively.
Effect of Food on Oral Absorption: When the oral solution was given under non-fasting conditions, peak ritonavir concentrations decreased 23% and the extent of absorption decreased 7% relative to fasting conditions. Dilution of the oral solution, within one hour of administration, with 240 mL of chocolate milk, Advera® or Ensure® did not significantly affect the extent and rate of ritonavir absorption. After a single 600 mg dose under non-fasting conditions, in two separate studies, the soft gelatin capsule (n=57) and oral solution (n=18) formulations yielded mean ± SD areas under the plasma concentration-time curve (AUCs) of 121.7 ± 53.8 and 129.0 ± 39.3 µg·h/mL, respectively. Relative to fasting conditions, the extent of absorption of ritonavir from the soft gelatin capsule formulation was 13% higher when administered with a meal (615 KCal; 14.5% fat, 9% protein, and 76% carbohydrate).
Metabolism: Nearly all of the plasma radioactivity after a single oral 600 mg dose of 14 C-ritonavir oral solution (n=5) was attributed to unchanged ritonavir. Five ritonavir metabolites have been identified in human urine and feces. The isopropylthiazole oxidation metabolite (M-2) is the major metabolite and has antiviral activity similar to that of parent drug; however, the concentrations of this metabolite in plasma are low. In vitro studies utilizing human liver microsomes have demonstrated that cytochrome P450 3A (CYP3A) is the major isoform involved in ritonavir metabolism, although CYP2D6 also contributes to the formation of M-2.
Elimination: In a study of five subjects receiving a 600 mg dose of 14 C-ritonavir oral solution, 11.3 ± 2.8% of the dose was excreted into the urine, with 3.5 ± 1.8% of the dose excreted as unchanged parent drug. In that study, 86.4 ± 2.9% of the dose was excreted in the feces with 33.8 ± 10.8% of the dose excreted as unchanged parent drug. Upon multiple dosing, ritonavir accumulation is less than predicted from a single dose possibly due to a time and dose-related increase in clearance.
Table 1
Ritonavir Pharmacokinetic CharacteristicsParametern Values (Mean ± SD) C max SS **/*10 11.2 ± 3.6 µg/mL C trough SS **/*10 3.7 ± 2.6 µg/mL V (beta) /F **/**91 0.41 ± 0.25 L/kg t 1/23-5 h CL/F SS **/*10 8.8 ± 3.2 L/h CL/F **/**91 4.6 ± 1.6 L/h CL R62 <0.1 L/h RBC/Plasma Ratio0.14 Percent Bound *98 to 99% **/* SS = steady state; patients taking ritonavir 600 mg q12h.**/** Single ritonavir 600 mg dose.*Primarily bound to human serum albumin and alpha-1 acid glycoprotein over the ritonavir concentration range of 0.01 to 30 µg/mL.Special Populations:
Gender, Race and Age : No age-related pharmacokinetic differences have been observed in adult patients (18 to 63 years). Ritonavir pharmacokinetics have not been studied in older patients. A study of ritonavir pharmacokinetics in healthy males and females showed no statistically significant differences in the pharmacokinetics of ritonavir. Pharmacokinetic differences due to race have not been identified.
Pediatric Patients : The pharmacokinetic profile of ritonavir in pediatric patients below the age of 2 years has not been established. Steady-state pharmacokinetics were evaluated in 37 HIV-infected patients ages 2 to 14 years receiving doses ranging from 250 mg/m 2 b.i.d. to 400 mg/m 2 b.i.d. Across dose groups, ritonavir steady-state oral clearance (CL/F/m 2 ) was approximately 1.5 times faster in pediatric patients than in adult subjects. Ritonavir concentrations obtained after 350 to 400 mg/m 2 twice daily in pediatric patients were comparable to those obtained in adults receiving 600 mg (approximately 330 mg/m 2 ) twice daily.
Renal Insufficiency : Ritonavir pharmacokinetics have not been studied in patients with renal insufficiency, however, since renal clearance is negligible, a decrease in total body clearance is not expected in patients with renalinsufficiency.
Hepatic Insufficiency : Dose-normalized steady-state ritonavir concentrations in subjects with mild hepatic insufficiency (400 mg BID, n=6) were similar to those in control subjects dosed with 500 mg BID. Dose-normalized steady-state ritonavir exposures in subjects with moderate hepatic impairment (400 mg BID, n=6) were about 40% lower than those in subjects with normal hepatic function (500 mg BID, n=6). Protein binding of ritonavir was not statistically significantly affected by mild or moderately impaired hepatic function. No dose adjustment is recommended in patients with mild or moderate hepatic impairment. However, health care providers should be aware of the potential for lower ritonavir concentrations in patients with moderate hepatic impairment and should monitor patient response carefully. Ritonavir has not been studied in patients with severe hepatic impairment.
Drug-Drug Interactions : See also CONTRAINDICATIONS , WARNINGS , and PRECAUTIONS : Drug Interactions .
Tables 2 and 3 summarize the effects on AUC and C max , with 95% confidence intervals (95% CI), of co-administration of ritonavir with a variety of drugs. For information about clinical recommendations see PRECAUTIONS - Drug Interactions .
Table 2: Drug Interactions:
Pharmacokinetic Parameters for Ritonavir in the Presence of the Co-administered Drug
(See Precautions, Table 6 for Recommended Alterations in the Dose or Regimen)Co-
administered
DrugDose of
Co-administered
Drug (mg)Dose of
NORVIR
(mg)n AUC %
(95% CI)C max
(95% CI)Clarithromycin500 q12h, 4 d 200 q8h, 4 d 22 up 12% (2, 23%) up 15% (2, 28%) Didanosine200 q12h, 4 d 600 q12h, 4 d 12 <-> <-> Fluconazole400 single dose, day
1; 200 daily, 4 d200 q6h, 4 d 8 up 12% (5, 20%) up 15% (7, 22%) Fluoxetine30 q12h, 8 d 600 single dose, 1 d 16 up 19% (7, 34%) <-> Ketoconazole200 daily, 7 d 500 q12h, 10 d 12 up 18% (-3, 52%) up 10% (-11, 36%) Rifampin600 or 300 daily,
10 d500 q12h, 20 d 7, 9 * down 35% (7, 55%) down 25% (-5, 46%) Zidovudine200 q8h, 4 d 300 q6h, 4 d 10 <-> <-> Table 3: Drug Interactions:
Pharmacokinetic Parameters for Co-administered Drug in the Presence of NORVIR
(See Precautions, Table 6 for Recommended Alterations in Dose or Regimen)Co-
administered
DrugDose of
Co-administered
Drug (mg)Dose of
NORVIR
(mg)n AUC % (95% CI) C max (95% CI) Alprazolam1, single dose 500 q12h, 10 d 12 down 12% (-5, 30%) down 16% (5, 27%) Clarithromycin500 q12h, 4 d 200 q8h, 4 d 22 up 77% (56, 103%) up 31% (15, 51%) 14-OH clarithromycin
metabolitedown 100% down 99% Desipramine100, single dose 500 q12h, 12 d 14 up 145% (103, 211%) up 22% (12, 35%) 2-OH
desipramine
metabolitedown 15% (3, 26%) down 67% (62, 72%) Didanosine200 q12h, 4 d 600 q12h, 4 d 12 down 13% (0, 23%) down 16% (5, 26%) Ethinyl
estradiol50 µg single dose 500 q12h, 16 d 23 down 40% (31, 49%) down 32% (24, 39%) Indinavir 1400 q12h, 15 d 400 q12h, 15 d 10 Day 14up 6% (-14, 29%) down 51% (40, 61%) Day 15down 7% (-25, 16%) down 62% (52, 70%) Ketoconazole200 daily, 7 d 500 q12h, 10 d 12 up 3.4-fold (2.8, 4.3X) up 55% (40, 72%) Meperidine50 oral single dose 500 q12h, 10 d 8 down 62% (59, 65%) down 59%
(42, 72%)Normeperidine
metabolite6 up 47% (-24, 345%) up 87% (42, 147%) Methadone 25, single dose 500 q12h, 15 d 11 down 36% (16, 52%) down 38%
(28, 46%)Rifabutin150 daily, 16 d 500 q12h, 10 d 5,
11 *up 4-fold (2.8, 6.1X) up 2.5-fold
(1.9, 3.4X)25-O-desacetyl
rifabutin
metaboliteup 35-fold
(25, 78X)up 16-fold
(14, 20X)Saquinavir 3400 BID steady-state 400 BID steady-state 7 up 17-fold (9, 31X) up 14-fold (7, 28X) Sildenafil100, single dose 500 BID, 8 d 28 up 11-fold up 4-fold Sulfa-
methoxazole 4800, single dose 500 q12h, 12 d 15 down 20% (16, 23%) <-> Theophylline3 mg/kg q8h, 15 d 500 q12h, 10 d 13,
11 *down 43% (42, 45%) down 32% (29, 34%) Trimethoprim 4160, single dose 500 q12h, 12 d 15 up 20% (3, 43%) <-> Warfarin5, single dose 400 q12h, 12d 12 S-Warfarinup 9% (-17, 44%) 5 down 9% (-16, -2%) 5 R-Warfarindown 33%
(-38, -27%) 5<-> Zidovudine200 q8h, 4 d 300 q6h, 4 d 9 down 25% (15, 34%) down 27% (4, 45%) 1 Ritonavir and indinavir were co-administered for 15 days; Day 14 doses were administered after a 15%-fat breakfast (757 Kcal) and 9%-fat evening snack (236 Kcal), and Day 15 doses were administered after a 15%-fat breakfast (757 Kcal) and 32%-fat dinner (815 Kcal). Indinavir C min was also increased 4-fold. Effects were assessed relative to an indinavir 800 mg q8h regimen under fasting conditions.2 Effects were assessed on a dose-normalized comparison to a methadone 20 mg single dose.3 Comparison to a standard saquinavir HGC 600 mg t.i.d. regimen (n=114). Saquinavir C min was 0.48 ± 0.36 µg/mL for 400/400 mg BID compared to below quantifiable limits for Saquinavir HGC 600 mg TID.4 Sulfamethoxazole and trimethoprim taken as single combination tablet.5 90% CI presented for R- and S-warfarin AUC and C max ratios.up Indicates increase.down Indicates decrease.<-> Indicates no change.* Parallel group design; entries are subjects receiving combination and control regimens, respectively.INDICATIONS AND USAGE
NORVIR is indicated in combination with other antiretroviral agents for the treatment of HIV-infection. This indication is based on the results from a study in patients with advanced HIV disease that showed a reduction in both mortality and AIDS-defining clinical events for patients who received NORVIR either alone or in combination with nucleoside analogues. Median duration of follow-up in this study was 13.5 months.
Description of Clinical Studies
The activity of NORVIR as monotherapy or in combination with nucleoside analogues has been evaluated in 1446 patients enrolled in two double-blind, randomized trials.
Advanced Patients with Prior Antiretroviral Therapy
Study 247 was a randomized, double-blind trial (with open-label follow-up) conducted in HIV-infected patients with at least nine months of prior antiretroviral therapy and baseline CD 4 cell counts </= 100 cells/µL. NORVIR 600 mg b.i.d. or placebo was added to each patient's baseline antiretroviral therapy regimen, which could have consisted of up to two approved antiretroviral agents. The study accrued 1090 patients, with mean baseline CD 4 cell count at study entry of 32 cells/µL. After the clinical benefit of NORVIR therapy was demonstrated, all patients were eligible to switch to open-label NORVIR for the duration of the follow-up period. Median duration of double-blind therapy with NORVIR and placebo was 6 months. The median duration of follow-up through the end of the open-label phase was 13.5 months for patients randomized to NORVIR and 14 months for patients randomized to placebo.
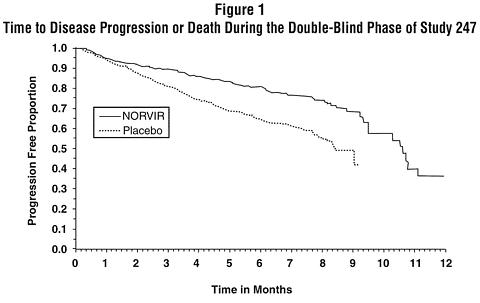
The cumulative incidence of clinical disease progression or death during the double-blind phase of Study 247 was 26% for patients initially randomized to NORVIR compared to 42% for patients initially randomized to placebo. This difference in rates was statistically significant (see Figure 1).
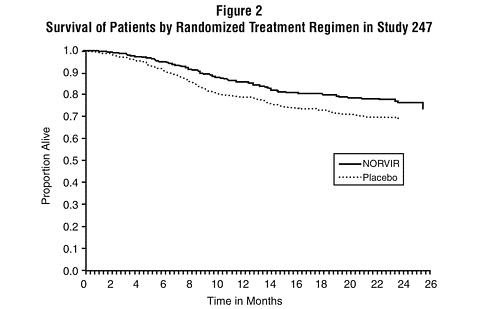
The cumulative mortality through the end of the open-label follow-up phase for patients enrolled in Study 247 was 18% for patients initially randomized to NORVIR compared to 26% for patients initially randomized to placebo. This difference in rates was statistically significant (see Figure 2).
Since the analysis at the end of the open-label phase includes patients in the placebo arm who were switched from placebo to NORVIR therapy, the survival benefit of NORVIR cannot be precisely estimated.
Figures 3 and 4 summarize the mean change from baseline for CD 4 cell count and plasma HIV RNA (copies/mL), respectively, during the first 24 weeks for the double-blind phase of Study 247.
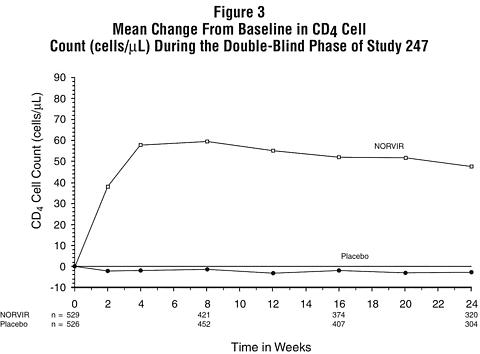
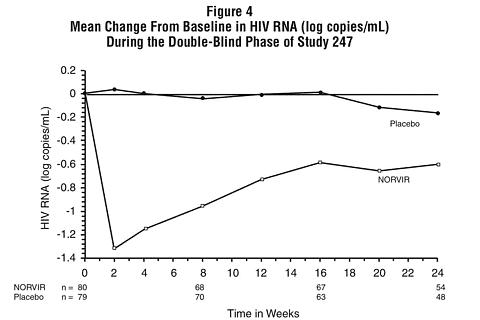
Patients Without Prior Antiretroviral Therapy
In Study 245, 356 antiretroviral-naive HIV-infected patients (mean baseline CD 4 = 364 cells/µL) were randomized to receive either NORVIR 600 mg b.i.d., zidovudine 200 mg t.i.d., or a combination of these drugs. Figures 5 and 6 summarize the mean change from baseline for CD 4 cell count and plasma HIV RNA (copies/mL), respectively, during the first 24 weeks for the double-blind phase of Study 245.
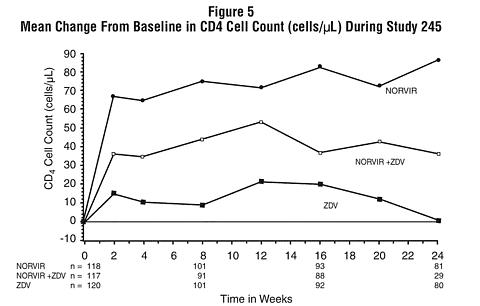
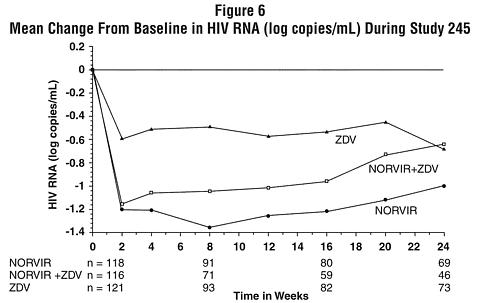
CONTRAINDICATIONS
NORVIR is contraindicated in patients with known hypersensitivity to ritonavir or any of its ingredients.
Co-administration of NORVIR is contraindicated with the drugs listed in Table 4 (also see PRECAUTIONS Table 5: Drugs That Should Not Be Co-administered with NORVIR ) because competition for primarily CYP3A by ritonavir could result in inhibition of the metabolism of these drugs and create the potential for serious and/or life-threatening reactions such as cardiac arrhythmias, prolonged or increased sedation, and respiratory depression.
Table 4
DRUGS THAT ARE CONTRAINDICATED WITH NORVIRDrug ClassDrugs Within Class That Are
CONTRAINDICATED With NORVIRAntiarrhythmicsamiodarone, bepridil, flecainide, propafenone, quinidineAntihistaminesastemizole, terfenadineErgot Derivativesdihydroergotamine, ergonovine, ergotamine, methylergonovineGI Motility AgentcisaprideNeurolepticpimozideSedative/hypnoticsmidazolam, triazolamWARNINGS
ALERT: Find out about medicines that should NOT be taken with NORVIR. This statement is included on the product's bottle label.
Drug Interactions
Ritonavir is an inhibitor of cytochrome P450 3A (CYP3A) both in vitro and in vivo . Ritonavir also inhibits CYP2D6 in vitro, but to a lesser extent than CYP3A. Co-administration of ritonavir and drugs primarily metabolized by CYP3A or CYP2D6 may result in increased plasma concentrations of other drugs that could increase or prolong its therapeutic and adverse effects (see Pharmacokinetics: Drug-Drug Interactions , CONTRAINDICATIONS - Table 4 Drugs That Are Contraindicated with NORVIR, PRECAUTIONS - Table 5 Drugs That Should Not Be Co-administered with NORVIR, Table 6 Established Drug Interactions and Tables 7 and 8 Predicted Drug Interactions: Use with Caution ).
The magnitude of the interactions and therapeutic consequences between ritonavir and the drugs listed in Tables 7 and 8 Predicted Drug Interactions: Use With Caution cannot be predicted with any certainty. When co-administering ritonavir with any agent listed in Tables 7 and 8 Predicted Drug Interactions: Use With Caution , special attention is warranted. Refer to PRECAUTIONS : Established Drug Interactions and Predicted Drug Interactions for additional information.
Cardiac and neurologic events have been reported with ritonavir when co-administered with disopyramide, mexiletine, nefazodone, fluoxetine and beta blockers. The possibility of drug interaction cannot be excluded.
Particular caution should be used when prescribing sildenafil in patients receiving NORVIR. Co-administration of NORVIR with sildenafil is expected to substantially increase sildenafil concentrations (11-fold increase in AUC) and may result in an increase in sildenafil-associated adverse events, including hypotension, syncope, visual changes, and prolonged erection (see PRECAUTIONS : Drug Interactions , Table 6 Established Drug Interactions: Alteration in Dose or Regimen Recommended Based on Drug Interaction Studies and the complete prescribing information for sildenafil).
Concomitant use of NORVIR with lovastatin or simvastatin is not recommended. Caution should be exercised if HIV protease inhibitors, including NORVIR, are used concurrently with other HMG-CoA reductase inhibitors that are also metabolized by the CYP3A4 pathway (e.g., atorvastatin or cerivastatin). The risk of myopathy including rhabdomyolysis may be increased when HIV protease inhibitors, including NORVIR, are used in combination with these drugs.
Concomitant use of NORVIR, and St. John's wort (hypericum perforatum) or products containing St. John's wort is not recommended. Co-administration of protease inhibitors, including NORVIR, with St. John's wort is expected to substantially decrease protease inhibitor concentrations and may result in sub-optimal levels of NORVIR and lead to loss of virologic response and possible resistance to NORVIR or to the class of protease inhibitors.
Allergic Reactions
Allergic reactions including urticaria, mild skin eruptions, bronchospasm, and angioedema have been reported. Rare cases of anaphylaxis and Stevens-Johnson syndrome have also been reported.
Hepatic Reactions
Hepatic transaminase elevations exceeding 5 times the upper limit of normal, clinical hepatitis, and jaundice have occurred in patients receiving NORVIR alone or in combination with other antiretroviral drugs (see Table 10). There may be an increased risk for transaminase elevations in patients with underlying hepatitis B or C. Therefore, caution should be exercised when administering NORVIR to patients with pre-existing liver diseases, liver enzyme abnormalities, or hepatitis. Increased AST/ALT monitoring should be considered in these patients, especially during the first three months of NORVIR treatment.
There have been postmarketing reports of hepatic dysfunction, including some fatalities. These have generally occurred in patients taking multiple concomitant medications and/or with advanced AIDS.
Pancreatitis
Pancreatitis has been observed in patients receiving NORVIR therapy, including those who developed hypertriglyceridemia. In some cases fatalities have been observed. Patients with advanced HIV disease may be at increased risk of elevated triglycerides and pancreatitis.
Pancreatitis should be considered if clinical symptoms (nausea, vomiting, abdominal pain) or abnormalities in laboratory values (such as increased serum lipase or amylase values) suggestive of pancreatitis should occur. Patients who exhibit these signs or symptoms should be evaluated and NORVIR therapy should be discontinued if a diagnosis of pancreatitis is made.
Diabetes Mellitus/Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV-infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
PRECAUTIONS
General
Ritonavir is principally metabolized by the liver. Therefore, caution should be exercised when administering this drug to patients with impaired hepatic function (see WARNINGS and CLINICAL PHARMACOLOGY : Hepatic Insufficiency ).
Resistance/Cross-resistance
Varying degrees of cross-resistance among protease inhibitors have been observed. Continued administration of ritonavir therapy following loss of viral suppression may increase the likelihood of cross-resistance to other protease inhibitors (see MICROBIOLOGY ).
Hemophilia
There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis, in patients with hemophilia type A and B treated with protease inhibitors. In some patients additional factor VIII was given. In more than half of the reported cases, treatment with protease inhibitors was continued or reintroduced. A causal relationship has not been established.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Lipid Disorders
Treatment with NORVIR therapy alone or in combination with saquinavir has resulted in substantial increases in the concentration of total triglycerides and cholesterol. Triglyceride and cholesterol testing should be performed prior to initiating NORVIR therapy and at periodic intervals during therapy. Lipid disorders should be managed as clinically appropriate. See PRECAUTIONS Tables 5 and 7 for additional information on potential drug interactions with NORVIR and HMG CoA reductase inhibitors.
Information For Patients
A statement to patients and health care providers is included on the product's bottle label: ALERT: Find out about medicines that should NOT be taken with NORVIR. A Patient Package Insert (PPI) for Norvir is available for patient information.
Patients should be informed that NORVIR is not a cure for HIV infection and that they may continue to acquire illnesses associated with advanced HIV infection, including opportunistic infections.
Patients should be told that the long-term effects of NORVIR are unknown at this time. They should be informed that NORVIR therapy has not been shown to reduce the risk of transmitting HIV to others through sexual contact or blood contamination.
Patients should be advised to take NORVIR with food, if possible.
Patients should be informed to take NORVIR every day as prescribed. Patients should not alter the dose or discontinue NORVIR without consulting their doctor. If a dose is missed, patients should take the next dose as soon as possible. However, if a dose is skipped, the patient should not double the next dose.
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long term health effects of these conditions are not known at this time.
NORVIR may interact with some drugs; therefore, patients should be advised to report to their doctor the use of any other prescription, non-prescription medication or herbal products, particularly St. John's wort.
Laboratory Tests
Ritonavir has been shown to increase triglycerides, cholesterol, SGOT (AST), SGPT (ALT), GGT, CPK, and uric acid. Appropriate laboratory testing should be performed prior to initiating NORVIR therapy and at periodic intervals or if any clinical signs or symptoms occur during therapy. For comprehensive information concerning laboratory test alterations associated with nucleoside analogues, physicians should refer to the complete product information for each of these drugs.
Drug Interactions
Ritonavir has been found to be an inhibitor of cytochrome P450 3A (CYP3A) both in vitro and in vivo (Table 3). Agents that are extensively metabolized by CYP3A and have high first pass metabolism appear to be the most susceptible to large increases in AUC (>3-fold) when co-administered with ritonavir. Ritonavir also inhibits CYP2D6 to a lesser extent. Co-administration of substrates of CYP2D6 with ritonavir could result in increases (up to 2-fold) in the AUC of the other agent, possibly requiring a proportional dosage reduction. Ritonavir also appears to induce CYP3A as well as other enzymes, including glucuronosyl transferase, CYP1A2, and possibly CYP2C9.
Drugs that are contraindicated specifically due to the expected magnitude of interaction and potential for serious adverse events are listed both in CONTRAINDICATIONS Table 4 and under Drugs That Should Not Be Co-administered with NORVIR in Table 5.
Those drug interactions that have been established based on drug interaction studies are listed with the pharmacokinetic results in CLINICAL PHARMACOLOGY , Tables 2 and 3. The clinical recommendations based on the results of these studies are listed in Table 6 Established Drug Interactions: Alteration in Dose or Regimen Recommended Based on Drug Interaction Studies .
A systematic review of over 200 medications prescribed to HIV-infected patients was performed to identify potential drug interactions with ritonavir. 2 There are a number of agents in which CYP3A or CYP2D6 partially contribute to the metabolism of the agent. In these cases, the magnitude of the interaction and therapeutic consequences cannot be predicted with any certainty.
When co-administering ritonavir with calcium channel blockers, immunosuppressants, some HMG-CoA reductase inhibitors (see WARNINGS , Drug Interactions ), some steroids, or other substrates of CYP3A, or most antidepressants, certain antiarrhythmics, and some narcotic analgesics which are partially mediated by CYP2D6 metabolism, it is possible that substantial increases in concentrations of these other agents may occur, possibly requiring a dosage reduction (>50%); examples are listed in Table 7 Predicted Drug Interactions: Use With Caution, Dose Decrease May Be Needed .
When co-administering ritonavir with any agent having a narrow therapeutic margin, such as anticoagulants, anticonvulsants, and antiarrhythmics, special attention is warranted. With some agents, the metabolism may be induced, resulting in decreased concentrations (see Table 8 Predicted Drug Interactions: Use With Caution, Dose Increase May Be Needed ).
Table 5
Drugs That Should Not Be Co-administered with NORVIRDrug Class: Drug NameClinical CommentAntiarrhythmics:
amiodarone, bepridil, flecainide, propafenone, quinidineCONTRAINDICATED due to potential for serious and/or life threatening reactions such as cardiac arrhythmias.Antihistamines:
astemizole, terfenadineCONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias.Ergot Derivatives:
dihydroergotamine, ergonovine, ergotamine, methylergonivineCONTRAINDICATED due to potential for serious and/or life-threatening reactions such as acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system.GI Motility Agent:
cisaprideCONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias.Herbal Products:
St. John's wort (hypericum perforatum)May lead to loss of virologic response and possible resistance to NORVIR or to the class of protease inhibitors.HMG-CoA Reductase Inhibitors:
lovastatin, simvastatinPotential for serious reactions such as risk of myopathy including rhabdomyolysis.Neuroleptic:
pimozideCONTRAINDICATED due to the potential for serious and/or life-threatening reactions such as cardiac arrhythmias.Sedative/hypnotics:
midazolam, triazolamCONTRAINDICATED due to potential for serious and/or life-threatening reactions such as prolonged or increased sedation or respiratory depression.Table 6: Established Drug Interactions: Alteration in Dose or
Regimen Recommended Based on Drug Interaction Studies
(see CLINICAL PHARMACOLOGY , Tables 2 and 3
for Magnitude or Interaction)Concomitant
Drug Class:
Drug NameEffect on Concentration of Ritonavir or Concomitant DrugClinical CommentHIV-Antiviral AgentsHIV Protease
Inhibitor:
indinavirWhen co-administered with reduced doses of indinavir and ritonavirAlterations in concentrations are noted when reduced doses of indinavir are co-administered with NORVIR.up indinavir (<-> AUC, down C max, up C min )Appropriate doses for this combination, with respect to efficacy and safety, have not been establishedHIV Protease
Inhibitor:
saquinavirWhen co-administered with reduced doses of saquinavir and ritonavirWhen used in combination therapy for up to 24 weeks, doses of 400 mg b.i.d. of ritonavir and saquinavir were better tolerated than the higher doses of the combination. Saquinavir plasma concentrations achieved with Invirase® (saquinavir mesylate) (400 mg b.i.d.) and ritonavir (400 mg b.i.d.) are similar to those achieved with Fortovase™ (saquinavir) (400 mg b.i.d.) and ritonavir (400 mg b.i.d.)up saquinavir (up AUC, up C max, up C min )Nucleoside
Reverse Transcriptase
Inhibitor: didanosineDosing of didanosine and ritonavir should be separated by 2.5 hours to avoid formulation incompatibilityOther AgentsAnesthetic:
meperidinedown meperidine/
up normeperidine (metabolite)Dosage increase and long-term use of meperidine with ritonavir are not recommended due to the increased concentrations of the metabolite normeperidine which has both analgesic activity and CNS stimulant activity (e.g., seizures)Antialcoholics:
disulfiram/metronidazoleRitonavir formulations contain alcohol, which can produce disulfiram-like reactions when co-administered with disulfiram or other drugs that produce this reaction (e.g., metronidazole)Anticoagulant:
warfarin
down R-warfarin
downup S-warfarinInitial frequent monitoring of the INR during ritonavir and warfarin co-administration is indicated.Antidepressant:
desipramineup desipramineDosage reduction and concentration monitoring of desipramine is recommendedAntifungal:
ketoconazoleup ketoconazoleHigh doses of ketoconazole (>200 mg/day) are not recommendedAnti-infective:
clarithromycinup clarithromycinFor patients with renal impairment the following dosage adjustments should be considered:
·For patients with CL CR 30 to 60 mL/min the dose of clarithromycin should be reduced by 50%.
·For patients with CL CR <30 mL/min the dose of clarithromycin should be decreased by 75%.
No dose adjustment for patients with normal renal function is necessary.Antimycobacterial:
rifabutinup rifabutin and rifabutin metaboliteDosage reduction of rifabutin by at least three-quarters of the usual dose of 300 mg/day is recommended (e.g., 150 mg every other day or three times a week). Further dosage reduction may be necessaryAntimycobacterial:
rifampindown ritonavirMay lead to loss of virologic response. Alternate antimycobacterial agents such as rifabutin should be considered (see Antimycobacterial: rifabutin, for dose reduction recommendations)Bronchodilator:
theophyllinedown theophyllineIncreased dosage of theophylline may be required; therapeutic monitoring should be consideredErectile
Dysfunction: sildenafilup sildenafilSildenafil should not exceed a maximum single dose of 25 mg in a 48-hour period in patients receiving concomitant ritonavir therapy (see WARNINGS )Narcotic
Analgesic:
methadonedown methadoneDosage increase of methadone may be consideredOral Contraceptive:
ethinyl estradioldown ethinyl estradiolDosage increase or alternate contraceptive measures should be consideredTable 7: Predicted Drug Interactions: Use With
Caution, Dose Decrease of Co-administered
Drug May Be Needed (see WARNINGS )Examples of Drugs in Which Plasma Concentrations May
Be Increased By Co-Administration With NORVIRDrug ClassExamples of DrugsAnalgesics, narcotictramadol, propoxypheneAntiarrhythmicsdisopyramide, lidocaine, mexilitineAnticonvulsantscarbamazepine, clonazepam, ethosuximideAntidepressantsbupropion, nefazodone, selective serotonin reuptake inhibitors (SSRIs), tricyclicsAntiemeticsdronabinolAntifungalsitraconazoleAntiparasiticsquinine(beta)-blockersmetoprolol, timololCalcium channel blockersdiltiazem, nifedipine, verapamilHypolipidemics, HMG CoA reductase inhibitors 1atorvastatin 2Immunosuppressantscyclosporine, tacrolimus, sirolimus (rapamycin)Neurolepticsperphenazine, risperidone, thioridazineSedative/hypnoticsclorazepate, diazepam, estazolam, flurazepam, zolpidemSteroidsdexamethasone, fluticasone, prednisoneStimulantsmethamphetamine1 Coadministration with lovastatin and simvastatin is not recommended (see WARNINGS , Drug Interactions ).2 Use lowest possible dose of atorvastatin with careful monitoring or consider HMG-CoA reductase inhibitor such as pravastatin or fluvastatin.Table 8: Predicted Drug Interactions: Use With Caution, Dose Increase
of Co-administered Drug May Be Needed
(see WARNINGS )Examples of Drugs in Which Plasma Concentrations
May Be Decreased By Co-Administration
With NORVIRAnticonvulsantsphenytoin, divalproex, lamotrigineAntiparasiticsatovaquoneCarcinogenesis and Mutagenesis
Carcinogenicity studies in mice and rats have been carried out on ritonavir. In male mice, at levels of 50, 100 or 200 mg/kg/day, there was a dose dependent increase in the incidence of both adenomas and combined adenomas and carcinomas in the liver. Based on AUC measurements, the exposure at the high dose was approximately 0.3-fold for males that of the exposure in humans with the recommended therapeutic dose (600 mg BID). There were no carcinogenic effects seen in females at the dosages tested. The exposure at the high dose was approximately 0.6-fold for the females that of the exposure in humans. In rats dosed at levels of 7, 15 or 30 mg/kg/day there were no carcinogenic effects. In this study, the exposure at the high dose was approximately 6% that of the exposure in humans with the recommended therapeutic dose. Based on the exposures achieved in the animal studies, the significance of the observed effects is not known. However, ritonavir was found to be negative for mutagenic or clastogenic activity in a battery of in vitro and in vivo assays including the Ames bacterial reverse mutation assay using S. typhimurium and E. coli , the mouse lymphoma assay, the mouse micronucleus test and chromosomal aberration assays in human lymphocytes.
Pregnancy, Fertility, and Reproduction
Pregnancy Category B: Ritonavir produced no effects on fertility in rats at drug exposures approximately 40% (male) and 60% (female) of that achieved with the proposed therapeutic dose. Higher dosages were not feasible due to hepatic toxicity.
No treatment-related malformations were observed when ritonavir was administered to pregnant rats or rabbits. Developmental toxicity observed in rats (early resorptions, decreased fetal body weight and ossification delays and developmental variations) occurred at a maternally toxic dosage at an exposure equivalent to approximately 30% of that achieved with the proposed therapeutic dose. A slight increase in the incidence of cryptorchidism was also noted in rats at an exposure approximately 22% of that achieved with the proposed therapeutic dose.
Developmental toxicity observed in rabbits (resorptions, decreased litter size and decreased fetal weights) also occurred at a maternally toxic dosage equivalent to 1.8 times the proposed therapeutic dose based on a body surface area conversion factor.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Antiretroviral Pregnancy Registry : To monitor maternal-fetal outcomes of pregnant women exposed to NORVIR, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
Nursing Mothers: The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV. It is not known whether ritonavir is secreted in human milk. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breast-feed if they are receiving NORVIR .
Pediatric Use
The safety and pharmacokinetic profile of ritonavir in pediatric patients below the age of 2 years have not been established. In HIV-infected patients age 2 to 16 years, the adverse event profile seen during a clinical trial and postmarketing experience was similar to that for adult patients. The evaluation of the antiviral activity of ritonavir in pediatric patients in clinical trials is ongoing.
Geriatric Use
Clinical studies of NORVIR did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The safety of NORVIR alone and in combination with nucleoside analogues was studied in 1270 patients. Table 9 lists treatment-emergent adverse events (at least possibly related and of at least moderate intensity) that occurred in 2% or greater of patients receiving NORVIR alone or in combination with nucleosides in Study 245 or Study 247 and in combination with saquinavir in ongoing study 462. In that study, 141 protease inhibitor-naive, HIV-infected patients with mean baseline CD 4 of 300 cells/µL were randomized to one of four regimens of NORVIR + saquinavir, including NORVIR 400 mg b.i.d. + saquinavir 400 mg b.i.d. Overall the most frequently reported clinical adverse events, other than asthenia, among patients receiving NORVIR were gastrointestinal and neurological disturbances including nausea, diarrhea, vomiting, anorexia, abdominal pain, taste perversion, and circumoral and peripheral paresthesias. Similar adverse event profiles were reported in patients receiving ritonavir in other trials.
Table 9
Percentage of Patients with Treatment-Emergent Adverse Events 1 of Moderate or
Severe Intensity Occurring in >/= 2% of Patients Receiving NORVIRAdverse
EventsStudy 245
Naive Patients 2Study 247
Advanced
Patients 3Study 462
PI-Naive
Patients 4NORVIR
+ ZDV
n=116NORVIR
n=117ZDV
n=119NORVIR
n=541Placebo
n=545NORVIR +
Saquinavir
n=141Body as a WholeAbdominal Pain5.2 6.0 5.9 8.3 5.1 2.1 Asthenia28.4 10.3 11.8 15.3 6.4 16.3 Fever1.7 0.9 1.7 5.0 2.4 0.7 Headache7.8 6.0 6.7 6.5 5.7 4.3 Malaise5.2 1.7 3.4 0.7 0.2 2.8 Pain (unspecified)0.9 1.7 0.8 2.2 1.8 4.3 CardiovascularSyncope0.9 1.7 0.8 0.6 0.0 2.1 Vasodilation3.4 1.7 0.8 1.7 0.0 3.5 DigestiveAnorexia8.6 1.7 4.2 7.8 4.2 4.3 Constipation3.4 0.0 0.8 0.2 0.4 1.4 Diarrhea25.0 15.4 2.5 23.3 7.9 22.7 Dyspepsia2.6 0.0 1.7 5.9 1.5 0.7 FecalIncontinence0.0 0.0 0.0 0.0 0.0 2.8 Flatulence2.6 0.9 1.7 1.7 0.7 3.5 Local Throat Irritation0.9 1.7 0.8 2.8 0.4 1.4 Nausea46.6 25.6 26.1 29.8 8.4 18.4 Vomiting23.3 13.7 12.6 17.4 4.4 7.1 Metabolic and NutritionalWeight Loss0.0 0.0 0.0 2.4 1.7 0.0 MusculoskeletalArthralgia0.0 0.0 0.0 1.7 0.7 2.1 Myalgia1.7 1.7 0.8 2.4 1.1 2.1 NervousAnxiety0.9 0.0 0.8 1.7 0.9 2.1 CircumoralParesthesia5.2 3.4 0.0 6.7 0.4 6.4 Confusion0.0 0.9 0.0 0.6 0.6 2.1 Depression1.7 1.7 2.5 1.7 0.7 7.1 Dizziness5.2 2.6 3.4 3.9 1.1 8.5 Insomnia3.4 2.6 0.8 2.0 1.8 2.8 Paresthesia5.2 2.6 0.0 3.0 0.4 2.1 PeripheralParesthesia0.0 6.0 0.8 5.0 1.1 5.7 Somnolence2.6 2.6 0.0 2.4 0.2 0.0 ThinkingAbnormal2.6 0.0 0.8 0.9 0.4 0.7 RespiratoryPharyngitis0.9 2.6 0.0 0.4 0.4 1.4 Skin and AppendagesRash0.9 0.0 0.8 3.5 1.5 0.7 Sweating3.4 2.6 1.7 1.7 1.1 2.8 Special SensesTaste Perversion17.2 11.1 8.4 7.0 2.2 5.0 UrogenitalNocturia0.0 0.0 0.0 0.2 0.0 2.8 1 Includes those adverse events at least possibly related to study drug or of unknown relationship and excludes concurrent HIV conditions.2 The median duration of treatment for patients randomized to regimens containing NORVIR in Study 245 was 9.1 months.3 The median duration of treatment for patients randomized to regimens containing NORVIR in Study 247 was 9.4 months.4 The median duration of treatment for patients in ongoing Study 462 was 48 weeks.
Adverse events occurring in less than 2% of patients receiving NORVIR in all phase II/phase III studies and considered at least possibly related or of unknown relationship to treatment and of at least moderate intensity are listed below by body system.
Body as a Whole: Abdomen enlarged, accidental injury, allergic reaction, back pain, cachexia, chest pain, chills, facial edema, facial pain, flu syndrome, hormone level altered, hypothermia, kidney pain, neck pain, neck rigidity, pelvic pain, photosensitivity reaction, and substernal chest pain.
Cardiovascular System: Cardiovascular disorder, cerebral ischemia, cerebral venous thrombosis, hypertension, hypotension, migraine, myocardial infarct, palpitation, peripheral vascular disorder, phlebitis, postural hypotension, tachycardia and vasospasm.
Digestive System: Abnormal stools, bloody diarrhea, cheilitis, cholestatic jaundice, colitis, dry mouth, dysphagia, eructation, esophageal ulcer, esophagitis, gastritis, gastroenteritis, gastrointestinal disorder, gastrointestinal hemorrhage, gingivitis, hepatic coma, hepatitis, hepatomegaly, hepatosplenomegaly, ileus, liver damage, melena, mouth ulcer, pancreatitis, pseudomembranous colitis, rectal disorder, rectal hemorrhage, sialadenitis, stomatitis, tenesmus, thirst, tongue edema, and ulcerative colitis.
Endocrine System: Adrenal cortex insufficiency and diabetes mellitus.
Hemic and Lymphatic System: Acute myeloblastic leukemia, anemia, ecchymosis, leukopenia, lymphadenopathy, lymphocytosis, myeloproliferative disorder, and thrombocytopenia.
Metabolic and Nutritional Disorders: Albuminuria, alcohol intolerance, avitaminosis, BUN increased, dehydration, edema, enzymatic abnormality, glycosuria, gout, hypercholesteremia, peripheral edema, and xanthomatosis.
Musculoskeletal System: Arthritis, arthrosis, bone disorder, bone pain, extraocular palsy, joint disorder, leg cramps, muscle cramps, muscle weakness, myositis, and twitching.
Nervous System: Abnormal dreams, abnormal gait, agitation, amnesia, aphasia, ataxia, coma, convulsion, dementia, depersonalization, diplopia, emotional lability, euphoria, grand mal convulsion, hallucinations, hyperesthesia, hyperkinesia, hypesthesia, incoordination, libido decreased, manic reaction, nervousness, neuralgia, neuropathy, paralysis, peripheral neuropathic pain, peripheral neuropathy, peripheral sensory neuropathy, personality disorder, sleep disorder, speech disorder, stupor, subdural hematoma, tremor, urinary retention, vertigo, and vestibular disorder.
Respiratory System: Asthma, bronchitis, dyspnea, epistaxis, hiccup, hypoventilation, increased cough, interstitial pneumonia, larynx edema, lung disorder, rhinitis, and sinusitis.
Skin and Appendages: Acne, contact dermatitis, dry skin, eczema, erythema multiforme, exfoliative dermatitis, folliculitis, fungal dermatitis, furunculosis, maculopapular rash, molluscum contagiosum, onychomycosis, pruritus, psoriasis, pustular rash, seborrhea, skin discoloration, skin disorder, skin hypertrophy, skin melanoma, urticaria, and vesiculobullous rash.
Special Senses: Abnormal electro-oculogram, abnormal electroretinogram, abnormal vision, amblyopia/blurred vision, blepharitis, conjunctivitis, ear pain, eye disorder, eye pain, hearing impairment, increased cerumen, iritis, parosmia, photophobia, taste loss, tinnitus, uveitis, visual field defect, and vitreous disorder.
Urogenital System: Acute kidney failure, breast pain, cystitis, dysuria, hematuria, impotence, kidney calculus, kidney failure, kidney function abnormal, kidney pain, menorrhagia, penis disorder, polyuria, urethritis, urinary frequency, urinary tract infection, and vaginitis.
Post-Marketing Experience: The following adverse events have been reported during post-marketing use of NORVIR. Because these reactions are reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or establish a causal relationship to NORVIR exposure.
Body as a whole: Dehydration, usually associated with gastrointestinal symptoms, and sometimes resulting in hypotension, syncope, or renal insufficiency has been reported. Syncope, orthostatic hypotension, and renal insufficiency have also been reported without known dehydration.
Co-administration of ritonavir with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system.
Redistribution/accumulation of body fat has been reported (see PRECAUTIONS , Fat Redistribution ).
Cardiovascular System: Cardiac and neurologic events have been reported when ritonavir has been co-administered with disopyramide, mexiletine, nefazodone, fluoxetine, and beta blockers. The possibility of drug interaction cannot be excluded.
Endocrine System: Cushing's syndrome and adrenal suppression have been reported when ritonavir, primarily at higher doses, has been co-administered with fluticasone propionate.
Hemic and Lymphatic System: There have been reports of increased bleeding in patients with hemophilia A or B (see PRECAUTIONS , Hemophilia ).
Nervous System: There have been postmarketing reports of seizure. Also, see Cardiovascular System .
Laboratory Abnormalities
Table 10 shows the percentage of patients who developed marked laboratory abnormalities.
Table 10
Percentage of Patients, by Study and Treatment Group, with Chemistry and Hematology Abnormalities Occurring in > 3% of Patients Receiving NORVIRStudy 245
Naive PatientsStudy 247
Advanced PatientsStudy 462
PI-Naive
PatientsVariableLimit NORVIR
+ ZDVNORVIR ZDV NORVIR Placebo NORVIR +
SaquinavirChemistryHigh Cholesterol>240 mg/dL 30.7 44.8 9.3 36.5 8.0 65.2 CPK>1000 IU/L 9.6 12.1 11.0 9.1 6.3 9.9 GGT>300 IU/L 1.8 5.2 1.7 19.6 11.3 9.2 SGOT (AST)>180 IU/L 5.3 9.5 2.5 6.4 7.0 7.8 SGPT (ALT)>215 IU/L 5.3 7.8 3.4 8.5 4.4 9.2 Triglycerides>800 mg/dL 9.6 17.2 3.4 33.6 9.4 23.4 Triglycerides>1500 mg/dL 1.8 2.6 - 12.6 0.4 11.3 Triglycerides
Fasting>1500 mg/dL 1.5 1.3 - 9.9 0.3 - Uric Acid>12 mg/dL - - - 3.8 0.2 1.4 HematologyLow Hematocrit<30% 2.6 - 0.8 17.3 22.0 0.7 Hemoglobin<8.0 g/dL 0.9 - - 3.8 3.9 - Neutrophils</=0.5 × 10 9 /L - - - 6.0 8.3 - RBC<3.0 × 10 12 /L 1.8 - 5.9 18.6 24.4 - WBC<2.5 × 10 9 /L - 0.9 6.8 36.9 59.4 3.5 1 ULN = upper limit of the normal range.-Indicates no events reported.OVERDOSAGE
Acute Overdosage
Human Overdose Experience: Human experience of acute overdose with NORVIR is limited. One patient in clinical trials took NORVIR 1500 mg/day for two days. The patient reported paresthesias which resolved after the dose was decreased. A post-marketing case of renal failure with eosinophilia has been reported with ritonavir overdose.
The approximate lethal dose was found to be greater than 20 times the related human dose in rats and 10 times the related human dose in mice.
Management of Overdosage
NORVIR oral solution contains 43% alcohol by volume. Accidental ingestion of the product by a young child could result in significant alcohol-related toxicity and could approach the potential lethal dose of alcohol.
Treatment of overdose with NORVIR consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. There is no specific antidote for overdose with NORVIR. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage; usual precautions should be observed to maintain the airway. Administration of activated charcoal may also be used to aid in removal of unabsorbed drug. Since ritonavir is extensively metabolized by the liver and is highly protein bound, dialysis is unlikely to be beneficial in significant removal of the drug. A Certified Poison Control Center should be consulted for up-to-date information on the management of overdose with NORVIR.
DOSAGE AND ADMINISTRATION
NORVIR is administered orally. It is recommended that NORVIR be taken with meals if possible. Patients may improve the taste of NORVIR oral solution by mixing with chocolate milk, Ensure®, or Advera® within one hour of dosing. The effects of antacids on the absorption of ritonavir have not been studied.
Adults
Recommended Dosage : The recommended dosage of ritonavir is 600 mg twice daily by mouth. Use of a dose titration schedule may help to reduce treatment-emergent adverse events while maintaining appropriate ritonavir plasma levels. Ritonavir should be started at no less than 300 mg twice daily and increased at 2 to 3 day intervals by 100 mg twice daily.
Concomitant Therapy : If saquinavir and ritonavir are used in combination, the dosage of saquinavir should be reduced to 400 mg twice daily. The optimum dosage of NORVIR (400 mg or 600 mg twice daily), in combination with saquinavir, has not been determined; however, the combination regimen was better tolerated in patients who received NORVIR 400 mg twice daily.
Pediatric Patients
Ritonavir should be used in combination with other antiretroviral agents (see General Dosing Guidelines ). The recommended dosage of ritonavir is 400 mg/m 2 twice daily by mouth and should not exceed 600 mg twice daily. Ritonavir should be started at 250 mg/m 2 and increased at 2 to 3 day intervals by 50 mg/m 2 twice daily. If patients do not tolerate 400 mg/m 2 twice daily due to adverse events, the highest tolerated dose may be used for maintenance therapy in combination with other antiretroviral agents, however, alternative therapy should be considered. When possible, dose should be administered using a calibrated dosing syringe.
General Dosing Guidelines
Patients should be aware that frequently observed adverse events, such as mild to moderate gastrointestinal disturbances and paraesthesias, may diminish as therapy is continued. In addition, patients initiating combination regimens with NORVIR and nucleosides may improve gastrointestinal tolerance by initiating NORVIR alone and subsequently adding nucleosides before completing two weeks of NORVIR monotherapy.
HOW SUPPLIED
NORVIR (ritonavir capsules) soft gelatin are white capsules imprinted with the corporate logo
, 100 and the Abbo-Code DS, available in the following package size:
Bottles of 120 capsules each...........................( NDC 0074-6633-22).
Bottles of 30 capsules each.............................( NDC 0074-6633-30).
Recommended storage: Store soft gelatin capsules in the refrigerator between 36-46°F (2-8°C) until dispensed. Refrigeration of NORVIR soft gelatin capsules by the patient is recommended, but not required if used within 30 days and stored below 77°F (25°C). Protect from light. Avoid exposure to excessive heat.
NORVIR (ritonavir oral solution) is an orange-colored liquid, supplied in amber-colored, multi-dose bottles containing 600 mg ritonavir per 7.5 mL marked dosage cup (80 mg/mL) in the following size:
240 mL bottles.............................................( NDC 0074-1940-63).
Recommended storage: Store NORVIR oral solution at room temperature 68°F to 77°F (20°C to 25°C). Do not refrigerate. Shake well before each use. Use by product expiration date.
Product should be stored and dispensed in the original container.
Avoid exposure to excessive heat. Keep cap tightly closed.
REFERENCES
- Sewester CS. Calculations. In: Drug Facts and Comparisons. St. Louis, MO: J.B. Lippincott Co; January, 1997:xix.
- Bertz RJ and Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet 1997; 32(3):210-258.
(Nos. 1940 and 6633)
03-5409-R20-Rev. January, 2005
Abbott LABORATORIES
NORTH CHICAGO, IL 60064, U.S.A.
PRINTED IN U.S.A.
NORVIR®
(ritonavir capsules) Soft Gelatin
(ritonavir oral solution)
ALERT: Find out about medicines that should NOT be taken with NORVIR. Please also read the section " MEDICINES YOU SHOULD NOT TAKE WITH NORVIR "
Patient Information
NORVIR®
(Nor-veer)
Generic Name: ritonavir
(rit-ON-uh-veer)
Please read this leaflet carefully before you start taking NORVIR. Also, read it each time you get your NORVIR prescription refilled, just in case something has changed. Remember that this information does not take the place of careful discussions with your doctor when you start this medication and at check ups.
You should remain under a doctor's care when taking NORVIR and you should not change or stop treatment without first talking with your doctor.
You should tell your doctor about any medicine you are taking or planning to take because taking NORVIR with some medications can result in serious or life-threatening problems.
Talk to your doctor if you have any questions about NORVIR. Your doctor or pharmacist can also give you more information about NORVIR.
What is NORVIR and how does it work?
NORVIR is in a class of medicines called the HIV protease (PRO-tee-ase) inhibitors. NORVIR is used in combination with other anti-HIV medicines to treat people with human immunodeficiency virus (HIV) infection. NORVIR is for adults and for children age 2 years and older.
HIV infection leads to the destruction of CD 4 (T) cells, which are important to the immune system. After a large number of CD 4 (T) cells have been destroyed, acquired immune deficiency syndrome (AIDS) develops.
NORVIR blocks HIV protease, a chemical which is needed for HIV to multiply. NORVIR reduces the amount of HIV in your blood and increases the number of CD 4 (T) cells. Patients who took NORVIR in clinical studies had significant reductions in both death and AIDS defining diseases; however NORVIR may not have these effects in all patients.
Does NORVIR cure HIV or AIDS?
NORVIR does not cure HIV infection or AIDS. The long-term effects of NORVIR are not known at this time. People taking NORVIR may still get opportunistic infections or other conditions that happen with HIV infection. Some of these conditions are pneumonia, herpes virus infections, and Mycobacterium avium complex (MAC) infections.
Does NORVIR reduce the risk of passing HIV to others?
NORVIR does not reduce the risk of passing HIV to others through sexual contact or blood contamination. Continue to practice safe sex and do not use or share dirty needles.
How should I take NORVIR?
- You should stay under a doctor's care when taking NORVIR. Do not change your treatment or stop treatment without first talking with your doctor.
- It is very important that you take NORVIR every day exactly as your doctor prescribed it.
- The usual dose for adults is six 100 mg capsules or 7.5 mL of the oral solution twice a day (morning and night), in combination with other anti-HIV medicines.
- The dosing of NORVIR may be different for you than for other patients. Follow the directions from your doctor, exactly as written on the label.
- Children from 2 to 16 years of age can also take NORVIR. The child's doctor will decide the right dose based on the child's height and weight.
- Take NORVIR with food if possible.
- NORVIR Oral Solution is peppermint/caramel flavored. You can take it alone, or improve the taste by mixing it with 8 ounces of chocolate milk, Ensure®, or Advera®. NORVIR Oral Solution should be taken within 1 hour if mixed with these items. Ask your doctor, nurse or pharmacist about other ways to improve the taste of NORVIR Oral Solution.
- Do not change or stop taking NORVIR without first talking with your health care provider.
- When your NORVIR supply starts to run low, get more from your doctor or pharmacy. This is very important because the amount of virus in your blood may increase if the medicine is stopped for even a short time. The virus may develop resistance to NORVIR and become harder to treat.
- Be sure to set up a schedule and follow it carefully.
- Only take medicine that has been prescribed specifically for you. Do not give NORVIR to others or take medicine prescribed for someone else.
What should I do if I miss a dose of NORVIR?
It is important that you do not miss any doses. If you miss a dose of NORVIR, take it as soon as possible and then take your next scheduled dose at its regular time. If it is almost time for your next dose, wait and take the next dose at the regular time. Do not double the next dose.
What happens if I take too much NORVIR?
If you think that you took more than the prescribed dose of this medicine, contact your local poison control center or emergency room immediately.
As with all prescription medicines, NORVIR should be kept out of the reach of young children. NORVIR liquid contains a large amount of alcohol. If a toddler or young child accidentally drinks more than the recommended dose of NORVIR, it could make him/her sick from too much alcohol. Contact your local poison control center or emergency room immediately if this happens.
Who should not take NORVIR?
Together with your doctor, you need to decide whether NORVIR is right for you.
-
Do not take NORVIR if you are taking certain medicines. These could cause serious side effects that could cause death. Before you take NORVIR, you must tell your doctor about all the medicines you are taking or are planning to take. These include other prescription and nonprescription medicines and herbal supplements.
For more information about medicines you should not take with NORVIR, please read the section " MEDICINES YOU SHOULD NOT TAKE WITH NORVIR " - Do not take NORVIR if you have had a serious allergic reaction to NORVIR or any of its ingredients.
Can I take NORVIR with other medications?*
NORVIR may interact with other medicines, including those you take without a prescription. You must tell your doctor about all the medicines you are taking or are planning to take.
MEDICINES YOU SHOULD NOT TAKE WITH NORVIR.
- Do not take the following medicines with NORVIR because they can cause serious or life-threatening problems such as irregular heartbeat, breathing difficulties or excessive sleepiness could occur:
Cordarone® (amiodarone)
Ergotamine, ergonovine, methylergonovine, and dihydroergotamine such as Cafergot®, Migranal®, D.H.E 45®, and others
Halcion® (triazolam)
Hismanal® (astemizole)
Orap® (pimozide)
Propulsid® (cisapride)
Quinidine, also known as Quinaglute®, Cardioquin®, Quinidex®, and others
Rythmol® (propafenone)
Seldane® (terfenadine)
Tambocor® (flecainide)
Vascor® (bepridil)
Versed® (midazolam)
- Do not take NORVIR with St. John's wort (hypericum perforatum), an herbal product sold as a dietary supplement or products containing St. John's wort. Talk with your doctor if you are taking or are planning to take St. John's wort. Taking St. John's wort may decrease NORVIR levels and lead to increased viral load and possible resistance to NORVIR or cross-resistance to other antiretroviral medicines.
- Do not take NORVIR with the cholesterol-lowering medicines Mevacor® (lovastatin) or Zocor® (simvastatin) because of possible serious reactions. There is also an increased risk of drug interactions between NORVIR and Lipitor® (atorvastatin); talk to your doctor before you take any of these cholesterol-reducing medicines with NORVIR.
Medicines that may require dosage adjustments:
It is possible that your doctor may need to increase or decrease the dose of other medicines when you are also taking NORVIR. Remember to tell your doctor all medicines you are taking or plan to take.
-
The following medicines require dose reduction if taken with NORVIR:
If you are taking Viagra® (sildenafil), your doctor may lower your dose of Viagra.
Before you take Viagra with NORVIR, talk to your doctor about possible drug interactions and side effects. If you take Viagra and NORVIR together, you may be at risk of side effects of Viagra such as low blood pressure, visual changes, and penile erection lasting more than 4 hours. If an erection lasts longer than 4 hours, you should get medical help immediately to avoid permanent damage to your penis. Your doctor can explain these symptoms to you.
- If you are taking Oral contraceptives ("the pill") to prevent pregnancy, your doctor should increase the dose or you should use a different type of contraception since NORVIR may reduce the effectiveness of oral contraceptives.
- If you are taking Mycobutin® (rifabutin), your doctor will lower the dose of Mycobutin.
-
Other Special Considerations:
NORVIR oral solution contains alcohol. Talk with your doctor if you are taking or planning to take metronidazole or disulfiram. Severe nausea and vomiting can occur. -
If you are taking both didanosine (Videx) and NORVIR:
Didanosine and NORVIR should be separated by at least 2.5 hours. - Rifampin, also known as Rimactane®, Rifadin®, Rifater®, or Rifamate®, may reduce blood levels of NORVIR. Be sure to tell your doctor if you are taking rifampin.
What are the possible side effects of NORVIR?
- This list of side effects is not complete. If you have questions about side effects, ask your doctor, nurse, or pharmacist. You should report any new or continuing symptoms to your doctor right away. Your doctor may be able to help you manage these side effects.
- The most commonly reported side effects are: feeling weak/tired, nausea, vomiting, diarrhea, loss of appetite, abdominal pain, changes in taste, tingling feeling or numbness in hands or feet or around the lips, headache, and dizziness.
- Blood tests in patients taking NORVIR may show possible liver problems. People with liver disease such as Hepatitis B and Hepatitis C who take NORVIR may have worsening liver disease. Liver problems including rare cases of death have occurred in patients taking NORVIR. It is unclear if NORVIR caused these liver problems because some patients had other illnesses or were taking other medicines.
- Some patients taking NORVIR can develop serious problems with their pancreas (pancreatitis) which may cause death. Tell your doctor if you have nausea, vomiting, or abdominal pain. These may be signs of pancreatitis.
- Some patients have large increases in triglycerides and cholesterol. The long-term chance of getting complications such as heart attacks or stroke due to increases in triglycerides and cholesterol caused by protease inhibitors is not known at this time.
- Diabetes and high blood sugar (hyperglycemia) have occurred in patients taking protease inhibitors. Some patients had diabetes before starting protease inhibitors, others did not. Some patients need changes in their diabetes medication. Others needed new diabetes medication.
- Changes in body fat have been seen in some patients taking antiretroviral therapy. These changes may include increased amount of fat in the upper back and neck ("buffalo hump"), breast and around the trunk. Loss of fat from the legs, arms and face may also happen. The cause and long term health effects of these conditions are not known at this time.
- Some patients with hemophilia have increased bleeding with protease inhibitors.
- Allergic reactions ranging from mild to severe have occurred in patients taking NORVIR.
There have been other side effects noted in patients receiving NORVIR; however, these side effects may have been due to other medicines that patients were taking or to the illness itself. Some of these side effects can be serious. If you have questions about side effects, ask your doctor, nurse, or pharmacist. You should report any new or persistent symptoms to your doctor immediately.
What should I tell my doctor before taking NORVIR?
- If you are pregnant or planning to become pregnant: The effects of NORVIR on pregnant women or their unborn babies are not known.
- If you are breast-feeding: Do not breast-feed if you are taking NORVIR. You should not breast-feed if you have HIV. If you are a woman who has or will have a baby, talk with your doctor about the best way to feed your baby. You should be aware that if your baby does not already have HIV, there is a chance that HIV can be transmitted through breast-feeding.
- If you have liver problems: If you have liver problems or are infected with Hepatitis B or Hepatitis C, you should tell your doctor before taking NORVIR.
- If you have diabetes: Some people taking protease inhibitors develop new or more serious diabetes or high blood sugar. Be sure to tell your doctor if you have diabetes or an increase in thirst and/or frequent urination.
- If you have hemophilia: Some people with hemophilia have had increased bleeding. It is not known whether the protease inhibitors caused these problems. Be sure to tell your doctor if you have hemophilia types A and B.
How do I store NORVIR?
- Keep NORVIR and all other medicines out of the reach of children.
- Store NORVIR Oral Solution at room temperature. Do not refrigerate NORVIR Oral Solution. Avoid exposing NORVIR Oral Solution to excessive heat or cold.
- Refrigeration of NORVIR soft gelatin capsules by the patient is recommended, but not required if used within 30 days and stored below 77°F (25°C). Avoid exposing NORVIR soft gelatin capsules to excessive heat or cold.
- Store NORVIR soft gelatin capsules and NORVIR Oral Solution in the original container.
- Shake NORVIR Oral Solution well before each use.
- Use NORVIR Oral Solution by the expiration date on the bottle.
Do not keep medicine that is out of date or that you no longer need. Be sure that if you throw any medicine away, it is out of the reach of children.
General advice about prescription medicines:
Talk to your doctor or other health care provider if you have any questions about this medicine or your condition. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. If you have any concerns about this medicine, ask your doctor. Your doctor or pharmacist can give you information about this medicine that was written for health care professionals. Do not use this medicine for a condition for which it was not prescribed. Do not share this medicine with other people.
*The brands listed are trademarks of their respective owners and are not trademarks of Abbott Laboratories. The makers of these brands are not affiliated with and do not endorse Abbott Laboratories or its products.
Ref: 03-5409-R20
Revised: January, 2005
Abbott LABORATORIES
NORTH CHICAGO, IL 60064, U.S.A.
PRINTED IN U.S.A.
Subscribe to the "News" RSS Feed 
TOP ۞