-
NuvaRing (Organon Usa)
delivers 0.120 mg/0.015 mg per day
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
FOR VAGINAL USE ONLY
DESCRIPTION
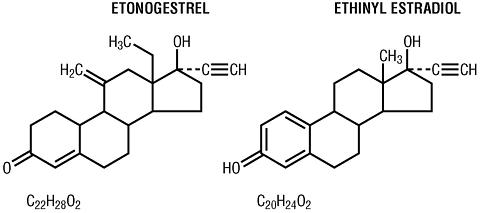
NuvaRing® (etonogestrel/ethinyl estradiol vaginal ring) is a non-biodegradable, flexible, transparent, colorless to almost colorless, combination contraceptive vaginal ring containing two active components, a progestin, etonogestrel (13-ethyl-17-hydroxy-11-methylene-18,19-dinor-17(alpha)-pregn-4-en-20-yn-3-one) and an estrogen, ethinyl estradiol (19-nor-17(alpha)-pregna-1,3,5(10)-trien-20-yne-3, 17-diol). When placed in the vagina, each ring releases on average 0.120 mg/day of etonogestrel and 0.015 mg/day of ethinyl estradiol over a three-week period of use. NuvaRing® is made of ethylene vinylacetate copolymers (28% and 9% vinyl acetate) and magnesium stearate and contains 11.7 mg etonogestrel and 2.7 mg ethinyl estradiol. NuvaRing® has an outer diameter of 54 mm and a cross-sectional diameter of 4 mm. The molecular weights for etonogestrel and ethinyl estradiol are 324.46 and 296.40, respectively. The structural formulas are as follows:
CLINICAL PHARMACOLOGY
Combination hormonal contraceptives act by suppression of gonadotropins. Although the primary effect of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
Receptor binding studies, as well as studies in animals, have shown that etonogestrel, the biologically active metabolite of desogestrel, combines high progestational activity with low intrinsic androgenicity. The relevance of this later finding in humans is unknown.
Pharmacokinetics
Absorption
Etonogestrel: Etonogestrel released by NuvaRing® is rapidly absorbed. Bioavailability of etonogestrel after vaginal administration is approximately 100%. The serum etonogestrel and ethinyl estradiol concentrations (pg/mL) observed during three weeks of NuvaRing® use are summarized in Table I.
Ethinyl estradiol: Ethinyl estradiol released by NuvaRing® is rapidly absorbed. Bioavailability of ethinyl estradiol after vaginal administration is approximately 55.6%, which is comparable to that with oral administration of ethinyl estradiol. The serum ethinyl estradiol concentrations observed during three weeks of NuvaRing® use are summarized in Table I.
TABLE I: MEAN (SD) SERUM ETONOGESTREL AND ETHINYL ESTRADIOL CONCENTRATIONS (n=16)1 week 2 weeks 3 weeks Etonogestrel (pg/mL)1578 (408) 1476 (362) 1374 (328) Ethinyl-estradiol (pg/mL)19.1 (4.5) 18.3 (4.3) 17.6 (4.3) The pharmacokinetic profile of etonogestrel and ethinyl estradiol during use of NuvaRing® is shown in Figure 1.
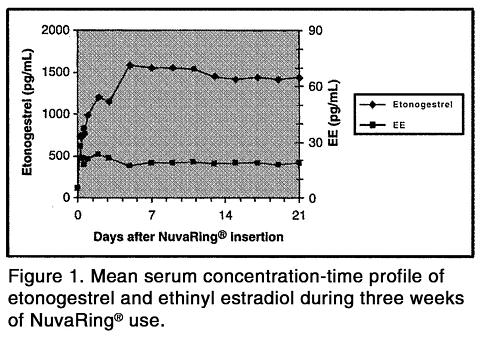
The pharmacokinetic parameters of etonogestrel and ethinyl estradiol were determined during one cycle of NuvaRing® use in 16 healthy female subjects and are summarized in Table II.
TABLE II: MEAN (SD) PHARMACOKINETIC PARAMETERS OF NuvaRing® (n=16)HormoneC max
pg/mLT max
hrt 1/2
hrCL
L/hrEtonogestrel1716 (445) 200.3 (69.6) 29.3 (6.1) 3.4 (0.8) Ethinyl Estradiol34.7 (17.5) 59.3 (67.5) 44.7 (28.8) 34.8 (11.6) C max - maximum serum drug concentrationT max - time at which maximum serum drug concentration occurst 1/2 - elimination half-life, calculated by 0.693/K elimCL - apparent clearanceDistribution
Etonogestrel: Etonogestrel is approximately 32% bound to sex hormone binding globulin (SHBG) and approximately 66% bound to albumin in blood.
Ethinyl estradiol: Ethinyl estradiol is highly but not specifically bound to serum albumin (approximately 98.5%) and induces an increase in the serum concentrations of SHBG.
Metabolism
In vitro data shows that both etonogestrel and ethinyl estradiol are metabolized in liver microsomes by the cytochrome P450 3A4 isoenzyme. Ethinyl estradiol is primarily metabolized by aromatic hydroxylation, but a wide variety of hydroxylated and methylated metabolites are formed. These are present as free metabolites and as sulfate and glucuronide conjugates. The hydroxylated ethinyl estradiol metabolites have weak estrogenic activity. The biological activity of etonogestrel metabolites is unknown.
Excretion
Etonogestrel and ethinyl estradiol are primarily eliminated in urine, bile and feces.
Special Populations
Race
No formal studies were conducted to evaluate the effect of race on the pharmacokinetics of NuvaRing® (etonogestrel/ethinyl estradiol vaginal ring).
Hepatic Insufficiency
No formal studies were conducted to evaluate the effect of hepatic disease on the pharmacokinetics, safety, and efficacy of NuvaRing®. However, steroid hormones may be poorly metabolized in patients with impaired liver function (see PRECAUTIONS ).
Renal Insufficiency
No formal studies were conducted to evaluate the effect of renal disease on the pharmacokinetics, safety, and efficacy of NuvaRing®.
Drug-Drug Interactions
Interactions between contraceptive steroids and other drugs have been reported in the literature (see PRECAUTIONS ) . The pharmacokinetics of NuvaRing® were evaluated in one cycle in 24 healthy female subjects randomized to a single-dose vaginal administration on Day 8 of 100 mg of a non-oxynol-9 spermicide gel or a 1200 mg miconazole nitrate antimycotic capsule. In this study, it was determined that the vaginally-administered, oil-based miconazole nitrate capsule increased the serum concentrations of etonogestrel and ethinyl estradiol by approximately 17% and 16%, respectively. The clinical significance of these findings is unknown; however, the contraceptive effectiveness of NuvaRing® is not expected to change. It was determined that the single dose of 100 mg vaginally-administered, water-based non-oxynol-9 gel did not affect the serum concentrations of etonogestrel or ethinyl estradiol. The effects of chronic administration of either of these products with NuvaRing® are unknown.
INDICATIONS AND USAGE
NuvaRing® is indicated for the prevention of pregnancy in women who elect to use this product as a method of contraception. Like oral contraceptives, NuvaRing® is highly effective if used as recommended in this label.
In two large clinical trials of 13 cycles of NuvaRing® use, pregnancy rates were between one and two per 100 women-years of use. Table III lists the pregnancy rates for users of various contraceptive methods.
TABLE III: PERCENTAGE OF WOMEN EXPERIENCING AN UNINTENDED PREGNANCY DURING THE FIRST YEAR OF TYPICAL USE AND THE FIRST YEAR OF PERFECT USE OF CONTRACEPTION AND THE PERCENTAGE CONTINUING USE AT THE END OF THE FIRST YEAR: UNITED STATES % of Women Experiencing an Unintended Pregnancy within the First Year of Use % of Women Continuing Use at One Year 3
(4)Method
(1)Typical Use 1
(2)Perfect Use 2
(3)Chance 485 85 Spermicides 526 6 40 Periodic abstinence25 63 Calendar9 Ovulation Method3 Sympto-Thermal 62 Post-Ovulation1 Cap 7Parous Women40 26 42 Nulliparous Women20 9 56 SpongeParous Women40 20 42 Nulliparous Women20 9 56 Diaphragm 720 6 56 Withdrawal19 4 Condom 8Female (Reality)21 5 56 Male14 3 61 Pill5 71 Progestin Only0.5 Combined0.1 IUDProgesterone T2.0 1.5 81 Copper T 380A0.8 0.6 78 LNg 200.1 0.1 81 Depo-Provera0.3 0.3 70 Norplant and Norplant-20.05 0.05 88 Female sterilization0.5 0.5 100 Male sterilization0.15 0.10 100 Emergency Contraceptive Pills: Treatment initiated within 72 hours after unprotected intercourse reduces the risk of pregnancy by at least 75%. 9Lactation Amenorrhea Method: LAM is a highly effective, temporary method of contraception. 10Adapted from Hatcher et al., Contraceptive Technology, 17 th Revised Edition. New York, NY: Irvington Publishers, 1998.1 Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.2 Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.3 Among couples attempting to avoid pregnancy, the percentage who continue to use a method for one year.4 The percents becoming pregnant in columns (2) and (3) are based on data from populations where contraception is not used and from women who cease using contraception in order to become pregnant. Among such populations, about 89% become pregnant within one year. This estimate was lowered slightly (to 85%) to represent the percent who would become pregnant within one year among women now relying on reversible methods of contraception if they abandoned contraception altogether.5 Foams, creams, gels, vaginal suppositories, and vaginal film.6 Cervical mucus (ovulation) method supplemented by calendar in the pre-ovulatory and basal body temperature in the post-ovulatory phases.7 With spermicidal cream or jelly.8 Without spermicides.9 The treatment schedule is one dose within 72 hours after unprotected intercourse, and a second dose 12 hours after the first dose. The FDA has declared the following brands of oral contraceptives to be safe and effective for emergency contraception. Ovral (one dose is two white pills), Alesse (one dose is five pink pills), Nordette or Levlen (one dose is four yellow pills).10 However, to maintain effective protection against pregnancy, another method of contraception must be used as soon as menstruation resumes, the frequency or duration of breastfeeds is reduced, bottle feeds are introduced, or the baby reaches six months of age.CONTRAINDICATIONS
NuvaRing® should not be used in women who currently have the following conditions:
- Thrombophlebitis or thromboembolic disorders
- A past history of deep vein thrombophlebitis or thromboembolic disorders
- Cerebral vascular or coronary artery disease (current or history)
- Valvular heart disease with complications
- Severe hypertension
- Diabetes with vascular involvement
- Headaches with focal neurological symptoms
- Major surgery with prolonged immobilization
- Known or suspected carcinoma of the breast or personal history of breast cancer
- Carcinoma of the endometrium or other known or suspected estrogen-dependent neoplasia
- Undiagnosed abnormal genital bleeding
- Cholestatic jaundice of pregnancy or jaundice with prior hormonal contraceptive use
- Hepatic tumors (benign or malignant), active liver disease
- Known or suspected pregnancy
- Heavy smoking (>/=15 cigarettes per day) and over age 35
- Hypersensitivity to any of the components of NuvaRing®
WARNINGS
Cigarette smoking increases the risk of serious cardiovascular side effects from combination oral contraceptive use. The risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use combination hormonal contraceptives, including NuvaRing®, should be strongly advised not to smoke. NuvaRing® and other contraceptives that contain both an estrogen and a progestin are called combination hormonal contraceptives. There is no epidemiologic data available to determine whether safety and efficacy with the vaginal route of administration of combination hormonal contraceptives would be different than the oral route. Practitioners prescribing NuvaRing® should be familiar with the following information relating to these risks.
The use of oral contraceptives is associated with increased risks of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases signficantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, obesity, and diabetes.
The information contained in this package insert is principally based on studies carried out in patients who used oral contraceptives with formulations of higher doses of estrogens and progestogens than those in common use today. The effect of long-term use of oral contraceptives with lower doses of both estrogens and progestogens remains to be determined.
Throughout this labeling, epidemiologic studies reported are of two types: retrospective or case control studies and prospective or cohort studies. Case control studies provide a measure of the relative risk of a disease, namely, a ratio of the incidence of a disease among oral contraceptive users to that among non-users. The relative risk does not provide information on the actual clinical occurrence of a disease. Cohort studies provide a measure of attributable risk, which is the difference in the incidence of disease between oral contraceptive users and non-users. The attributable risk does provide information about the actual occurrence of a disease in the population. For further information, the reader is referred to a text on epidemiologic methods.
-
THROMBOEMBOLIC DISORDERS AND OTHER VASCULAR PROBLEMS
-
Thromboembolism
An increased risk of thromboembolic and thrombotic disease associated with the use of oral contraceptives is well established. Case control studies have found the relative risk of users compared to non-users to be 3 for the first episode of superficial venous thrombosis, 4 to 11 for deep vein thrombosis or pulmonary embolism, and 1.5 to 6 for women with predisposing conditions for venous thromboembolic disease. Cohort studies have shown the relative risk to be somewhat lower, about 3 for new cases and about 4.5 for new cases requiring hospitalization. The risk of thromboembolic disease associated with oral contraceptives is not related to length of use and disappears after pill use is stopped.
Several epidemiology studies indicate that third generation oral contraceptives, including those containing desogestrel (etonogestrel, the progestin in NuvaRing®, is the biologically active metabolite of desogestrel), are associated with a higher risk of venous thromboembolism than certain second generation oral contraceptives. In general, these studies indicate an approximate two-fold increased risk, which corresponds to an additional one to two cases of venous thromboembolism per 10,000 women-years of use. However, data from additional studies have not shown this two-fold increase in risk. It is unknown if NuvaRing® has a different risk of venous thromboembolism than second generation oral contraceptives.
A two- to four-fold increase in relative risk of post-operative thromboembolic complications has been reported with the use of oral contraceptives. The relative risk of venous thrombosis in women who have predisposing conditions is twice that of women without such medical conditions. If feasible, combination hormonal contraceptives, including NuvaRing®, should be discontinued at least four weeks prior to and for two weeks after elective surgery of a type associated with an increase in risk of thromboembolism and during and following prolonged immobilization. Since the immediate postpartum period is also associated with an increased risk of thromboembolism, combination hormonal contraceptives, such as NuvaRing®, should be started no earlier than four weeks after delivery in women who elect not to breast feed.
The clinician should be alert to the earliest manifestations of thrombotic disorders (thrombophlebitis, pulmonary embolism, cerebrovascular disorders, and retinal thrombosis). Should any of these occur or be suspected, NuvaRing® should be discontinued immediately. -
Myocardial infarction
An increased risk of myocardial infarction has been attributed to oral contraceptive use. This risk is primarily in smokers or women with other underlying risk factors for coronary artery disease such as hypertension, hypercholesterolemia, morbid obesity, and diabetes. The relative risk of heart attack for current combination oral contraceptive users has been estimated to be 2 to 6. The risk is very low in women under the age of 30.
Smoking in combination with oral contraceptive use has been shown to contribute substantially to the incidence of myocardial infarction in women in their mid-thirties or older with smoking accounting for the majority of excess cases. Mortality rates associated with circulatory disease have been shown to increase substantially in smokers, over the age of 35 and non-smokers over the age of 40 among women who use oral contraceptives (see Table IV) .
TABLE IV: CIRCULATORY DISEASE MORTALITY RATES PER 100,000 WOMAN-YEARS BY AGE, SMOKING STATUS, AND COMBINATION ORAL CONTRACEPTIVE USEAge Ever-Users
Non-SmokersEver-Users
SmokersControls
Non-SmokersControls
Smokers15-24 0.0 10.5 0.0 0.0 25-34 4.4 14.2 2.7 4.2 35-44 21.5 63.4 6.4 15.2 45+ 52.4 206.7 11.4 27.9 (Adapted from P.M. Layde and V. Beral, Lancet, 1981;1:541-546.)
Oral contraceptives may compound the effects of well-known risk factors, such as hypertension, diabetes, hyperlipidemias, age, and obesity. In particular, some progestogens are known to decrease HDL cholesterol and cause glucose intolerance, while estrogens may create a state of hyperinsulinism. Oral contraceptives have been shown to increase blood pressure among users (see WARNINGS ) . Similar effects on risk factors have been associated with an increased risk of heart disease. NuvaRing® must be used with caution in women with cardiovascular disease risk factors. -
Cerebrovascular diseases
Oral contraceptives have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes), although, in general, the risk is greatest among older (>35 years), hypertensive women who also smoke. Hypertension was found to be a risk factor for both users and non-users, for both types of strokes, while smoking interacted to increase the risk for hemorrhagic strokes.
In a large study, the relative risk of thrombotic strokes has been shown to range from 3 for normotensive users to 14 for users with severe hypertension. The relative risk of hemorrhagic stroke is reported to be 1.2 for non-smokers who used oral contraceptives, 2.6 for smokers who did not use oral contraceptives, 7.6 for smokers who used oral contraceptives, 1.8 for normotensive users and 25.7 for users with severe hypertension. The attributable risk is also greater in older women. -
Dose-related risk of vascular disease from oral contraceptives
A positive association has been observed between the amount of estrogen and progestogen in oral contraceptives and the risk of vascular disease. A decline in serum high-density lipoproteins (HDL) has been reported with many progestational agents. A decline in serum high-density lipoproteins has been associated with an increased incidence of ischemic heart disease. Because estrogens increase HDL cholesterol, the net effect of an oral contraceptive depends on a balance achieved between doses of estrogen and progestogen and the nature and absolute amount of progestogens used in the contraceptives. The activity and amount of both hormones should be considered in the choice of a hormonal contraceptive. -
Persistence of risk of vascular disease
There are two studies that have shown persistence of risk of vascular disease for ever-users of oral contraceptives. In a study in the United States, the risk of developing myocardial infarction after discontinuing oral contraceptives persists for at least nine years for women 40-49 years old who had used oral contraceptives for five or more years, but this increased risk was not demonstrated in other age groups. In another study in Great Britain, the risk of developing cerebrovascular disease persisted for at least six years after discontinuation of oral contraceptives, although excess risk was very small. However, both studies were performed with oral contraceptive formulations containing 50 micrograms or more of estrogen.
It is unknown whether NuvaRing® is distinct from combination oral contraceptives with regard to the occurrence of venous or arterial thrombosis.
-
Thromboembolism
-
ESTIMATES OF MORTALITY FROM CONTRACEPTIVE USE
One study gathered data from a variety of sources that have estimated the mortality rate associated with different methods of contraception at different ages (Table V). These estimates include the combined risk of death associated with contraceptive methods plus the risk attributable to pregnancy in the event of method failure. Each method of contraception has its specific benefits and risks. The study concluded that with the exception of oral contraceptive users age 35 and older who smoke and age 40 and older who did not smoke, mortality associated with all methods of birth control is low and below that associated with childbirth.
The observation of a possible increase in risk of mortality with age for oral contraceptive users is based on data gathered in the 1970's, but not reported until 1983. However, current clinical practice involves the use of lower estrogen-dose formulations combined with careful consideration of risk factors.
Because of these changes in practice and, also, because of some limited new data which suggest that the risk of cardiovascular disease with the use of oral contraceptives may now be less than previously observed, the Fertility and Maternal Health Drugs Advisory Committee was asked to review the topic in 1989. The Committee concluded that although cardiovascular disease risks may be increased with oral contraceptive use after age 40 in healthy non-smoking women (even with the newer low-dose formulations), there are also greater potential health risks associated with pregnancy in older women and with the alternative surgical and medical procedures which may be necessary if such women do not have access to effective and acceptable means of contraception. Therefore, the Committee recommended that the benefits of low-dose oral contraceptive use by healthy non-smoking women over 40 may outweigh the possible risks. Although the data are mainly obtained with oral contraceptives, this is likely to apply to NuvaRing® as well. Women of all ages who take hormonal contraceptives, should take the lowest possible dose formulation that is effective and meets the needs of the individual patient.
TABLE V: ANNUAL NUMBER OF BIRTH-RELATED OR METHOD-RELATED DEATHS ASSOCIATED WITH CONTROL OF FERTILITY PER 100,000 NON-STERILE WOMEN, BY FERTILITY CONTROL METHOD ACCORDING TO AGE Method of control and outcome15-19 20-24 25-29 30-34 35-39 40-44 No fertility control methods *7.0 7.4 9.1 14.8 25.7 28.2 Oral contraceptives non-smoker **0.3 0.5 0.9 1.9 13.8 31.6 Oral contraceptives smoker **2.2 3.4 6.6 13.5 51.1 117.2 IUD **0.8 0.8 1.0 1.0 1.4 1.4 Condom *1.1 1.6 0.7 0.2 0.3 0.4 Diaphragm/spermicide *1.9 1.2 1.2 1.3 2.2 2.8 Periodic abstinence *2.5 1.6 1.6 1.7 2.9 3.6 *Deaths are birth related**Deaths are method relatedAdapted from H.W. Ory, Family Planning Perspectives 1983;15:50-56.
-
CARCINOMA OF THE REPRODUCTIVE ORGANS AND BREASTS
Numerous epidemiologic studies have been performed on the incidence of breast, endometrial, ovarian, and cervical cancer in women using combination oral contraceptives.
The risk of having breast cancer diagnosed may be slightly increased among current and recent users of combination oral contraceptives. However, this excess risk appears to decrease over time after COC discontinuation and by 10 years after cessation the increased risk disappears. Some studies report an increased risk with duration of use while other studies do not and no consistent relationships have been found with dose or type of steroid. Some studies have found a small increase in risk for women who first use COCs before age 20. Most studies show a similar pattern of risk with COC use regardless of a woman's reproductive history or her family breast cancer history.
In addition, breast cancers diagnosed in current or ever oral contraceptive users may be less clinically advanced than in never-users.
Women who currently have or have had breast cancer should not use hormonal contraceptives because breast cancer is usually a hormonally sensitive tumor.
Some studies suggest that combination oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
In spite of many studies of the relationship between oral contraceptive use and breast and cervical cancers, a cause-and-effect relationship has not been established.
It is unknown whether NuvaRing® is distinct from oral contraceptives with regard to the above statements. -
HEPATIC NEOPLASIA
Benign hepatic adenomas are associated with oral contraceptive use, although the incidence of benign tumors is rare in the United States. Indirect calculations have estimated the attributable risk to be in the range of 3.3 cases per 100,000 for users, a risk that increases after four or more years of use. Rupture of rare, benign, hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies from Britain have shown an increased risk of developing hepatocellular carcinoma in long term (>8 years) oral contraceptive users. However, these cancers are extremely rare in the US and the attributable risk (the excess incidence) of liver cancers in oral contraceptive users approaches less than one per million users. It is unknown whether NuvaRing® is distinct from oral contraceptives in this regard. -
OCULAR LESIONS
There have been clinical case reports of retinal thrombosis associated with the use of oral contraceptives. NuvaRing® should be discontinued if there is unexplained partial or complete loss of vision, onset of proptosis or diplopia, papilledema, or retinal vascular lesions. Appropriate diagnostic and therapeutic measures should be undertaken immediately. -
HORMONAL CONTRACEPTIVE USE BEFORE OR DURING EARLY PREGNANCY
Hormonal contraceptives should not be used during pregnancy.
Extensive epidemiologic studies have revealed no increased risk of birth defects in women who have used oral contraceptives prior to pregnancy. Studies also do not suggest a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned, when oral contraceptives are taken inadvertently during early pregnancy.
Combination hormonal contraceptives, such as NuvaRing®, should not be used to induce withdrawal bleeding as a test for pregnancy. NuvaRing® should not be used during pregnancy to treat threatened or habitual abortion. It is recommended that for any patient who has not adhered to the prescribed regimen for use of NuvaRing® and has missed a menstrual period or who has missed two consecutive periods, pregnancy should be ruled out. -
GALLBLADDER DISEASE
Combination hormonal contraceptives, such as NuvaRing®, may worsen existing gallbladder disease and may accelerate the development of this disease in previously asymptomatic women. Women with a history of combination hormonal contraceptive-related cholestasis are more likely to have the condition recur with subsequent combination hormonal contraceptive use. -
CARBOHYDRATE AND LIPID METABOLIC EFFECTS
Hormonal contraceptives have been shown to cause a decrease in glucose tolerance in some users. However, in the non-diabetic woman, combination hormonal con-traceptives appear to have no effect on fasting blood glucose. Prediabetic and diabetic women should be carefully observed while taking combination hormonal contraceptives, such as NuvaRing®. In a clinical study involving 37 NuvaRing®-treated subjects, glucose tolerance tests showed no clinically significant changes in serum glucose levels from baseline to cycle six.
A small proportion of women will have persistent hypertriglyceridemia while using oral contraceptives. Changes in serum triglycerides and lipoprotein levels have been reported in combination hormonal contraceptive users. -
ELEVATED BLOOD PRESSURE
An increase in blood pressure has been reported in women taking oral contraceptives and this increase is more likely in older oral contraceptive users and with continued use. Data from the Royal College of General Practitioners and subsequent randomized trials have shown that the incidence of hypertension increases with increasing concentrations of progestogens.
Women with a history of hypertension or hypertension-related diseases, or renal disease should be encouraged to use another method of contraception. If these women elect to use NuvaRing®, they should be monitored closely and if significant elevation of blood pressure occurs, NuvaRing® should be discontinued. For most women, elevated blood pressure will return to normal after stopping hormonal contraceptives, and there is no difference in the occurrence of hypertension between former and never-users. -
HEADACHE
The onset or exacerbation of migraine or development of headache with a new pattern which is recurrent, persistent, or severe requires discontinuation of NuvaRing® and evaluation of the cause. -
BLEEDING IRREGULARITIES
Bleeding Patterns
Breakthrough bleeding and spotting are sometimes encountered in women using NuvaRing®. If abnormal bleeding while using NuvaRing® persists or is severe, appropriate investigation should be instituted to rule out the possibility of organic pathology or pregnancy, and appropriate treatment should be instituted when necessary. In the event of amenorrhea, pregnancy should be ruled out.
Bleeding patterns were evaluated in two large clinical studies. During cycles 1 through 13, breakthrough bleeding/spotting occurred in 7.2 to 11.7% of cycles in a study of 1177 US and Canadian subjects, and in 2.6 to 6.4% of cycles in a second study of 1145 European and Israeli subjects. Absence of withdrawal bleeding occurred in 2.3 to 3.8% of cycles in the US and Canadian trial subjects and in 0.6 to 2.1% of cycles in the European and Israeli trials subjects. Bleeding patterns for individual women over multiple cycles were not evaluated.
Some women may encounter amenorrhea or oligomenorrhea after discontinuing use of NuvaRing®, especially when such a condition was pre-existent. -
ECTOPIC PREGNANCY
Ectopic as well as intrauterine pregnancy may occur in contraceptive failures.
PRECAUTIONS
1. GENERAL
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
2. PHYSICAL EXAMINATION AND FOLLOW-UP
It is good medical practice for women using NuvaRing®, as for all women, to have an annual medical evaluation including physical examination and relevant laboratory tests. The physical examination should include special reference to blood pressure, breasts, abdomen, pelvic organs and vagina (including cervical cytology). In case of undiagnosed, persistent or recurrent abnormal vaginal bleeding, appropriate measures should be conducted to rule out malignancy. Women with a family history of breast cancer or who have breast nodules should be monitored with particular care.
3. LIPID DISORDERS
Women who are being treated for hyperlipidemias should be followed closely if they elect to use NuvaRing®. Some progestogens may elevate LDL lev-els and may render the control of hyperlipidemias more difficult.
4.LIVER FUNCTION
If jaundice develops in any woman using NuvaRing®, product use should be discontinued. The hormones in NuvaRing® may be poorly metabolized in patients with impaired liver function.
5. FLUID RETENTION
Steroid hormones like those in NuvaRing®, may cause some degree of fluid retention. NuvaRing® should be prescribed with caution, and only with careful monitoring, in patients with conditions which might be aggravated by fluid retention.
6. EMOTIONAL DISORDERS
Women who become significantly depressed while using combination hormonal contraceptives, such as NuvaRing®, should stop the medication and use another method of contraception in an attempt to determine whether the symptom is drug related. Women with a history of depression should be carefully observed and NuvaRing® discontinued if significant depression occurs.
7. CONTACT LENSES
Contact lens wearers who develop visual changes or changes in lens tolerance should be assessed by an ophthalmologist.
8. DRUG INTERACTIONS
Changes in Contraceptive Effectiveness Associated with Co-Administration of Other Drugs
Contraceptive effectiveness may be reduced when hormonal contraceptives are co-administered with some antibiotics, antifungals, anticonvulsants, and other drugs that increase metabolism of contraceptive steroids. This could result in unintended pregnancy or breakthrough bleeding. Examples include barbiturates, griseofulvin, rifampin, phenylbutazone, phenytoin, carbamazepine, felbamate, oxcarbazepine, topiramate and possibly with ampicillin and tetracyclines. Women may need to use an additional contraceptive method when taking medications which may make hormonal contraceptives less effective.
Several of the anti-HIV protease inhibitors have been studied with co-administration of oral combination hormonal contraceptives; significant changes (increase and decrease) in the mean AUC of the estrogen and progestin have been noted in some cases. The efficacy and safety of oral contraceptive products may be affected; it is unknown whether this applies to NuvaRing®. Healthcare providers should refer to the label of the individual anti-HIV protease inhibitors for further drug-drug interaction information.
Herbal products containing St. John's Wort (hypericum perforatum) may induce hepatic enzymes (cytochrome P450) and p-glycoprotein transporter and may reduce the effectiveness of contraceptive steroids. This may also result in breakthrough bleeding.
Increase in Plasma Hormone Levels Associated with Co-Administered Drugs
Co-administration of atorvastatin and certain oral contraceptives containing ethinyl estradiol increase AUC values for ethinyl estradiol by approximately 20%. Ascorbic acid and acetaminophen may increase plasma ethinyl estradiol levels, possibly by inhibition of conjugation. CYP 3A4 inhibitors such as itraconazole or ketoconazole may increase plasma hormone levels.
Changes in Plasma Levels of Co-Administered Drugs
Combination hormonal contraceptives containing some synthetic estrogens (e.g., ethinyl estradiol) may inhibit the metabolism of other compounds. Increased plasma concentrations of cyclosporine, prednisolone, and theophylline have been reported with concomitant administration of oral contraceptives. In addition, oral contraceptives may induce the conjugation of other compounds. Decreased plasma concentrations of acetaminophen and increased clearance of temazepam, salicylic acid, morphine and clofibric acid have been noted when these drugs were administered with oral contraceptives.
9.INTERACTIONS WITH LABORATORY TESTS
Certain endocrine and liver function tests and blood components may be affected by combined hormonal contraceptives:
a. Increased prothrombin and factors VII, VIII, IX and X; decreased antithrombin 3, increased norepinephrine-induced platelet aggregability.
b. Increased thyroid binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4 by column or by radioimmunoassay. Free T3 resin uptake is decreased, reflecting the elevated TBG; free T4 concentration is unaltered.
c. Other binding proteins may be elevated in serum.
d. Sex hormone-binding globulins are increased and result in elevated levels of total circulating sex steroids; however, free or biologically active levels either decrease or remain unchanged.
e. Triglycerides may be increased and levels of various other lipids and lipoproteins may be affected.
f. Glucose tolerance may be decreased.
g. Serum folate levels may be depressed by oral contraceptive therapy. This may be of clinical significance if a woman becomes pregnant shortly after discontinuing NuvaRing®.
10. CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
In a 24-month carcinogenicity study in rats with subdermal implants releasing 10 and 20 µg etonogestrel per day, (approximately 0.3 and 0.6 times the systemic steady state exposure of women using NuvaRing®), no drug-related carcinogenic potential was observed. Etonogestrel was not genotoxic in the in-vitro Ames/Salmonella reverse mutation assay, the chromosomal aberration assay in Chinese hamster ovary cells or in the in-vivo mouse micronucleus test. Fertility returned after withdrawal from treatment. See WARNINGS .
11. PREGNANCY
Pregnancy Category X (see CONTRAINDICATIONS and WARNINGS) .
Teratology studies have been performed in rats and rabbits using the oral route of administration at doses up to 130 and 260 times, respectively, the human NuvaRing® dose (based on body surface area) and have revealed no evidence of harm to the fetus due to etonogestrel.
12. NURSING MOTHERS
The effects of NuvaRing® in nursing mothers have not been evaluated and are unknown. Small amounts of contraceptive steroids have been identified in the milk of nursing mothers and a few adverse effects on the child have been reported, including jaundice and breast enlargement. In addition, contraceptive steroids given in the postpartum period may interfere with lactation by decreasing the quantity and quality of breast milk. Long-term follow-up of children whose mothers used combination hormonal contraceptives while breast feeding has shown no deleterious effects on infants. However, women who are breast feeding should be advised not to use NuvaRing® but to use other forms of contraception until the child is weaned.
13. PEDIATRIC USE
Safety and efficacy of NuvaRing® have been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under the age of 16 and for users 16 years and older. Use of this product before menarche is not indicated.
14. GERIATRIC USE
This product has not been studied in women over 65 years of age and is not indicated in this population.
15. VAGINAL USE
NuvaRing® may not be suitable for women with conditions that make the vagina more susceptible to vaginal irritation or ulceration. Some women are aware of the ring at random times during the 21 days of use or during intercourse. During intercourse some sexual partners may feel NuvaRing® in the vagina. However, clinical studies revealed that 90% of couples did not find this to be a problem.
If NuvaRing® has been removed or expelled during the three-week use period, it should be rinsed with cool to lukewarm (not hot) water and reinserted as soon as possible, but at the latest within three hours of removal or expulsion. If NuvaRing® is lost, a new vaginal ring should be inserted and the regimen should be continued without alteration. If the ring has been out of the vagina for more than three hours, contraceptive effectiveness may be reduced and an additional method of contraception, such as male condoms or spermicide, must be used until the ring has been used continuously for seven days . NuvaRing® may interfere with the correct placement and position of a diaphragm. A diaphragm is therefore not recommended as a backup method with NuvaRing® use.
16. EXPULSION
NuvaRing® can be accidentally expelled, for example, when it has not been inserted properly, or while removing a tampon, moving the bowels, straining, or with severe constipation. If this occurs, the vaginal ring can be rinsed with cool to lukewarm (not hot) water and re-inserted promptly (see VAGINAL USE above and INFORMATION FOR THE PATIENT , DOSAGE AND ADMINISTRATION ). If NuvaRing® is lost, a new vaginal ring should be inserted and the regimen should be continued without alteration. If the ring has been out of the vagina for more than three hours, contraceptive effectiveness may be reduced and an additional method of contraception, such as male condoms or spermicide, MUST be used until NuvaRing® has been used continuously for seven days. Vaginal stenosis, cervical prolapse, rectoceles, and cystoceles are conditions that under some circumstances may make expulsion more likely to occur.
INFORMATION FOR THE PATIENT
The patient should be instructed regarding the proper use of NuvaRing® (see Patient Information printed below).
ADVERSE REACTIONS
The most common adverse events reported by 5 to 14% of women using NuvaRing® in clinical trials (n=2501) were the following: vaginitis, headache, upper respiratory tract infection, leukorrhea, sinusitis, weight gain, and nausea.
The most frequent system-organ class adverse events leading to discontinuation in 1 to 2.5% of women using NuvaRing® in the trials included the following: device related events (foreign body sensation, coital problems, device expulsion), vaginal symptoms (discomfort/vaginitis/leukorrhea), headache, emotional lability, and weight gain.
Listed below are adverse reactions that have been associated with the use of combination hormonal contraceptives. These are also likely to apply to combination vaginal hormonal contraceptives, such as NuvaRing®.
An increased risk of the following serious adverse reactions has been associated with the use of combination hormonal contraceptives (see CONTRAINDICATIONS and WARNINGS ):
- Thrombophlebitis and venous thrombosis with or without embolism
- Arterial thromboembolism
- Pulmonary embolism
- Myocardial infarction
- Cerebral hemorrhage
- Cerebral thrombosis
- Hypertension
- Gallbladder disease
- Hepatic adenomas or benign liver tumors
There is evidence of an association between the following conditions and the use of combination hormonal contraceptives:
- Mesenteric thrombosis
- Retinal thrombosis
The following additional adverse reactions have been reported in users of combination hormonal contraceptives and are believed to be drug-related:
- Nausea
- Vomiting
- Gastrointestinal symptoms (such as abdominal cramps and bloating)
- Breakthrough bleeding
- Spotting
- Change in menstrual flow
- Amenorrhea
- Temporary infertility after discontinuation of treatment
- Edema
- Melasma which may persist
- Breast changes: tenderness, enlargement, secretion
- Intolerance to contact lenses
- Change in weight (increase or decrease)
- Change in cervical erosion and secretion
- Diminution in lactation when given immediately postpartum
- Cholestatic jaundice
- Migraine
- Rash (allergic)
- Mental depression
- Reduced tolerance to carbohydrates
- Vaginal candidiasis
- Change in corneal curvature (steepening)
The following additional adverse reactions have been reported in users of combination hormonal contraceptives and a causal association has been neither confirmed nor refuted:
- Pre-menstrual syndrome
- Cataracts
- Changes in appetite
- Cystitis-like syndrome
- Headache
- Nervousness
- Dizziness
- Hirsutism
- Loss of scalp hair
- Erythema multiforme
- Erythema nodosum
- Hemorrhagic eruption
- Vaginitis
- Porphyria
- Impaired renal function
- Hemolytic uremic syndrome
- Acne
- Changes in libido
- Colitis
- Budd-Chiari Syndrome
OVERDOSAGE
Overdosage of combination hormonal contraceptives may cause nausea, vomiting, vaginal bleeding, or other menstrual irregularities. Given the nature and design of NuvaRing® it is unlikely that overdosage will occur. If NuvaRing® is broken, it does not release a higher dose of hormones. Serious ill effects have not been reported following acute ingestion of large doses of oral contraceptives by young children. There are no antidotes and further treatment should be symptomatic.
DOSAGE AND ADMINISTRATION
To achieve maximum contraceptive effectiveness, NuvaRing® must be used as directed (see When to Start NuvaRing® below) . One NuvaRing® is inserted in the vagina by the woman herself. The ring is to remain in place continuously for three weeks. It is removed for a one-week break, during which a withdrawal bleed usually occurs. A new ring is inserted one week after the last ring was removed.
The user can choose the insertion position that is most comfortable to her, for example, standing with one leg up, squatting, or lying down. The ring is to be compressed and inserted into the vagina. The exact position of NuvaRing® inside the vagina is not critical for its function. The vaginal ring must be inserted on the appropriate day and left in place for three consecutive weeks. This means that the ring is removed three weeks later on the same day of the week as it was inserted and at about the same time. NuvaRing® can be removed by hooking the index finger under the forward rim or by grasping the rim between the index and middle finger and pulling it out. The used ring should be placed in the sachet (foil pouch) and discarded in a waste receptacle out of the reach of children and pets (do not flush in toilet). After a one-week break, during which a withdrawal bleed usually occurs, a new ring is inserted on the same day of the week as it was inserted in the previous cycle. The withdrawal bleed usually starts on day 2-3 after removal of the ring and may not have finished before the next ring is inserted. In order to maintain contraceptive effectiveness, the new ring must be inserted one week after the previous one was removed even if menstrual bleeding has not finished.
When to Start NuvaRing®
IMPORTANT: The possibility of ovulation and conception prior to the first use of NuvaRing® should be considered.
No preceding hormonal contraceptive use in the past month
Counting the first day of menstruation as "Day 1". NuvaRing® should be inserted on or prior to Day 5 of the cycle, even if the patient has not finished bleeding. During the first cycle, an additional method of contraception, such as male condoms or spermicides, is recommended until after the first seven days of continuous ring use.
Switching from a combination oral contraceptive
NuvaRing® may be inserted anytime within seven days after the last combined (estrogen plus progestin) oral contraceptive tablet and no later than the day that a new cycle of pills would have been started. No backup method is needed.
Switching from a progestin-only method
There are several types of progestin-only methods. Women should insert the first NuvaRing® as follows:
- Any day of the month when switching from a progestin-only pill; do not skip any days between the last pill and the first day of NuvaRing® use
- On the same day as contraceptive implant removal
- On the same day as removal of a progestin-containing IUD, or
- On the day when the next contraceptive injection would be due
In all of these cases, the patient should be advised to use an additional method of contraception, such as male condoms or spermicide, for the first seven days after insertion of the ring.
Following complete first trimester abortion
The patient may start using NuvaRing® within the first five days following a complete first trimester abortion and does not need to use an additional method of contraception. If use of NuvaRing® is not started within five days following a first trimester abortion, the patient should follow the instructions for "No preceding hormonal contraceptive use in the past month." In the meantime she should be advised to use a non-hormonal contraceptive method.
Following delivery or second trimester abortion
The use of NuvaRing® for contraception may be initiated four weeks postpartum in women who elect not to breast feed. Women who are breast feeding should be advised not to use NuvaRing® but to use other forms of contraception until the child is weaned. NuvaRing® use may be initiated four weeks after a second trimester abortion. When NuvaRing® is used postpartum or postabortion, the increased risk of thromboembolic disease must be considered. (See CONTRAINDICATIONS and WARNINGS concerning thromboembolic disease. See PRECAUTIONS for "Nursing Mothers".) If the patient begins using NuvaRing® postpartum, and has not yet had a period, the possibility of ovulation and conception occurring prior to initiation of NuvaRing® should be considered, and she should be instructed to use an additional method of contraception, such as male condoms or spermicide, for the first seven days.
Deviations from the Recommended Regimen
To prevent loss of contraceptive efficacy patients should not deviate from the recommended regimen.
Inadvertent removal, expulsion, or prolonged ring-free interval
If NuvaRing® has been out of the vagina during the three-week use period, it can be rinsed with cool to lukewarm (not hot) water and should be reinserted as soon as possible, at the latest within three hours. If the ring has been out of the vagina for more than three hours, contraceptive effectiveness may be reduced and an additional method of contraception, such as male condoms or spermicide, MUST be used until NuvaRing® has been used continuously for seven days.
If the ring-free interval has been extended beyond one week, the possibility of pregnancy should be considered, and an additional method of contraception, such as male condoms or spermicide, MUST be used until NuvaRing® has been used continuously for seven days.
Prolonged Use of NuvaRing®
If NuvaRing® has been left in place for up to one extra week (i.e., up to four weeks total), it should be removed and the patient should insert a new ring after the one-week ring-free interval. The mean serum etonogestrel concentration during the fourth week of continuous use of NuvaRing® was 1272 ± 311 pg/mL compared to a mean concentration range of 1578 ± 408 to 1374 ± 328 pg/mL during weeks one to three. The mean serum ethinyl estradiol concentration during the fourth week of continuous use of NuvaRing® was 16.8 ± 4.6 pg/mL compared to a mean concentration range of 19.1 ± 4.5 to 17.6 ± 4.3 pg/mL during weeks one to three. If NuvaRing® has been left in place for longer than four weeks, pregnancy should be ruled out, and an additional method of contraception, such as male condoms or spermicide, MUST be used until a new NuvaRing® has been used continuously for seven days.
In the event of a missed menstrual period
- If the patient has not adhered to the prescribed regimen (NuvaRing® has been out of the vagina for more than three hours or the preceding ring-free interval was extended beyond one week) the possibility of pregnancy should be considered at the time of the first missed period and NuvaRing® use should be discontinued if pregnancy is confirmed.
- If the patient has adhered to the prescribed regimen and misses two consecutive periods, pregnancy should be ruled out.
- If the patient has retained one NuvaRing® for longer than four weeks, pregnancy should be ruled out.
HOW SUPPLIED
Each NuvaRing® (etonogestrel/ethinyl estradiol vaginal ring) is individually packaged in a reclosable aluminum laminate sachet consisting of three layers, from outside to inside: polyester, aluminum foil, and low-density polyethylene. The ring should be replaced in this reclosable sachet after use for convenient disposal.
Box of 3 sachets NDC 0052-0273-03
Box of 1 sachet NDC 0052-0273-01
Storage
Prior to dispensing to the user, store refrigerated 2-8°C (36-46°F). After dispensing to the user, NuvaRing® can be stored for up to 4 months at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Avoid storing NuvaRing® in direct sunlight or at temperatures above 30°C (86°F). For the Dispenser: When NuvaRing® is dispensed to the user, place an expiration date on the label. The date should not exceed either 4 months from the date of dispensing or the expiration date, whichever comes first.
Rx only
REFERENCES FURNISHED UPON REQUEST
PATIENT INFORMATION
NuvaRing® (etonogestrel/ethinyl estradiol vaginal ring)
Rx only
Read this leaflet carefully before you use NuvaRing® so that you understand the benefits and risks of using this form of birth control. The leaflet gives you information about the possible serious side effects of NuvaRing®. This leaflet will also tell you how to use NuvaRing® properly so that it will give you the best possible protection against pregnancy. Read the information you get whenever you get a new prescription or refill, because there may be new information. This information does not take the place of talking with your healthcare provider.
What is NuvaRing®?
NuvaRing® (NEW-vah-ring) is a flexible combined contraceptive vaginal ring. It is used to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases (STD's) such as chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
NuvaRing® contains a combination of a progestin and estrogen, two kinds of female hormones. You insert the ring in your vagina and leave it there for three weeks. You then remove it for a one-week ring-free period. After the ring is inserted, it releases a continuous low dose of hormones into your body.
Contraceptives that contain both an estrogen and a progestin are called combination hormonal contraceptives. Most studies on combination contraceptives have used oral (taken by mouth) contraceptives. NuvaRing® may have the same risks that have been found for combination oral contraceptives. This leaflet will tell you about risks of taking combination oral contraceptives that may also apply to NuvaRing® users. In addition it will tell you how to use NuvaRing® properly so that it willl give you the best possible protection against pregnancy.
Who should not use NuvaRing®?
Cigarette smoking increases the risk of serious cardiovascular side effects when you use combination oral contraceptives. This risk increases even more if you are over age 35 and if you smoke 15 or more cigarettes a day. Women who use combination hormonal contraceptives, including NuvaRing®, are strongly advised not to smoke. Do not use NuvaRing® if you have any of the following conditions:
- pregnancy or suspected pregnancy
- blood clots in your legs (thrombosis), lungs (pulmonary embolism), or eyes now or in the past
- chest pain (angina pectoris)
- heart attack or stroke
- severe high blood pressure
- diabetes with complications of the kidneys, eyes, nerves, or blood vessels
- headaches with neurological symptoms
- known or suspected breast cancer or cancer of the lining of the uterus, cervix, or vagina (now or in the past)
- unexplained vaginal bleeding
- yellowing of the whites of the eyes or of the skin (jaundice) during pregnancy or during past use of oral contraceptives (birth control pills)
- liver tumors or active liver disease
- disease of the heart valves with complications
- need for a long period of bedrest following major surgery
- an allergic reaction to any of the components of NuvaRing®
Tell your healthcare provider if you have ever had any of the conditions just listed. Your healthcare provider can suggest another method of birth control.
Talk with your healthcare provider about when to start NuvaRing® if you are recovering from the birth of a child or a second trimester miscarriage or abortion or if you are breast feeding.
In addition, talk to your healthcare provider about using NuvaRing® if you have any of the following conditions. Women with any of these conditions should be checked often by their doctor or healthcare provider if they choose to use NuvaRing®.
- a family history of breast cancer
- breast nodules, fibrocystic disease, an abnormal breast x-ray, or abnormal mammogram
- diabetes
- high blood pressure
- high cholesterol or triglycerides
- headaches or epilepsy
- mental depression
- gallbladder or kidney disease
- major surgery (You may need to stop using NuvaRing® for a while to reduce your chance of getting blood clots.)
- any condition that makes the vagina get irritated easily
- prolapsed (dropped) uterus, dropped bladder (cystocele), or rectal prolapse (rectocele)
- severe constipation
How should I use NuvaRing®?
For the best protection from pregnancy, use NuvaRing® exactly as directed. Insert one NuvaRing® in the vagina and keep it in place for three weeks in a row. Remove it for a one-week break and then insert a new ring. During the one-week break, you will usually have your menstrual period. Your healthcare provider should examine you at least once a year to see if there are any signs of side effects of NuvaRing® use.
When should I start NuvaRing®?
Follow the instructions in one of the sections below to find out when to start using NuvaRing®.
If you did not use a hormonal contraceptive in the past month
Counting the first day of your menstrual period as "Day 1", insert your first NuvaRing® between Day 1 and Day 5 of the cycle, but at the latest on Day 5, even if you have not finished bleeding. During this first cycle, use an extra method of birth control, such as male condoms or spermicide, for the first seven days of ring use.
If you are switching from a combination oral contraceptive (birth control pill containing both progestin and estrogen)
Insert NuvaRing® anytime during the first seven days after the last combined (estrogen and progestin) oral contraceptive tablet and no later than the day when you would have started a new pill cycle. No extra birth control method is needed.
If you are switching from a progestin-only contraceptive (mini-pill, implant, injection, or IUD)
- When switching from a mini-pill, start using NuvaRing® on any day of the month. Do not skip days between your last pill and first day of NuvaRing® use.
- When switching from an implant, start using NuvaRing® on the same day you have your implant removed.
- When switching from an injectable contraceptive, start using NuvaRing® on the day when your next injection is due.
- When switching from a progestin-containing IUD, start using NuvaRing® on the same day you have your IUD removed.
When you are switching from a progestin-only contraceptive, use an extra method of birth control, such as male condoms or spermicide, for the first seven days after inserting NuvaRing®.
Following first trimester abortion or miscarriage
If you start using NuvaRing® within five days after a complete first trimester abortion or miscarriage, you do not need to use an extra method of contraception.
If NuvaRing® is not started within five days after a first trimester abortion or miscarriage, begin NuvaRing® at the time of your next menstrual period. Counting the first day of your menstrual period as "Day 1", insert NuvaRing® on or before Day 5 of the cycle, even if you have not finished bleeding. During this first cycle, use an extra method of birth control, such as male condoms or spermicide, for the first seven days of ring use.
How do I insert NuvaRing®?
-
Each NuvaRing® comes in a reclosable foil pouch. After washing and drying your hands, remove NuvaRing® from its foil pouch. Keep the foil pouch for proper disposal of the ring after use. Choose the position that is most comfortable for you. For example, lying down, squatting, or standing with one leg up (Figures 1a, 1b, and 1c, respectively).
-
Hold NuvaRing® between your thumb and index finger (Figure 2a) and press the opposite sides of the ring together (Figure 2b).
-
Gently push the folded ring into your vagina (Figures 3a, 3b, and 3c). The exact position of NuvaRing® in the vagina is not important for it to work.
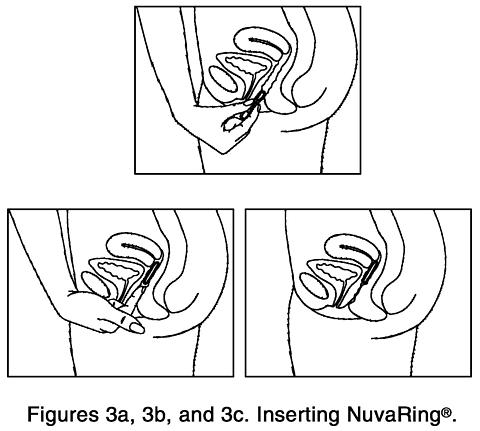
Although some women may be aware of NuvaRing® in the vagina, most women do not feel it once it is in place. If you feel discomfort, NuvaRing® if probably not inserted back far enough in the vagina. Use your finger to gently push NuvaRing® further into your vagina. There is no danger of NuvaRing® being pushed too far up in the vagina or getting lost. NuvaRing® can be inserted only as far as the end of the vagina, where the cervix (the narrow, lower end of the uterus) will block NuvaRing® from going any further. - Once inserted, keep NuvaRing® in place for three weeks in a row.
How do I remove NuvaRing®?
-
Remove the ring three weeks after insertion on the same day of the week as it was inserted, at about the same time. For example, when NuvaRing® is inserted on a Sunday at about 10:00 PM, the ring should be removed on the Sunday three weeks later at about 10:00 PM.
You can remove NuvaRing® by hooking the index finger under the forward rim or by holding the rim between the index and middle finger and pulling it out. -
Place the used ring in the foil pouch and properly dispose of it in a waste receptacle out of the reach of children and pets. Do not throw it in the toilet.
Your menstrual period will usually start two to three days after the ring is removed and may not have finished before the next ring is inserted. To continue to have pregnancy protection, you must insert the new ring one week after the last one was removed, even if your menstrual period has not stopped.
When do I insert a new ring?
After a one-week ring-free break, insert a new ring on the same day of the week as it was inserted in the last cycle. For example, if NuvaRing® was inserted on a Sunday at about 10:00 PM, after the one-week break you should insert a new ring on a Sunday at about 10:00 PM.
If NuvaRing® slips out:
Rarely, NuvaRing® can slip out of the vagina if it has not been inserted properly, or while removing a tampon, moving the bowels, straining, or with severe constipation.
If NuvaRing® slips out of the vagina, and it has been out less than three hours, you should still be protected from pregnancy. NuvaRing® can be rinsed with cool to lukewarm (not hot) water and should be reinserted as soon as possible, and at the latest within three hours. If you have lost NuvaRing®, you must insert a new NuvaRing® and use it on the same schedule as you would have used the lost ring. If NuvaRing® has been out of the vagina for more than three hours, you may not be adequately protected from pregnancy. NuvaRing® can be rinsed with cool to lukewarm (not hot) water and reinserted as soon as possible. You must use an extra method of birth control, such as male condoms or spermicide, until the NuvaRing® has been in place for seven days in a row.
Women with conditions affecting the vagina, such as prolapsed (dropped) uterus, may be more likely to have NuvaRing® slip out of the vagina. If NuvaRing® slips out repeatedly, you should consult with your healthcare provider.
If NuvaRing® is in your vagina too long:
If NuvaRing® has been left in your vagina for an extra week or less (four weeks total or less), remove it and insert a new ring after a one-week ring-free break.
If NuvaRing® has been left in place for more than four weeks, you may not be adequately protected from pregnancy and you must check to be sure you are not pregnant. You must use an extra method of birth control, such as male condoms or spermicide, until the new NuvaRing® has been in place for seven days in a row.
If you miss a menstrual period:
You must check to be sure that you are not pregnant if:
- you miss a period and NuvaRing® was out of the vagina for more than three hours during the three weeks of ring use
- you miss a period and you had waited longer than one week to insert a new ring
- you have followed the instructions and you miss two periods in a row
- you have left NuvaRing® in place for longer than four weeks
Overdose
NuvaRing® is unlikely to cause an overdose because the ring holding the medicine releases a steady amount of contraceptive hormones. Do not use more than one ring at a time. Overdose of combination hormonal contraceptives may cause nausea, vomiting, or vaginal bleeding.
What should I avoid while using NuvaRing®?
- Smoking may increase your risk of heart attack or stroke while using combination hormonal contraceptives, including NuvaRing®. The risk increases with age and number of cigarettes smoked a day.
Cigarette smoking increases the risk of serious cardiovascular side effects when you use combination oral contraceptives. This risk increases even more if you are over age 35 and if you smoke 15 or more cigarettes a day. Women who use combination hormonal contraceptives, like NuvaRing®, are strongly advised not to smoke. Do not breast feed while using NuvaRing®. Some of the medicine may pass through the milk to the baby and could cause yellowing of the skin (jaundice) and breast enlargement. NuvaRing® could also decrease the amount and quality of your breast milk.
The hormones in NuvaRing® can interact with many other medicines and herbal supplements. Tell your healthcare provider about any medicines you are taking, including prescription medicines, over-the-counter medicines, herbal remedies, and vitamins.
The blood levels of the hormones released by NuvaRing® were increased when women used an oil-based vaginal medication (miconazole nitrate) for a yeast infection while NuvaRing® was in place. The pregnancy protection of NuvaRing® is not likely to be changed by use of these products. The blood levels of the hormones released by NuvaRing® were not changed when women used vaginal, water-based spermicides (nonoxynol or N-9 products) along with NuvaRing®.
While using NuvaRing®, you should not rely upon a diaphragm when you need a backup method of birth control because NuvaRing® may interfere with the correct placement and position of a diaphragm.
If you are scheduled for any laboratory tests, tell your doctor or healthcare provider you are using NuvaRing®. Contraceptive hormones may change certain blood tests results.
What are the possible risks and side effects of NuvaRing®?
-
Blood clots
The hormones in NuvaRing® may cause changes in your blood clotting system which may allow your blood to clot more easily. If blood clots form in your legs, they can travel to the lungs and cause a sudden blockage of a vessel carrying blood to the lungs. Rarely, clots occur in the blood vessels of the eye and may cause blindness, double vision, or other vision problems. The risk of getting blood clots may be greater with the type of progestin in NuvaRing® than with some other progestins in certain low-dose birth control pills. It is unknown if the risk of blood clots is different with NuvaRing® use than with the use of certain birth control pills. -
Heart attacks and strokes
Hormonal contraceptives may increase your risk of strokes (blockage of blood flow to the brain) or heart attacks (blockage of blood flow to the heart). Any of these conditions can cause death or serious disability. Smoking greatly increases the risk of having heart attacks and strokes. Furthermore, smoking and the use of combination hormonal contraceptives, like NuvaRing®, greatly increases the chances of developing and dying of heart disease. If you use combination hormonal contraceptives, including NuvaRing®, you should not smoke. -
High blood pressure and heart disease
Combination hormonal contraceptives, including NuvaRing®, can worsen conditions like high blood pressure, diabetes, and problems with cholesterol and triglycerides. -
Cancer of the breast
Various studies give conflicting reports on the relationship between breast cancer and hormone contraceptive use. Combination hormonal contraceptives, including NuvaRing®, may slightly increase your chance of having breast cancer diagnosed. After you stop using hormonal contraceptives, the chance of having breast cancer diagnosed begins to go back down. You should have regular breast examinations by a healthcare provider and examine your own breasts monthly. Tell your healthcare provider if you have a family history of breast cancer or if you have had breast nodules or an abnormal mammogram. -
Gallbladder disease
Combination hormonal contraceptive users may have a higher chance of having gallbladder disease. -
Liver tumors
In rare cases, combination hormonal contraceptives, like NuvaRing®, can cause non-cancerous (benign) but dangerous liver tumors. These benign liver tumors can break and cause fatal internal bleeding. In addition, it is possible that women who use combination hormonal contraceptives, like NuvaRing®, have a higher chance of getting liver cancer. However, liver cancers are extremely rare.
The common side effects reported by NuvaRing® users are:
- vaginal infections and irritation
- vaginal discharge (leukorrhea)
- headache
- weight gain
- nausea
In addition to the risks and side effects listed above, users of combination hormonal contraceptives have reported the following side effects:
- vomiting
- change in appetite
- abdominal cramps and bloating
- breast tenderness or enlargement
- irregular vaginal bleeding or spotting
- changes in menstrual cycle
- temporary infertility after treatment
- fluid retention (edema)
- spotty darkening of the skin, particularly on the face
- rash
- weight changes
- depression
- intolerance to contact lenses
Call your healthcare provider right away if you get any of the symptoms listed below. They may be signs of a serious problem:
- sharp chest pain, coughing blood, or sudden shortness of breath (possible clot in the lung)
- pain in the calf (back of lower leg; possible clot in the leg)
- crushing chest pain or heaviness in the chest (possible heart attack)
- sudden severe headache or vomiting, dizziness or fainting, problems with vision or speech, weakness, or numbness in an arm or leg (possible stroke)
- sudden partial or complete loss of vision (possible clot in the eye)
- yellowing of the skin or whites of the eyes (jaundice), especially with fever, tiredness, loss of appetite, dark colored urine, or light colored bowel movements (possible liver problems)
- severe pain, swelling or tenderness in the abdomen (gallbladder or liver problems)
- breast lumps (possible breast cancer or benign breast disease)
- irregular vaginal bleeding or spotting that happens in more than one menstrual cycle or lasts for more than a few days
- swelling (edema) of your fingers or ankles
- difficulty in sleeping, weakness, lack of energy, fatigue, or a change in mood (possible severe depression)
How effective is NuvaRing®?
If NuvaRing® is used according to the directions, your chance of getting pregnant is about 1 to 2% a year. This means that, for every 100 women who use NuvaRing® for a year, about one or two will become pregnant. Your chance of getting pregnant increases if NuvaRing® is not used exactly according to the directions.
By comparison, the chances of getting pregnant in the first year of typical use (not always following directions exactly) of other methods of birth control are as follows:
No birth control method:85%Spermicides alone:26%Periodic abstinence methods(calendar, ovulation, thermometer):25%Withdrawal:19%Cervical Cap with spermicides:20 to 40%Vaginal sponge:20 to 40%Diaphragm with spermicides:20%Condom alone (male):14%Condom alone (female):21%Oral contraceptives:5%IUD:less than 1 to 2%Implants:less than 1%Injection:less than 1%Sterilization:less than 1%Other information
- Place the used ring in the reclosable foil pouch and properly dispose of it in a waste receptacle out of the reach of children and pets.
- Store NuvaRing® at room temperature, 25°C (77°F). Temperatures can be from 59-86°F (15-30°C). Avoid direct sunlight or storing above 86°F (30°C).
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use NuvaRing® for a condition for which it was not prescribed. Do not give NuvaRing® to anyone else who may want to use it.
This leaflet summarizes the most important information about NuvaRing®. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NuvaRing® that is written for health professionals.
ORGANON
Manufactured for Organon Usa Inc.
West Orange, NJ 07052
by N.V. Organon, Oss, The Netherlands
©2004 Organon Usa Inc. 5310220 10/04 13
Subscribe to the "News" RSS Feed 
TOP ۞
