-
Ortho Evra Transdermal System (Ortho Women's Health & Urology)
Prescribing Information
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Rx only
DESCRIPTION
ORTHO EVRA® is a combination transdermal contraceptive patch with a contact surface area of 20 cm 2 . It contains 6.00 mg norelgestromin and 0.75 mg ethinyl estradiol (EE), and releases 150 micrograms of norelgestromin and 20 micrograms of EE to the bloodstream per 24 hours. This level of transdermal release of EE results in exposure to EE greater than that produced by an oral contraceptive product containing 20 micrograms of EE (See Pharmacokinetics , Absorption ).
ORTHO EVRA® is a thin, matrix-type transdermal contraceptive patch consisting of three layers. The backing layer is composed of a beige flexible film consisting of a low-density pigmented polyethylene outer layer and a polyester inner layer. It provides structural support and protects the middle adhesive layer from the environment. The middle layer contains polyisobutylene/polybutene adhesive, crospovidone, non-woven polyester fabric and lauryl lactate as inactive components. The active components in this layer are the hormones, norelgestromin and ethinyl estradiol. The third layer is the release liner , which protects the adhesive layer during storage and is removed just prior to application. It is a transparent polyethylene terephthalate (PET) film with a polydimethylsiloxane coating on the side that is in contact with the middle adhesive layer.
The outside of the backing layer is heat-stamped "ORTHO EVRA® 150/20."
The structural formulas of the components are:
Molecular weight, norelgestromin: 327.47
Molecular weight, ethinyl estradiol: 296.41
Chemical name for norelgestromin: 18, 19-dinorpregn-4-en-20-yn-3-one, 13-ethyl- 17-hydroxy-, 3-oxime, (17(alpha))
Chemical name for ethinyl estradiol: 19-Norpregna-1, 3, 5 (10)-trien-20-yne-3, 17-diol, (17(alpha))
CLINICAL PHARMACOLOGY
Pharmacodynamics
Norelgestromin is the active progestin largely responsible for the progestational activity that occurs in women following application of ORTHO EVRA®. Norelgestromin is also the primary active metabolite produced following oral administration of norgestimate (NGM), the progestin component of the oral contraceptive products ORTHO-CYCLEN® and ORTHO TRI-CYCLEN®.
Combination oral contraceptives act by suppression of go-nadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
Receptor and human sex hormone-binding globulin (SHBG) binding studies, as well as studies in animals and humans, have shown that both norgestimate and norelgestromin exhibit high progestational activity with minimal intrinsic androgenicity 90-93 . Transdermally-administered norelgestromin, in combination with ethinyl estradiol, does not counteract the estrogen-induced increases in SHBG, resulting in lower levels of free testosterone in serum compared to baseline.
Pharmacokinetic studies with ORTHO EVRA® demonstrated consistent elimination kinetics for norelgestromin and EE with half-life values of approximately 28 hours and 17 hours, respectively. One clinical trial assessed the return of hypothalamic-pituitary-ovarian axis function post-therapy and found that FSH, LH, and Estradiol mean values, though suppressed during therapy, returned to near baseline values during the 6 weeks post therapy.
Pharmacokinetics
Absorption
Following application of ORTHO EVRA®, both norelgestromin and EE rapidly appear in the serum, reach a plateau by approximately 48 hours, and are maintained at an approximate steady-state throughout the wear period. C SS concentrations for norelgestromin and EE during one week of patch wear are approximately 0.6-0.8 ng/ml and 40-50 pg/ml, respectively, and are generally consistent from all studies and application sites. These C SS concentrations are within the reference ranges for norelgestromin (0.6 to 1.2 ng/ml) and EE (25 to 75 pg/ml) established based upon the C ave concentrations observed with subjects taking ORTHO-CYCLEN®.
Daily absorption of norelgestromin and EE from ORTHO EVRA® was determined by comparison to an intravenous infusion of norelgestromin and EE. The results indicated that the average dose of norelgestromin and EE absorbed into the systemic circulation is 150 mcg/day and 20 mcg/day, respectively. This level of transdermal release of EE results in exposure to EE greater than that produced by an oral contraceptive product containing 20 micrograms of EE.
The absorption of norelgestromin and EE following application of ORTHO EVRA® to the abdomen, buttock, upper outer arm and upper torso (excluding breast) was evaluated in a cross-over design study. The results of this study indicated that C SS and AUC for the buttock, upper arm and torso for each analyte were equivalent. While C SS values for the abdomen were within reference ranges for EE 35 mcg/NGM 250 mcg oral contraceptive users, exposure to the drugs was lower and strict bioequivalence requirements for AUC were not met in this study. However, in a separate parallel group multiple application pharmacokinetic study, C SS and AUC for the buttock and abdomen were not statistically different. Therefore, all four sites may be considered therapeutically equivalent.
The absorption of norelgestromin and EE following application of ORTHO EVRA® was studied under conditions encountered in a health club (sauna, whirlpool and treadmill) and in a cold water bath. The results indicated that for norelgestromin there were no significant treatment effects on C SS or AUC when compared to normal wear. For EE, slight increases were observed due to sauna, whirlpool and treadmill, however, the C SS values following these treatments were within the reference range. There was no significant effect of cold water on these parameters.
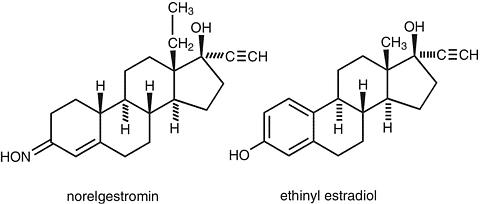
In multiple dose studies, C SS and AUC for norelgestromin and EE were found to increase slightly over time when compared to Week 1 of Cycle 1. In a three-cycle study, these pharmacokinetic parameters reached steady-state conditions during all three weeks of Cycle 3. (See Table 1, Figures 1 and 2).
Table 1: Mean (SD) Pharmacokinetic Parameters of Norelgestromin and EE Following 3 Consecutive Cycles of ORTHO EVRA® Wear on the ButtockAnalyteParameterCycle 1
Week 1Cycle 3
Week 1Cycle 3
Week 2Cycle 3
Week 3NorelgestrominC ss a0.70 (0.28)0.70 (0.29)0.80 (0.23)0.70 (0.32)AUC 0-168 b107 (44.2)105 (45.5)132 (57.1)120 (52.8)t ½ cncncnc32.1 (12.9)EEC ss d46.4 (17.9)47.6 (17.3)59.0 (25.1)49.6 (27.0)AUC 0-168 e6796 (2673)7160 (2893)10054 (4205)8840 (5176)t ½ cncncnc21.0 (9.07)a ng/mLb ng·h/mLc hd pg/mLe pg·h/mLnc = not calculated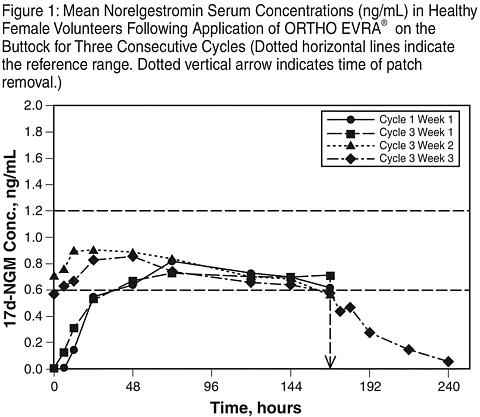
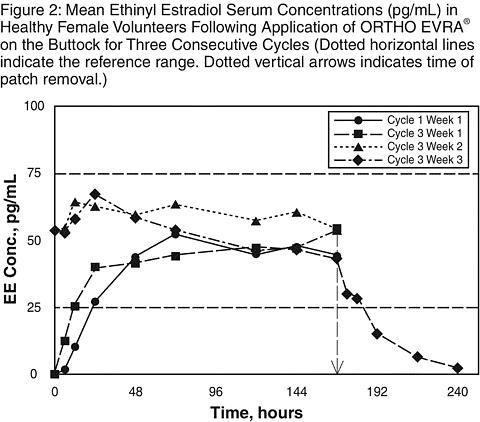
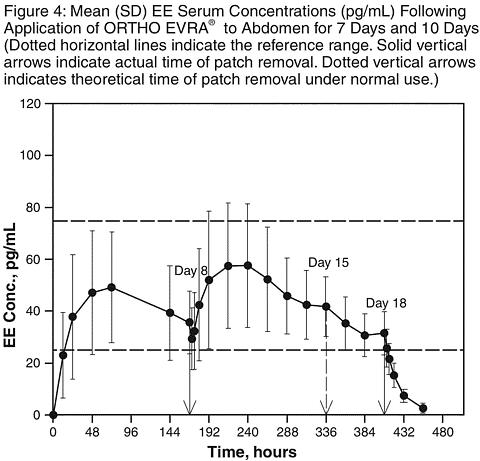
Results from a study of consecutive ORTHO EVRA® wear for 7 days and 10 days indicated that serum concentrations of norelgestromin and EE dropped slightly during the first 6 hours after the patch replacement, still stayed within the reference range and recovered within 12 hours. Target C ss of norelgestromin and EE were maintained during 2 days of extended wear of ORTHO EVRA®.
Metabolism
Since ORTHO EVRA® is applied transdermally, first-pass metabolism (via the gastrointestinal tract and/or liver) of norelgestromin and EE that would be expected with oral administration is avoided. Hepatic metabolism of norelgestromin occurs and metabolites include norgestrel, which is highly bound to SHBG, and various hydroxylated and conjugated metabolites. Ethinyl estradiol is also metabolized to various hydroxylated products and their glucuronide and sulfate conjugates.
Distribution
Norelgestromin and norgestrel (a serum metabolite of norelgestromin) are highly bound (>97%) to serum proteins. Norelgestromin is bound to albumin and not to SHBG, while norgestrel is bound primarily to SHBG, which limits its biological activity. Ethinyl estradiol is extensively bound to serum albumin.
Elimination
Following removal of patches, the elimination kinetics of norelgestromin and EE were consistent for all studies with half-life values of approximately 28 hours and 17 hours, respectively. The metabolites of norelgestromin and EE are eliminated by renal and fecal pathways.
Special Populations
Effects of Age, Body Weight, Body Surface Area and Race: The effects of age, body weight, body surface area and race on the pharmacokinetics of norelgestromin and EE were evaluated in 230 healthy women from nine pharmacokinetic studies of single 7-day applications of ORTHO EVRA®. For both norelgestromin and EE, increasing age, body weight and body surface area each were associated with slight decreases in C ss and AUC values. However, only a small fraction (10-25%) of the overall variability in the pharmacokinetics of norelgestromin and EE following application of ORTHO EVRA® may be associated with any or all of the above demographic parameters. There was no significant effect of race with respect to Caucasians, Hispanics and Blacks.
Renal and Hepatic Impairment:
No formal studies were conducted with ORTHO EVRA® to evaluate the pharmacokinetics, safety, and efficacy in women with renal or hepatic impairment. Steroid hormones may be poorly metabolized in patients with impaired liver function (see PRECAUTIONS ).
Drug Interactions
The metabolism of hormonal contraceptives may be influenced by various drugs. Of potential clinical importance are drugs that cause the induction of enzymes that are responsible for the degradation of estrogens and progestins, and drugs that interrupt entero-hepatic recirculation of estrogen (e.g. certain antibiotics) 72 .
The proposed mechanism of interaction of antibiotics is different from that of liver enzyme-inducing drugs. Literature suggests possible interactions with the concomitant use of hormonal contraceptives and ampicillin or tetracycline. In a pharmacokinetic drug interaction study, oral administration of tetracycline HCl, 500 mg q.i.d. for 3 days prior to and 7 days during wear of ORTHO EVRA® did not significantly affect the pharmacokinetics of norelgestromin or EE.
The major target for enzyme inducers is the hepatic microsomal estrogen-2-hydroxylase (cytochrome P450 3A4) 99 . See also PRECAUTIONS , Drug Interactions .
Patch Adhesion
In the clinical trials with ORTHO EVRA®, approximately 2% of the cumulative number of patches completely detached. The proportion of subjects with at least 1 patch that completely detached ranged from 2% to 6%, with a reduction from Cycle 1 (6%) to Cycle 13 (2%). For instructions on how to manage detachment of patches, refer to the DOSAGE AND ADMINISTRATION section.
INDICATIONS AND USAGE
ORTHO EVRA® is indicated for the prevention of pregnancy.
Like oral contraceptives, ORTHO EVRA® is highly effective if used as recommended in this label.
In 3 large clinical trials in North America, Europe and South Africa, 3,330 women (ages 18-45) completed 22,155 cycles of ORTHO EVRA® use, pregnancy rates were approximately 1 per 100 women-years of ORTHO EVRA® use. The racial distribution was 91% Caucasian, 4.9% Black, 1.6% Asian, and 2.4% Other.
With respect to weight, 5 of the 15 pregnancies reported with ORTHO EVRA® use were among women with a baseline body weight >/=198 lbs. (90kg), which constituted <3% of the study population. The greater proportion of pregnancies among women at or above 198 lbs. was statistically significant and suggests that ORTHO EVRA® may be less effective in these women.
Health Care Professionals who consider ORTHO EVRA® for women at or above 198 lbs. should discuss the patient's individual needs in choosing the most appropriate contraceptive option.
Table 2 lists the accidental pregnancy rates for users of various methods of contraception. The efficacy of these contraceptive methods, except sterilization, IUD, and Norplant depends upon the reliability with which they are used. Correct and consistent use of methods can result in lower failure rates.
Table 2: Percentage of Women Experiencing an Unintended Pregnancy During the First Year of Typical Use and the First Year of Perfect Use of Contraception and the Percentage Continuing Use at the End of the First Year. United States.% of Women Experiencing an Unintended Pregnancy
within the First Year of Use% of Women
Continuing Use
at One Year 3Method
(1)Typical Use 1
(2)Perfect Use 2
(3)(4) Chance 485 85 Spermicides 526 6 40 Periodic abstinence25 63 Calendar9 Ovulation Method3 Sympto-Thermal 62 Post-Ovulation1 Cap 7Parous Women40 26 42 Nulliparous Women20 9 56 SpongeParous Women40 20 42 Nulliparous Women20 9 56 Diaphragm 720 6 56 Withdrawal19 4 Condom 8Female (Reality)21 5 56 Male14 3 61 Pill5 71 Progestin Only0.5 Combined0.1 IUDProgesterone T2.0 1.5 81 Copper T380A0.8 0.6 78 LNg 200.1 0.1 81 Depo-Provera0.3 0.3 70 Norplant and Norplant-20.05 0.05 88 Female Sterilization0.5 0.5 100 Male Sterilization0.15 0.10 100 Hatcher et al, 1998, Ref. # 1.Emergency Contraceptive Pills: Treatment initiated within 72 hours after unprotected intercourse reduces the risk of pregnancy by at least 75%. 9Lactational Amenorrhea Method: LAM is highly effective, temporary method of contraception. 10Source: Trussell J, Contraceptive efficacy. In Hatcher RA, Trussell J, Stewart F, Cates W, Stewart GK, Kowal D, Guest F, Contraceptive Technology: Seventeenth Revised Edition. New York NY: Irvington Publishers, 1998.1 Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.2 Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.3 Among couples attempting to avoid pregnancy, the percentage who continue to use a method for one year.4 The percents becoming pregnant in columns (2) and (3) are based on data from populations where contraception is not used and from women who cease using contraception in order to become pregnant. Among such populations, about 89% become pregnant within one year. This estimate was lowered slightly (to 85%) to represent the percent who would become pregnant within one year among women now relying on reversible methods of contraception if they abandoned contraception altogether.5 Foams, creams, gels, vaginal suppositories, and vaginal film.6 Cervical mucus (ovulation) method supplemented by calendar in the pre-ovulatory and basal body temperature in the post-ovulatory phases.7 With spermicidal cream or jelly.8 Without spermicides.9 The treatment schedule is one dose within 72 hours after unprotected intercourse, and a second dose 12 hours after the first dose. The Food and Drug Administration has declared the following brands of oral contraceptives to be safe and effective for emergency contraception: Ovral (1 dose is 2 white pills), Alesse (1 dose is 5 pink pills), Nordette or Levlen (1 dose is 2 light-orange pills), Lo/Ovral (1 dose is 4 white pills), Triphasil or Tri-Levlen (1 dose is 4 yellow pills).10 However, to maintain effective protection against pregnancy, another method of contraception must be used as soon as menstruation resumes, the frequency or duration of breastfeeds is reduced, bottle feeds are introduced, or the baby reaches six months of age.
ORTHO EVRA® has not been studied for and is not indicated for use in emergency contraception.
CONTRAINDICATIONS
ORTHO EVRA® should not be used in women who currently have the following conditions:
- Thrombophlebitis, thromboembolic disorders
- A past history of deep vein thrombophlebitis or thromboembolic disorders
- Cerebrovascular or coronary artery disease (current or past history)
- Valvular heart disease with complications 103
- Severe hypertension 103
- Diabetes with vascular involvement 103
- Headaches with focal neurological symptoms
- Major surgery with prolonged immobilization
- Known or suspected carcinoma of the breast or personal history of breast cancer
- Carcinoma of the endometrium or other known or suspected estrogen-dependent neoplasia
- Undiagnosed abnormal genital bleeding
- Cholestatic jaundice of pregnancy or jaundice with prior hormonal contraceptive use
- Acute or chronic hepatocellular disease with abnormal liver function 103
- Hepatic adenomas or carcinomas
- Known or suspected pregnancy
- Hypersensitivity to any component of this product
WARNINGS
Cigarette smoking increases the risk of serious cardiovascular side effects from hormonal contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use hormonal contraceptives, including ORTHO EVRA®, should be strongly advised not to smoke. ORTHO EVRA® and other contraceptives that contain both an estrogen and a progestin are called combination hormonal contraceptives. There is no epidemiologic data available to determine whether safety and efficacy with the transdermal route of administration would be different than the oral route. Practitioners prescribing ORTHO EVRA® should be familiar with the following information relating to risks.
The use of combination hormonal contraceptives is associated with increased risks of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, obesity and diabetes.
The information contained in this package insert is principally based on studies carried out in women who used combination oral contraceptives with higher formulations of estrogens and progestins than those in common use today. The effect of long-term use of combination hormonal contraceptives with lower doses of both estrogen and progestin administered by any route remains to be determined.
Throughout this labeling, epidemiological studies reported are of two types: retrospective or case control studies and prospective or cohort studies. Case control studies provide a measure of the relative risk of a disease, namely, a ratio of the incidence of a disease among oral contraceptive users to that among nonusers. The relative risk does not provide information on the actual clinical occurrence of a disease. Cohort studies provide a measure of attributable risk, which is the difference in the incidence of disease between hormonal contraceptive users and nonusers. The attributable risk does provide information about the actual occurrence of a disease in the population (adapted from refs. 2 and 3 with the author's permission). For further information, the reader is referred to a text on epidemiological methods.
-
Thromboembolic Disorders and Other Vascular Problems
-
Thromboembolism
An increased risk of thromboembolic and thrombotic disease associated with the use of hormonal contraceptives is well established. Case control studies have found the relative risk of users compared to nonusers to be 3 for the first episode of superficial venous thrombosis, 4 to 11 for deep vein thrombosis or pulmonary embolism, and 1.5 to 6 for women with predisposing conditions for venous thromboembolic disease 2,3,19-24 . Cohort studies have shown the relative risk to be somewhat lower, about 3 for new cases and about 4.5 for new cases requiring hospitalization 25 . The risk of thromboembolic disease associated with hormonal contraceptives is not related to length of use and disappears after hormonal contraceptive use is stopped 2 . A two- to four-fold increase in relative risk of post-operative thromboembolic complications has been reported with the use of hormonal contraceptives 9,26 . The relative risk of venous thrombosis in women who have predisposing conditions is twice that of women without such medical conditions 9,26 . If feasible, hormonal contraceptives should be discontinued at least four weeks prior to and for two weeks after elective surgery of a type associated with an increase in risk of thromboembolism and during and following prolonged immobilization. Since the immediate postpartum period is also associated with an increased risk of thromboembolism, hormonal contraceptives should be started no earlier than four weeks after delivery in women who elect not to breast-feed.
In the large clinical trials (N= 3,330 with 1,704 women-years of exposure), one case of non-fatal pulmonary embolism occurred during ORTHO EVRA® use, and one case of post-operative non-fatal pulmonary embolism was reported following ORTHO EVRA® use. It is unknown if the risk of venous thromboembolism with ORTHO EVRA® use is different than with use of combination oral contraceptives.
As with any combination hormonal contraceptives, the clinician should be alert to the earliest manifestations of thrombotic disorders (thrombophlebitis, pulmonary embolism, cerebrovascular disorders, and retinal thrombosis). Should any of these occur or be suspected, ORTHO EVRA® should be discontinued immediately. -
Myocardial Infarction
An increased risk of myocardial infarction has been attributed to hormonal contraceptive use. This risk is primarily in smokers or women with other underlying risk factors for coronary artery disease such as hypertension, hypercholesterolemia, morbid obesity, and diabetes. The relative risk of heart attack for current hormonal contraceptive users has been estimated to be two to six 4-10 compared to non-users. The risk is very low under the age of 30.
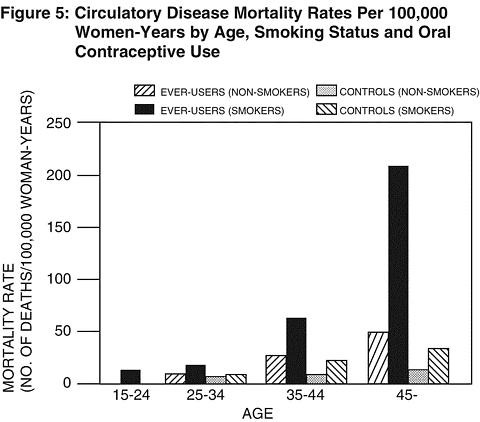
Smoking in combination with oral contraceptive use has been shown to contribute substantially to the incidence of myocardial infarctions in women in their mid-thirties or older with smoking accounting for the majority of excess cases 11 . Mortality rates associated with circulatory disease have been shown to increase substantially in smokers, especially in those 35 years of age and older among women who use oral contraceptives. (See Figure 5)
Hormonal contraceptives may compound the effects of well-known risk factors, such as hypertension, diabetes, hyperlipidemias, age and obesity 13 . In particular, some progestins are known to decrease HDL cholesterol and cause glucose intolerance, while estrogens may create a state of hyperinsulinism 14-18 . Hormonal contraceptives have been shown to increase blood pressure among some users (see Section 9 in WARNINGS ). Similar effects on risk factors have been associated with an increased risk of heart disease. Hormonal contraceptives, including ORTHO EVRA®, must be used with caution in women with cardiovascular disease risk factors.
Norgestimate and norelgestromin have minimal androgenic activity (see CLINICAL PHARMACOLOGY ). There is some evidence that the risk of myocardial infarction associated with hormonal contraceptives is lower when the progestin has minimal androgenic activity than when the activity is greater 97 . -
Cerebrovascular Diseases
Hormonal contraceptives have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes), although, in general, the risk is greatest among older (>35 years), hypertensive women who also smoke. Hypertension was found to be a risk factor for both users and nonusers, for both types of strokes, and smoking interacted to increase the risk of stroke 27-29 .
In a large study, the relative risk of thrombotic strokes has been shown to range from 3 for normotensive users to 14 for users with severe hypertension 30 . The relative risk of hemorrhagic stroke is reported to be 1.2 for non-smokers who used hormonal contraceptives, 2.6 for smokers who did not use hormonal contraceptives, 7.6 for smokers who used hormonal contraceptives, 1.8 for normotensive users and 25.7 for users with severe hypertension 30 . The attributable risk is also greater in older women 3 . -
Dose-Related Risk of Vascular Disease From Hormonal Contraceptives
A positive association has been observed between the amount of estrogen and progestin in hormonal contraceptives and the risk of vascular disease 31-33 . A decline in serum high-density lipoproteins (HDL) has been reported with many progestational agents 14-16 . A decline in serum high-density lipoproteins has been associated with an increased incidence of ischemic heart disease. Because estrogens increase HDL cholesterol, the net effect of a hormonal contraceptive depends on a balance achieved between doses of estrogen and progestin and the activity of the progestin used in the contraceptives. The activity and amount of both hormones should be considered in the choice of a hormonal contraceptive. -
Persistence of Risk of Vascular Disease
There are two studies that have shown persistence of risk of vascular disease for ever-users of combination hormonal contraceptives. In a study in the United States, the risk of developing myocardial infarction after discontinuing combination hormonal contraceptives persists for at least 9 years for women 40-49 years who had used combination hormonal contraceptives for five or more years, but this increased risk was not demonstrated in other age groups 8 . In another study in Great Britain, the risk of developing cerebrovascular disease persisted for at least 6 years after discontinuation of combination hormonal contraceptives, although excess risk was very small 34 . However, both studies were performed with combination hormonal contraceptive formulations containing 50 micrograms or higher of estrogens.
It is unknown whether ORTHO EVRA® is distinct from other combination hormonal contraceptives with regard to the occurrence of venous and arterial thrombosis.
-
Thromboembolism
-
Estimates of Mortality From Combination Hormonal Contraceptive Use
One study gathered data from a variety of sources that have estimated the mortality rate associated with different methods of contraception at different ages (Table 3). These estimates include the combined risk of death associated with contraceptive methods plus the risk attributable to pregnancy in the event of method failure. Each method of contraception has its specific benefits and risks. The study concluded that with the exception of combination oral contraceptive users 35 and older who smoke, and 40 and older who do not smoke, mortality associated with all methods of birth control is low and below that associated with childbirth.
The observation of a possible increase in risk of mortality with age for combination oral contraceptive users is based on data gathered in the 1970's but not reported until 1983 35 . Current clinical recommendation involves the use of lower estrogen dose formulations and a careful consideration of risk factors. In 1989, the Fertility and Maternal Health Drugs Advisory Committee was asked to review the use of combination hormonal contraceptives in women 40 years of age and over. The Committee concluded that although cardiovascular disease risks may be increased with combination hormonal contraceptive use after age 40 in healthy non-smoking women (even with the newer low-dose formulations), there are also greater potential health risks associated with pregnancy in older women and with the alternative surgical and medical procedures that may be necessary if such women do not have access to effective and acceptable means of contraception. The Committee recommended that the benefits of low-dose combination hormonal contraceptive use by healthy non-smoking women over 40 may outweigh the possible risks 36,37 .
Although the data are mainly obtained with oral contraceptives, this is likely to apply to ORTHO EVRA® as well. Women of all ages who use combination hormonal contraceptives, should use the lowest possible dose formulation that is effective and meets the individual patient needs.
Table 3. Annual Number of Birth-Related or Method-Related Deaths Associated With Control of Fertility Per 100,000 Non-Sterile Women, by Fertility Control Method According to AgeMethod of control
and outcome15-19 20-24 25-29 30-34 35-39 40-44 No fertility control
methods *7.0 7.4 9.1 14.8 25.7 28.2 Oral contraceptives,
non-smoker **0.3 0.5 0.9 1.9 13.8 31.6 Oral contraceptives,
smoker **2.2 3.4 6.6 13.5 51.1 117.2 IUD **0.8 0.8 1.0 1.0 1.4 1.4 Condom *1.1 1.6 0.7 0.2 0.3 0.4 Diaphragm/spermicide *1.9 1.2 1.2 1.3 2.2 2.8 Periodic abstinence *2.5 1.6 1.6 1.7 2.9 3.6 *Deaths are birth-related**Deaths are method-related
Adapted from H.W. Ory, ref. # 35.
-
Carcinoma of the Reproductive Organs and Breasts
Numerous epidemiological studies give conflicting reports on the relationship between breast cancer and COC use. The risk of having breast cancer diagnosed may be slightly increased among current and recent users of combination oral contraceptives. However, this excess risk appears to decrease over time after COC discontinuation and by 10 years after cessation the increased risk disappears. Some studies report an increased risk with duration of use while other studies do not and no consistent relationships have been found with dose or type of steroid. Some studies have found a small increase in risk for women who first use COCs before age 20. Most studies show a similar pattern of risk with COC use regardless of a woman's reproductive history or her family breast cancer history.
In addition, breast cancers diagnosed in current or ever oral contraceptive users may be less clinically advanced than in never-users.
Women who currently have or have had breast cancer should not use hormonal contraceptives because breast cancer is usually a hormonally sensitive tumor.
Some studies suggest that combination oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women 45-48 . However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
In spite of many studies of the relationship between oral contraceptive use and breast and cervical cancers, a cause-and-effect relationship has not been established. It is not known whether ORTHO EVRA® is distinct from oral contraceptives with regard to the above statements. -
Hepatic Neoplasia
Benign hepatic adenomas are associated with hormonal contraceptive use, although the incidence of benign tumors is rare in the United States. Indirect calculations have estimated the attributable risk to be in the range of 3.3 cases/100,000 for users, a risk that increases after four or more years of use, especially with hormonal contraceptives containing 50 micrograms or more of estrogen 49 . Rupture of benign, hepatic adenomas may cause death through intra-abdominal hemorrhage 50,51 .
Studies from Britain and the US have shown an increased risk of developing hepatocellular carcinoma in long term (>/= 8 years) 52-54,96 oral contraceptive users. However, these cancers are extremely rare in the U.S. and the attributable risk (the excess incidence) of liver cancers in oral contraceptive users approaches less than one per million users. It is unknown whether ORTHO EVRA® is distinct from oral contraceptives in this regard. -
Ocular Lesions
There have been clinical case reports of retinal thrombosis associated with the use of hormonal contraceptives. ORTHO EVRA® should be discontinued if there is unexplained partial or complete loss of vision; onset of proptosis or diplopia; papilledema; or retinal vascular lesions. Appropriate diagnostic and therapeutic measures should be undertaken immediately. -
Hormonal Contraceptive Use Before or During Early Pregnancy
Extensive epidemiological studies have revealed no increased risk of birth defects in women who have used oral contraceptives prior to pregnancy 56,57 . Studies also do not indicate a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned 55,56,58,59 , when oral contraceptives are taken inadvertently during early pregnancy.
Combination hormonal contraceptives such as ORTHO EVRA® should not be used to induce withdrawal bleeding as a test for pregnancy. ORTHO EVRA® should not be used during pregnancy to treat threatened or habitual abortion. It is recommended that for any patient who has missed two consecutive periods, pregnancy should be ruled out. If the patient has not adhered to the prescribed schedule for the use of ORTHO EVRA® the possibility of pregnancy should be considered at the time of the first missed period. Hormonal contraceptive use should be discontinued if pregnancy is confirmed. -
Gallbladder Disease
Earlier studies have reported an increased lifetime relative risk of gallbladder surgery in users of hormonal contraceptives and estrogens 60,61 . More recent studies, however, have shown that the relative risk of developing gallbladder disease among hormonal contraceptive users may be minimal 62-64 . The recent findings of minimal risk may be related to the use of hormonal contraceptive formulations containing lower hormonal doses of estrogens and progestins.
Combination hormonal contraceptives such as ORTHO EVRA® may worsen existing gallbladder disease and may accelerate the development of this disease in previously asymptomatic women. Women with a history of combination hormonal contraceptive-related cholestasis are more likely to have the condition recur with subsequent combination hormonal contraceptive use. -
Carbohydrate and Lipid Metabolic Effects
Hormonal contraceptives have been shown to cause a decrease in glucose tolerance in some users 17 . However, in the non-diabetic woman, combination hormonal contraceptives appear to have no effect on fasting blood glucose 67 . Prediabetic and diabetic women in particular should be carefully monitored while taking combination hormonal contraceptives such as ORTHO EVRA®.
In clinical trials with oral contraceptives containing ethinyl estradiol and norgestimate there were no clinically significant changes in fasting blood glucose levels. There were no clinically significant changes in glucose levels over 24 cycles of use. Moreover, glucose tolerance tests showed no clinically significant changes from baseline to cycles 3, 12 and 24. In a 6-cycle clinical trial with ORTHO EVRA® there were no clinically significant changes in fasting blood glucose from baseline to end of treatment.
A small proportion of women will have persistent hypertriglyceridemia while taking hormonal contraceptives. As discussed earlier (see WARNINGS 1a and 1d), changes in serum triglycerides and lipoprotein levels have been reported in hormonal contraceptive users. -
Elevated Blood Pressure
Women with significant hypertension should not be started on hormonal contraception 103 . Women with a history of hypertension or hypertension-related diseases, or renal disease 70 should be encouraged to use another method of contraception. If women elect to use ORTHO EVRA®, they should be monitored closely and if a clinically significant elevation of blood pressure occurs, ORTHO EVRA® should be discontinued. For most women, elevated blood pressure will return to normal after stopping hormonal contraceptives, and there is no difference in the occurrence of hypertension between former and never users 68-71 .
An increase in blood pressure has been reported in women taking hormonal contraceptives 68 and this increase is more likely in older hormonal contraceptive users 69 and with extended duration of use 61 . Data from the Royal College of General Practitioners 12 and subsequent randomized trials have shown that the incidence of hypertension increases with increasing progestational activity. -
Headache
The onset or exacerbation of migraine headache or the development of headache with a new pattern that is recurrent, persistent or severe requires discontinuation of ORTHO EVRA® and evaluation of the cause. -
Bleeding Irregularities
Breakthrough bleeding and spotting are sometimes encountered in women using ORTHO EVRA®. Non-hormonal causes should be considered and adequate diagnostic measures taken to rule out malignancy, other pathology, or pregnancy in the event of breakthrough bleeding, as in the case of any abnormal vaginal bleeding. If pathology has been excluded, time or a change to another contraceptive product may resolve the bleeding. In the event of amenorrhea, pregnancy should be ruled out before initiating use of ORTHO EVRA®.
Some women may encounter amenorrhea or oligomenorrhea after discontinuation of hormonal contraceptive use, especially when such a condition was pre-existent.
Bleeding Patterns:
In the clinical trials most women started their withdrawal bleeding on the fourth day of the drug-free interval, and the median duration of withdrawal bleeding was 5 to 6 days. On average 26% of women per cycle had 7 or more total days of bleeding and/or spotting (this includes both withdrawal flow and breakthrough bleeding and/or spotting). -
Ectopic Pregnancy
Ectopic as well as intrauterine pregnancy may occur in contraceptive failures.
PRECAUTIONS
Women should be counseled that ORTHO EVRA® does not protect against HIV infection (AIDS) and other sexually transmitted infections.
1. Body Weight >/=198 lbs. (90 kg)
Results of clinical trials suggest that ORTHO EVRA® may be less effective in women with body weight >/=198 lbs. (90 kg) than in women with lower body weights.
2. Physical Examination and Follow-Up
It is good medical practice for women using ORTHO EVRA®, as for all women, to have annual medical evaluation and physical examinations. The physical examination, however, may be deferred until after initiation of hormonal contraceptives if requested by the woman and judged appropriate by the clinician. The physical examination should include special reference to blood pressure, breasts, abdomen and pelvic organs, including cervical cytology, and relevant laboratory tests. In case of undiagnosed, persistent or recurrent abnormal vaginal bleeding, appropriate measures should be conducted to rule out malignancy or other pathology. Women with a strong family history of breast cancer or who have breast nodules should be monitored with particular care.
3. Lipid Disorders
Women who are being treated for hyperlipidemias should be followed closely if they elect to use ORTHO EVRA®. Some progestins may elevate LDL levels and may render the control of hyperlipidemias more difficult.
4. Liver Function
If jaundice develops in any woman using ORTHO EVRA®, the medication should be discontinued. The hormones in ORTHO EVRA® may be poorly metabolized in patients with impaired liver function.
5. Fluid Retention
Steroid hormones like those in ORTHO EVRA® may cause some degree of fluid retention. ORTHO EVRA® should be prescribed with caution, and only with careful monitoring, in patients with conditions which might be aggravated by fluid retention.
6. Emotional Disorders
Women who become significantly depressed while using combination hormonal contraceptives such as ORTHO EVRA® should stop the medication and use another method of contraception in an attempt to determine whether the symptom is drug related. Women with a history of depression should be carefully observed and ORTHO EVRA® discontinued if significant depression occurs.
7. Contact Lenses
Contact lens wearers who develop visual changes or changes in lens tolerance should be assessed by an ophthalmologist.
8. Drug Interactions
Changes in Contraceptive Effectiveness Associated with Co-Administration of Other Drugs:
Contraceptive effectiveness may be reduced when hormonal contraceptives are co-administered with some antibiotics, antifungals, anticonvulsants, and other drugs that increase metabolism of contraceptive steroids. This could result in unintended pregnancy or breakthrough bleeding. Examples include barbiturates, griseofulvin, rifampin, phenylbutazone, phenytoin, carbamazepine, felbamate, oxcarbazepine, topiramate and possibly with ampicillin.
The proposed mechanism of interaction of antibiotics is different from that of liver enzyme-inducing drugs. Literature suggests possible interactions with the concomitant use of hormonal contraceptives and ampicillin or tetracycline. In a pharmacokinetic drug interaction study, oral administration of tetracycline HCl, 500 mg q.i.d. for 3 days prior to and 7 days during wear of ORTHO EVRA® did not significantly affect the pharmacokinetics of norelgestromin or EE.
Several of the anti-HIV protease inhibitors have been studied with co-administration of oral combination hormonal contraceptives; significant changes (increase and decrease) in the mean AUC of the estrogen and progestin have been noted in some cases. The efficacy and safety of oral contraceptive products may be affected; it is unknown whether this applies to ORTHO EVRA®. Healthcare professionals should refer to the label of the individual anti-HIV protease inhibitors for further drug-drug interaction information.
Herbal products containing St. John's Wort (hypericum perforatum) may induce hepatic enzymes (cytochrome P450) and p-glycoprotein transporter and may reduce the effectiveness of contraceptive steroids. This may also result in breakthrough bleeding.
Increase in Plasma Hormone Levels Associated with Co-Administered Drugs:
Co-administration of atorvastatin and certain oral contraceptives containing ethinyl estradiol increase AUC values for ethinyl estradiol by approximately 20%. Ascorbic acid and acetaminophen may increase plasma ethinyl estradiol levels, possibly by inhibition of conjugation. CYP 3A4 inhibitors such as itraconazole or ketoconazole may increase plasma hormone levels.
Changes in Plasma Levels of Co-Administered Drugs:
Combination hormonal contraceptives containing some synthetic estrogens (e.g., ethinyl estradiol) may inhibit the metabolism of other compounds. Increased plasma concentrations of cyclosporine, prednisolone, and theophylline have been reported with concomitant administration of oral contraceptives. In addition, oral contraceptives may induce the conjugation of other compounds. Decreased plasma concentrations of acetaminophen and increased clearance of temazepam, salicylic acid, morphine and clofibric acid have been noted when these drugs were administered with oral contraceptives.
Although norelgestromin and its metabolites inhibit a variety of P450 enzymes in human liver microsomes, the clinical consequence of such an interaction on the levels of other concomitant medications is likely to be insignificant. Under the recommended dosing regimen, the in vivo concentrations of norelgestromin and its metabolites, even at the peak serum levels, are relatively low compared to the inhibitory constant (Ki) (based on results of in vitro studies).
Health care professionals are advised to also refer to prescribing information of co-administered drugs for recommendations regarding management of concomitant therapy.
9. Interactions With Laboratory Tests
Certain endocrine and liver function tests and blood components may be affected by hormonal contraceptives:
- Increased prothrombin and factors VII, VIII, IX, and X; decreased antithrombin 3; increased norepinephrine-induced platelet aggregability.
- Increased thyroid binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4 by column or by radioimmunoassay. Free T3 resin uptake is decreased, reflecting the elevated TBG, free T4 concentration is unaltered.
- Other binding proteins may be elevated in serum.
- Sex hormone binding globulins are increased and result in elevated levels of total circulating endogenous sex steroids and corticoids; however, free or biologically active levels either decrease or remain unchanged.
- Triglycerides may be increased and levels of various other lipids and lipoproteins may be affected.
- Glucose tolerance may be decreased.
- Serum folate levels may be depressed by hormonal contraceptive therapy. This may be of clinical significance if a woman becomes pregnant shortly after discontinuing ORTHO EVRA®.
10. Carcinogenesis
No carcinogenicity studies were conducted with norelgestromin. However, bridging PK studies were conducted using doses of NGM/EE which were used previously in the 2-year rat carcinogenicity study and 10-year monkey toxicity study to support the approval of ORTHO-CYCLEN® and ORTHO TRI-CYCLEN® under NDAs 19-653 and 19-697, respectively. The PK studies demonstrated that rats and monkeys were exposed to 16 and 8 times the human exposure, respectively, with the proposed ORTHO EVRA® transdermal contraceptive system.
Norelgestromin was tested in in-vitro mutagenicity assays (bacterial plate incorporation mutation assay, CHO/HGPRT mutation assay, chromosomal aberration assay using cultured human peripheral lymphocytes) and in one in-vivo test (rat micronucleus assay) and found to have no genotoxic potential.
See WARNINGS Section.
11. Pregnancy
Pregnancy Category X. See CONTRAINDICATIONS and WARNINGS Sections.
Norelgestromin was tested for its reproductive toxicity in a rabbit developmental toxicity study by the SC route of administration. Doses of 0, 1, 2, 4 and 6 mg/kg body weight, which gave systemic exposure of approximately 25 to 125 times the human exposure with ORTHO EVRA®, were administered daily on gestation days 7-19. Malformations reported were paw hyperflexion at 4 and 6 mg/kg and paw hyperextension and cleft palate at 6 mg/kg.
12. Nursing Mothers
The effects of ORTHO EVRA® in nursing mothers have not been evaluated and are unknown. Small amounts of combination hormonal contraceptive steroids have been identified in the milk of nursing mothers and a few adverse effects on the child have been reported, including jaundice and breast enlargement. In addition, combination hormonal contraceptives given in the postpartum period may interfere with lactation by decreasing the quantity and quality of breast milk. Long-term follow-up of infants whose mothers used combination hormonal contraceptives while breast feeding has shown no deleterious effects. However, the nursing mother should be advised not to use ORTHO EVRA® but to use other forms of contraception until she has completely weaned her child.
13. Pediatric Use
Safety and efficacy of ORTHO EVRA® have been established in women of reproductive age. Safety and efficacy are expected to be the same for post-pubertal adolescents under the age of 16 and for users 16 years and older. Use of this product before menarche is not indicated.
14. Geriatric Use
This product has not been studied in women over 65 years of age and is not indicated in this population.
15. Sexually Transmitted Diseases
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
16. Patch Adhesion
Experience with more than 70,000 ORTHO EVRA® patches worn for contraception for 6-13 cycles showed that 4.7% of patches were replaced because they either fell off (1.8%) or were partly detached (2.9%). Similarly, in a small study of patch wear under conditions of physical exertion and variable temperature and humidity, less than 2% of patches were replaced for complete or partial detachment.
If the ORTHO EVRA® patch becomes partially or completely detached and remains detached, insufficient drug delivery occurs. A patch should not be re-applied if it is no longer sticky, if it has become stuck to itself or another surface, if it has other material stuck to it, or if it has become loose or fallen off before. If a patch cannot be re-applied, a new patch should be applied immediately. Supplemental adhesives or wraps should not be used to hold the ORTHO EVRA® patch in place.
If a patch is partially or completely detached for more than one day (24 hours or more) OR if the woman is not sure how long the patch has been detached, she may not be protected from pregnancy. She should stop the current contraceptive cycle and start a new cycle immediately by applying a new patch. Back-up contraception, such as condoms, spermicide, or diaphragm, must be used for the first week of the new cycle.
INFORMATION FOR THE PATIENT
See Patient Labeling printed below.
ADVERSE REACTIONS
The most common adverse events reported by 9 to 22% of women using ORTHO EVRA® in clinical trials (N= 3,330) were the following, in order of decreasing incidence: breast symptoms, headache, application site reaction, nausea, upper respiratory infection, menstrual cramps, and abdominal pain.
The most frequent adverse events leading to discontinuation in 1 to 2.4% of women using ORTHO EVRA® in the trials included the following: nausea and/or vomiting, application site reaction, breast symptoms, headache, and emotional lability.
Listed below are adverse events that have been associated with the use of combination hormonal contraceptives. These are also likely to apply to combination transdermal hormonal contraceptives such as ORTHO EVRA®.
An increased risk of the following serious adverse reactions has been associated with the use of combination hormonal contraceptives (see WARNINGS Section).
- Thrombophlebitis and venous thrombosis with or without embolism
- Arterial thromboembolism
- Pulmonary embolism
- Myocardial infarction
- Cerebral hemorrhage
- Cerebral thrombosis
- Hypertension
- Gallbladder disease
- Hepatic adenomas or benign liver tumors
There is evidence of an association between the following conditions and the use of combination hormonal contraceptives:
- Mesenteric thrombosis
- Retinal thrombosis
The following adverse reactions have been reported in users of combination hormonal contraceptives and are believed to be drug-related:
- Nausea
- Vomiting
- Gastrointestinal symptoms (such as abdominal cramps and bloating)
- Breakthrough bleeding
- Spotting
- Change in menstrual flow
- Amenorrhea
- Temporary infertility after discontinuation of treatment
- Edema
- Melasma which may persist
- Breast changes: tenderness, enlargement, secretion
- Change in weight (increase or decrease)
- Change in cervical erosion and secretion
- Diminution in lactation when given immediately postpartum
- Cholestatic jaundice
- Migraine
- Rash (allergic)
- Mental depression
- Reduced tolerance to carbohydrates
- Vaginal candidiasis
- Change in corneal curvature (steepening)
- Intolerance to contact lenses
The following adverse reactions have been reported in users of combination hormonal contraceptives and a cause and effect association has been neither confirmed nor refuted:
- Pre-menstrual syndrome
- Cataracts
- Changes in appetite
- Cystitis-like syndrome
- Headache
- Nervousness
- Dizziness
- Hirsutism
- Loss of scalp hair
- Erythema multiforme
- Erythema nodosum
- Hemorrhagic eruption
- Vaginitis
- Porphyria
- Impaired renal function
- Hemolytic uremic syndrome
- Acne
- Changes in libido
- Colitis
- Budd-Chiari Syndrome
OVERDOSAGE
Serious ill effects have not been reported following accidental ingestion of large doses of hormonal contraceptives. Overdosage may cause nausea and vomiting, and withdrawal bleeding may occur in females. Given the nature and design of the ORTHO EVRA® patch, it is unlikely that overdosage will occur. Serious ill effects have not been reported following acute ingestion of large doses of oral contraceptives by young children. In case of suspected overdose, all ORTHO EVRA® patches should be removed and symptomatic treatment given.
DOSAGE AND ADMINISTRATION
To achieve maximum contraceptive effectiveness, ORTHO EVRA® must be used exactly as directed.
Complete instructions to facilitate patient counseling on proper system usage may be found in the Detailed Patient Labeling .
Transdermal Contraceptive System Overview
ORTHO EVRA® is a combination transdermal contraceptive that contains 6.00 mg norelgestromin and 0.75 mg ethinyl estradiol (EE), and releases 150 micrograms of norelgestromin and 20 micrograms of EE to the blood-stream per 24 hours. This level of transdermal release of EE results in exposure to EE greater than that produced by an oral contraceptive product containing 20 micrograms of EE (See Pharmacokinetics , Absorption ).
This system uses a 28-day (four-week) cycle. A new patch is applied each week for three weeks (21 total days). Week Four is patch-free. Withdrawal bleeding is expected during this time.
Every new patch should be applied on the same day of the week. This day is known as the "Patch Change Day." For example, if the first patch is applied on a Monday, all subsequent patches should be applied on a Monday. Only one patch should be worn at a time.
On the day after Week Four ends a new four-week cycle is started by applying a new patch. Under no circumstances should there be more than a seven-day patch-free interval between dosing cycles.
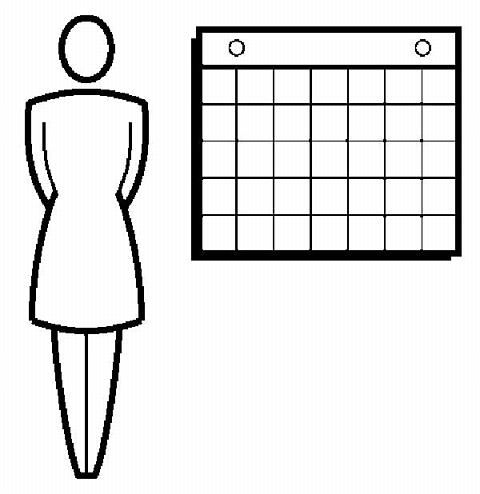
If the woman is starting ORTHO EVRA® for the first time , she should wait until the day she begins her menstrual period . Either a First Day start or Sunday start may be chosen (see below). The day she applies her first patch will be Day 1. Her "Patch Change Day" will be on this day every week.

·for First Day Start: the patient should apply her first patch during the first 24 hours of her menstrual period.
If therapy starts after Day 1 of the menstrual cycle, a non-hormonal back-up contraceptive (such as a condoms, spermicide, or diaphragm) should be used concurrently for the first 7 consecutive days of the first treatment cycle.
OR
· for Sunday Start: the woman should apply her first patch on the first Sunday after her menstrual period starts. She must use back-up contraception for the first week of her first cycle.
If the menstrual period begins on a Sunday, the first patch should be applied on that day, and no back-up contraception is needed.
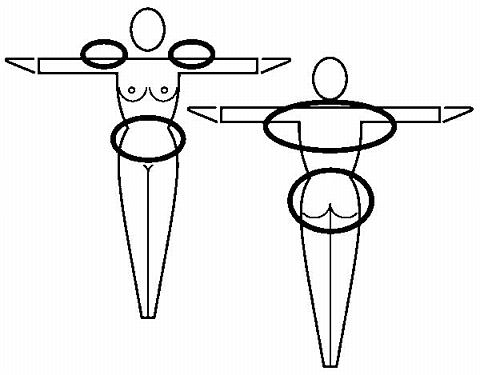
Where to apply the patch. The patch should be applied to clean, dry, intact healthy skin on the buttock, abdomen, upper outer arm or upper torso, in a place where it won't be rubbed by tight clothing. ORTHO EVRA® should not be placed on skin that is red, irritated or cut, nor should it be placed on the breasts.
To prevent interference with the adhesive properties of ORTHO EVRA®, no make-up, creams, lotions, powders or other topical products should be applied to the skin area where the ORTHO EVRA® patch is or will be placed.
Application of the ORTHO EVRA® patch
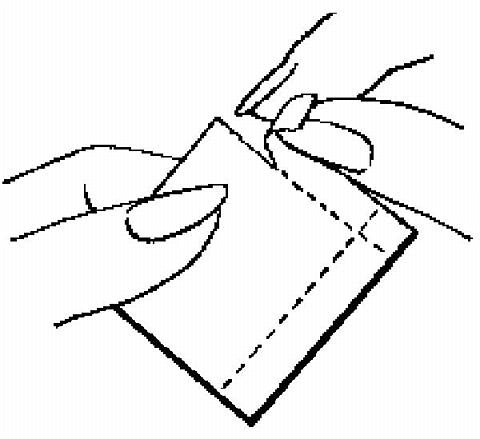
The foil pouch is opened by tearing it along the edge using the fingers.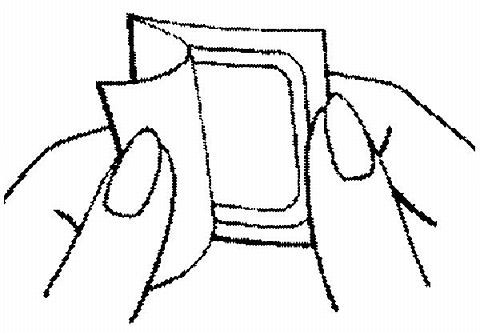
The foil pouch should be peeled apart and open flat.
A corner of the patch is grasped firmly and it is gently removed from the foil pouch.
The woman should be instructed to use her fingernail, to lift one corner of the patch and peel the patch and the plastic liner off the foil liner. Sometimes patches can stick to the inside of the pouch - the woman should be careful not to accidentally remove the clear liner as she removes the patch.
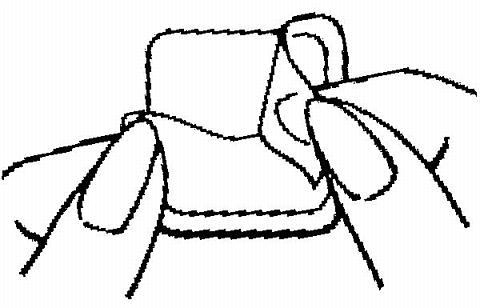
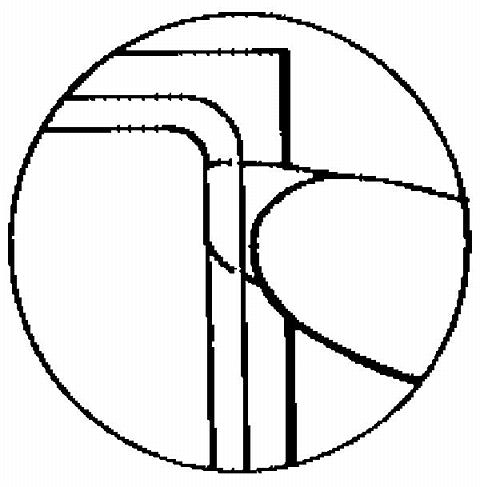
Half of the clear protective liner is to be peeled away. (The woman should avoid touching the sticky surface of the patch).
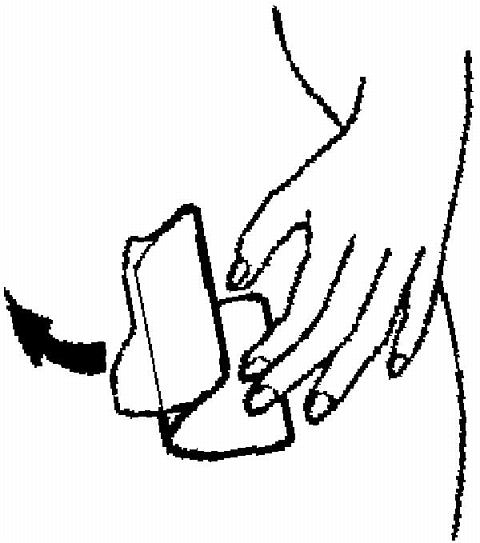
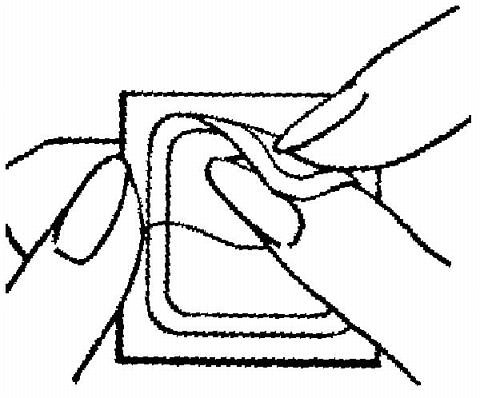
The sticky surface of the patch is applied to the skin and the other half of the liner is removed. The woman should press down firmly on the patch with the palm of her hand for 10 seconds, making sure that the edges stick well. She should check her patch every day to make sure it is sticking.
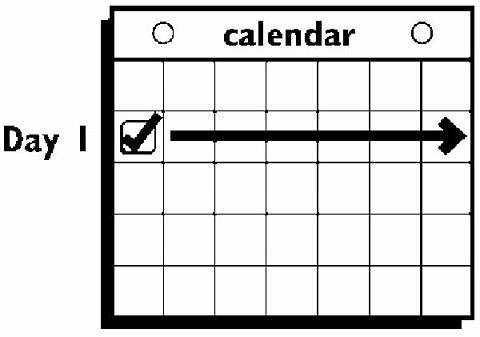
The patch is worn for seven days (one week). On the "Patch Change Day", Day 8, the used patch is removed and a new one is applied immediately. The used patch still contains some active hormones - it should be carefully folded in half so that it sticks to itself before safely disposing of it in the trash. Used patches should not be flushed down the toilet.
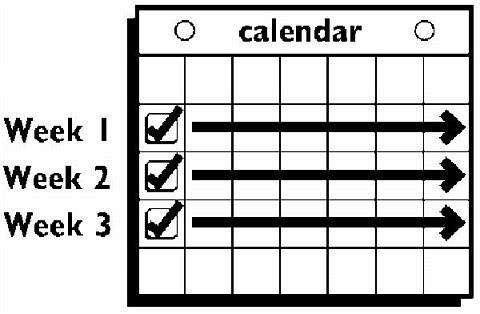
A new patch is applied for Week Two (on Day 8) and again for Week Three (on Day 15), on the usual "Patch Change Day". Patch changes may occur at any time on the Change Day. Each new ORTHO EVRA® patch should be applied to a new spot on the skin to help avoid irritation, although they may be kept within the same anatomic area.
Week Four is patch-free (Day 22 through Day 28), thus completing the four-week contraceptive cycle. Bleeding is expected to begin during this time.
The next four-week cycle is started by applying a new patch on the usual "Patch Change Day," the day after Day 28, no matter when the menstrual period begins or ends.
Under no circumstances should there be more than a seven-day patch-free interval between patch cycles.
If the ORTHO EVRA® patch becomes partially or completely detached and remains detached, insufficient drug delivery occurs.
If a patch is partially or completely detached:
- for less than one day (up to 24 hours), the woman should try to reapply it to the same place or replace it with a new patch immediately. No back-up contraception is needed. The woman's "Patch Change Day" will remain the same.
- for more than one day (24 hours or more) OR if the woman is not sure how long the patch has been detached , SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should stop the current contraceptive cycle and start a new cycle immediately by applying a new patch. There is now a new "Day 1" and a new "Patch Change Day." Back-up contraception, such as condoms, spermicide, or diaphragm, must be used for the first week of the new cycle.
A patch should not be re-applied if it is no longer sticky, if it has become stuck to itself or another surface, if it has other material stuck to it or if it has previously become loose or fallen off. If a patch cannot be re-applied, a new patch should be applied immediately. Supplemental adhesives or wraps should not be used to hold the ORTHO EVRA® patch in place.
If the woman forgets to change her patch...
- at the start of any patch cycle (Week One /Day 1): SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should apply the first patch of her new cycle as soon as she remembers. There is now a new "Patch Change Day" and a new "Day 1." The woman must use back-up contraception, such as condoms, spermicide, or diaphragm, for the first week of the new cycle.
-
in the middle of the patch cycle (Week Two/Day 8 or Week Three/Day 15),
- for one or two days (up to 48 hours), she should apply a new patch immediately. The next patch should be applied on the usual "Patch Change Day." No back-up contraception is needed. (See Figures 3 and 4 in the Clinical Pharmacology section.)
- for more than two days (48 hours or more), SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should stop the current contraceptive cycle and start a new four-week cycle immediately by putting on a new patch. There is now a new "Patch Change Day" and a new "Day 1." The woman must use back-up contraception for one week.
-
at the end of the patch cycle (Week Four/Day 22),
Week Four (Day 22): If the woman forgets to remove her patch, she should take it off as soon as she remembers. The next cycle should be started on the usual "Patch Change Day," which is the day after Day 28. No back-up contraception is needed.
Under no circumstances should there be more than a seven-day patch-free interval between cycles . If there are more than seven patch-free days, THE WOMAN MAY NOT BE PROTECTED FROM PREGNANCY and back-up contraception, such as condoms, spermicide, or diaphragm, must be used for seven days. As with combined oral contraceptives, the risk of ovulation increases with each day beyond the recommended drug-free period. If coital exposure has occurred during such an extended patch-free interval, the possibility of fertilization should be considered.
Change Day Adjustment
If the woman wishes to change her Patch Change Day she should complete her current cycle, removing the third ORTHO EVRA® patch on the correct day. During the patch-free week, she may select an earlier Patch Day Change by applying a new ORTHO EVRA® patch on the desired day. In no case should there be more than 7 consecutive patch-free days.
Switching From an Oral Contraceptive
Treatment with ORTHO EVRA® should begin on the first day of withdrawal bleeding. If there is no withdrawal bleeding within 5 days of the last active (hormone-containing) tablet, pregnancy must be ruled out. If therapy starts later than the first day of withdrawal bleeding, a non-hormonal contraceptive should be used concurrently for 7 days. If more than 7 days elapse after taking the last active oral contraceptive tablet, the possibility of ovulation and conception should be considered.
Use After Childbirth
Women who elect not to breast-feed should start contraceptive therapy with ORTHO EVRA® no sooner than 4 weeks after childbirth. If a woman begins using ORTHO EVRA® postpartum, and has not yet had a period, the possibility of ovulation and conception occurring prior to use of ORTHO EVRA® should be considered, and she should be instructed to use an additional method of contraception, such as condoms, spermicide, or diaphragm, for the first seven days. (See Precautions : Nursing Mothers , and Warnings : Thromboembolic and Other Vascular Problems .)
Use After Abortion or Miscarriage 106
After an abortion or miscarriage that occurs in the first trimester, ORTHO EVRA® may be started immediately. An additional method of contraception is not needed if ORTHO EVRA® is started immediately. If use of ORTHO EVRA® is not started within 5 days following a first trimester abortion, the woman should follow the instructions for a woman starting ORTHO EVRA® for the first time. In the meantime she should be advised to use a non-hormonal contraceptive method. Ovulation may occur within 10 days of an abortion or miscarriage.
ORTHO EVRA® should be started no earlier than 4 weeks after a second trimester abortion or miscarriage. When ORTHO EVRA® is used postpartum or postabortion, the increased risk of thromboembolic disease must be considered. (See CONTRAINDICATIONS and WARNINGS concerning thromboembolic disease. See PRECAUTIONS for " Nursing Mothers ".)
Breakthrough Bleeding or Spotting
In the event of breakthrough bleeding or spotting (bleeding that occurs on the days that ORTHO EVRA® is worn), treatment should be continued. If breakthrough bleeding persists longer than a few cycles, a cause other than ORTHO EVRA® should be considered.
In the event of no withdrawal bleeding (bleeding that should occur during the patch-free week), treatment should be resumed on the next scheduled Change Day. If ORTHO EVRA® has been used correctly, the absence of withdrawal bleeding is not necessarily an indication of pregnancy. Nevertheless, the possibility of pregnancy should be considered, especially if absence of withdrawal bleeding occurs in 2 consecutive cycles. ORTHO EVRA® should be discontinued if pregnancy is confirmed.
In Case of Vomiting or Diarrhea
Given the nature of transdermal application, dose delivery should be unaffected by vomiting.
In Case of Skin Irritation
If patch use results in uncomfortable irritation, the patch may be removed and a new patch may be applied to a different location until the next Change Day. Only one patch should be worn at a time.
ADDITIONAL INSTRUCTIONS FOR DOSING
Breakthrough bleeding, spotting, and amenorrhea are frequent reasons for patients discontinuing hormonal contraceptives. In case of breakthrough bleeding, as in all cases of irregular bleeding from the vagina, nonfunctional causes should considered. In case of undiagnosed persistent or recurrent abnormal bleeding from the vagina, adequate diagnostic measures are indicated to rule out pregnancy or malignancy. If pathology has been excluded, time or a change to another method of contraception may solve the problem.
Use of Hormonal Contraceptives in the Event of a Missed Menstrual Period:
- If the woman has not adhered to the prescribed schedule, the possibility of pregnancy should be considered at the time of the first missed period. Hormonal contraceptive use should be discontinued if pregnancy is confirmed.
- If the woman has adhered to the prescribed regimen and misses one period, she should continue using her contraceptive patches.
- If the woman has adhered to the prescribed regimen and misses two consecutive periods, pregnancy should be ruled out. ORTHO EVRA® use should be discontinued if pregnancy is confirmed.
HOW SUPPLIED
Each beige ORTHO EVRA® patch contains 6.0 mg norelgestromin and 0.75 mg EE, and releases 150 micrograms of norelgestromin and 20 micrograms of EE to the bloodstream per 24 hours. This level of transdermal release of EE results in exposure to EE greater than that procuded by an oral contraceptive product containing 20 micrograms of EE (See Pharmacokinetics , Absorption ).
Each patch surface is heat stamped with ORTHO EVRA® 150/20. Each patch is packaged in a protective pouch.
ORTHO EVRA® is available in folding cartons of 1 cycle each (NDC # 0062-1920-15); each cycle contains 3 patches.
ORTHO EVRA® is also available in folding cartons containing a single patch (NDC # 0062-1920-01), intended for use as a replacement in the event that a patch is inadvertently lost or destroyed.
Special Precautions for Storage and Disposal
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Store patches in their protective pouches. Apply immediately upon removal from the protective pouch.
Do not store in the refrigerator or freezer.
Used patches still contain some active hormones. Each patch should be carefully folded in half so that it sticks to itself before safely disposing of it in the trash. Used patches should not be flushed down the toilet.
REFERENCES
- Trussel J. Contraceptive efficacy. In Hatcher RA, Trussel J, Stewart F, Cates W, Stewart GK, Kowal D, Guest F. Contraceptive Technology: Seventeenth Revised Edition . New York NY: Irvington Publishers, 1998.
- Stadel BV. Oral contraceptives and cardiovascular disease. (Pt.1). N Engl J Med 1981; 305:612-618.
- Stadel BV. Oral contraceptives and cardiovascular disease. (Pt.2). N Engl J Med 1981; 305:672-677.
- Adam SA, Thorogood M. Oral contraception and myocardial infarction revisited: the effects of new preparations and prescribing patterns. Br J Obstet Gynaecol 1981; 88:838-845.
- Mann Jl, Inman WH. Oral contraceptives and death from myocardial infarction. Br Med J 1975; 2(5965):245-248.
- Mann Jl, Vessey MP, Thorogood M, Doll R. Myocardial infarction in young women with special reference to oral contraceptive practice. Br Med J 1975; 2(5956):241-245.
- Royal College of General Practitioners' Oral Contraception Study: Further analyses of mortality in oral contraceptive users. Lancet 1981; 1:541-546.
- Slone D, Shapiro S, Kaufman DW, Rosenberg L, Miettinen OS, Stolley PD. Risk of myocardial infarction in relation to current and discontinued use of oral contraceptives. N Engl J Med 1981:305:420-424.
- Vessey MP. Female hormones and vascular disease-an epidemiological overview. Br J Fam Plann 1980; 6 (Supplement): 1-12.
- Russell-Briefel RG, Ezzati TM, Fulwood R, Perlman JA, Murphy RS. Cardiovascular risk status and oral contraceptive use, United States, 1976-80. Prevent Med 1986; 15:352-362.
- Goldbaum GM, Kendrick JS, Hogelin GC, Gentry EM. The relative impact of smoking and oral contraceptive use on women in the United States. JAMA 1987; 258:1339-1342.
- Layde PM, Beral V. Further analyses of mortality in oral contraceptive users; Royal College of General Practitioners' Oral Contraception Study. (Table 5) Lancet 1981; 1:541-546.
- Knopp RH. Arteriosclerosis risk: the roles of oral contraceptives and postmenopausal estrogens. J Reprod Med 1986; 31(9) (Supplement):913-921.
- Krauss RM, Roy S, Mishell DR, Casagrande J, Pike MC. Effects of two low-dose oral contraceptives on serum lipids and lipoproteins: Differential changes in high-density lipoproteins subclasses. Am J Obstet 1983; 145:446-452.
- Wahl P, Walden C, Knopp R, Hoover J, Wallace R, Heiss G, Rifkind B. Effect of estrogen/progestin potency on lipid/lipoprotein cholesterol. N Engl J Med 1983; 308:862-867.
- Wynn V, Niththyananthan R. The effect of progestin in combined oral contraceptives on serum lipids with special reference to high density lipoproteins. Am J Obstet Gynecol 1982;142:766-771.
- Wynn V, Godsland I. Effects of oral contraceptives on carbohydrate metabolism. J Reprod Med 1986;31(9)(Supplement):892-897.
- LaRosa JC. Atherosclerotic risk factors in cardiovascular disease. J Reprod Med 1986;31(9)(Supplement):906-912.
- Inman WH, Vessey MP. Investigation of death from pulmonary, coronary, and cerebral thrombosis and embolism in women of child-bearing age. Br Med J 1968;2(5599):193-199.
- Maguire MG, Tonascia J, Sartwell PE, Stolley PD, Tockman MS. Increased risk of thrombosis due to oral contraceptives: a further report. Am J Epidemiol 1979;110(2):188-195.
- Petitti DB, Wingerd J, Pellegrin F, Ramacharan S. Risk of vascular disease in women: smoking, oral contraceptives, noncontraceptive estrogens, and other factors. JAMA 1979;242:1150-
- Vessey MP, Doll R. Investigation of relation between use of oral contraceptives and thromboembolic disease. Br Med J 1968;2(5599):199-205.
- Vessey MP, Doll R. Investigation of relation between use of oral contraceptives and thromboembolic disease. A further report. Br Med J 1969; 2(5658):651-657.
- Porter JB, Hunter JR, Danielson DA, Jick H, Stergachis A. Oral contraceptives and non-fatal vascular disease-recent experience. Obstet Gynecol 1982;59(3):299-302.
- Vessey M, Doll R, Peto R, Johnson B, Wiggins P. A long-term follow-up study of women using different methods of contraception: an interim report. J Biosocial Sci 1976;8:375-427.
- Royal College of General Practitioners: Oral Contraceptives, venous thrombosis, and varicose veins. J Royal Coll Gen Pract 1978; 28:393-399.
- Collaborative Group for the Study of Stroke in Young Women: Oral contraception and increased risk of cerebral ischemia or thrombosis. N Engl J Med 1973;288:871-878.
- Petitti DB, Wingerd J. Use of oral contraceptives, cigarette smoking, and risk of subarachnoid hemorrhage. Lancet 1978;2:234-236.
- Inman WH. Oral contraceptives and fatal subarachnoid hemorrhage. Br Med J 1979:2(6203):1468-1470.
- Collaborative Group for the Study of Stroke in Young Women: Oral Contraceptives and stroke in young women: associated risk factors. JAMA 1975; 231:718-722.
- Inman WH, Vessey MP, Westerholm B, Engelund A. Thromboembolic disease and the steroidal content of oral contraceptives. A report to the Committee on Safety of Drugs. Br Med J 1970;2:203-209.
- Meade TW, Greenberg G, Thompson SG. Progestogens and cardiovascular reactions associated with oral contraceptives and a comparison of the safety of 50- and 35-mcg oestrogen preparations. Br Med J 1980;280(6224):1157-1161.
- Kay CR. Progestogens and arterial disease-evidence from the Royal College of General Practitioners' Study. Am J Obstet Gynecol 1982;142:762-765.
- Royal College of General Practitioners: Incidence of arterial disease among oral contraceptive users. J Royal Coll Gen Pract 1983;33:75-82.
- Ory HW. Mortality associated with fertility and fertility control: 1983. Family Planning Perspectives 1983;15:50-56.
- The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development: Oral contraceptive use and the risk of breast cancer. N Engl J Med 1986;315:405-411.
- Pike MC, Henderson BE, Krailo MD, Duke A, Roy S. Breast cancer in young women and use of oral contraceptives: possible modifying effect of formulation and age at use. Lancet 1983;2:926-929.
- Paul C, Skegg DG, Spears GFS, Kaldor JM. Oral contraceptives and breast cancer: A national study. Br Med J 1986; 293:723-725.
- Miller DR, Rosenberg L, Kaufman DW, Schottenfeld D, Stolley PD, Shapiro S. Breast cancer risk in relation to early oral contraceptive use. Obstet Gynecol 1986;68:863-868.
- Olson H, Olson KL, Moller TR, Ranstam J, Holm P. Oral contraceptive use and breast cancer in young women in Sweden (letter). Lancet 1985; 2:748-749.
- McPherson K, Vessey M, Neil A, Doll R, Jones L, Roberts M. Early contraceptive use and breast cancer: Results of another case-control study. Br J Cancer 1987; 56:653-660.
- Huggins GR, Zucker PF. Oral contraceptives and neoplasia; 1987 update. Fertil Steril 1987; 47:733-761.
- McPherson K, Drife JO. The pill and breast cancer: why the uncertainty? Br Med J 1986; 293:709-710.
- Shapiro S. Oral contraceptives-time to take stock. N Engl J Med 1987; 315:450-451.
- Ory H, Naib Z, Conger SB, Hatcher RA, Tyler CW. Contraceptive choice and prevalence of cervical dysplasia and carcinoma in situ. Am J Obstet Gynecol 1976; 124:573-577.
- Vessey MP, Lawless M, McPherson K, Yeates D. Neoplasia of the cervix uteri and contraception: a possible adverse effect of the pill. Lancet 1983; 2:930.
- Brinton LA, Huggins GR, Lehman HF, Malli K, Savitz DA, Trapido E, Rosenthal J, Hoover R. Long term use of oral contraceptives and risk of invasive cervical cancer. Int J Cancer 1986;38:339-344.
- WHO Collaborative Study of Neoplasia and Steroid Contraceptives: Invasive cervical cancer and combined oral contraceptives. Br Med J 1985; 290:961-965.
- Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW. Epidemiology of hepatocellular adenoma: the role of oral contraceptive use. JAMA 1979; 242:644-648.
- Bein NN, Goldsmith HS. Recurrent massive hemorrhage from benign hepatic tumors secondary to oral contraceptives. Br J Surg 1977; 64:433-435.
- Klatskin G. Hepatic tumors: possible relationship to use of oral contraceptives. Gastroenterology 1977; 73:386-394.
- Henderson BE, Preston-Martin S, Edmondson HA, Peters RL, Pike MC. Hepatocellular carcinoma and oral contraceptives. Br J Cancer 1983;48:437-440.
- Neuberger J, Forman D, Doll R, Williams R. Oral contraceptives and hepatocellular carcinoma. Br Med J 1986; 292:1355-1357.
- Forman D, Vincent TJ, Doll R. Cancer of the liver and oral contraceptives. Br Med J 1986; 292:1357-1361.
- Harlap S, Eldor J. Births following oral contraceptive failures. Obstet Gynecol 1980; 55:447-452.
- Savolainen E, Saksela E, Saxen L. Teratogenic hazards of oral contraceptives analyzed in a national malformation register. Am J Obstet Gynecol 1981:140:521-524.
- Janerich DT, Piper JM, Glebatis DM. Oral contraceptives and birth defects. Am J Epidemiol 1980; 112:73-79.
- Ferencz C, Matanoski GM, Wilson PD, Rubin JD, Neill CA, Gutberlet R. Maternal hormone therapy and congenital heart disease. Teratology 1980; 21:225-239.
- Rothman KJ, Fyler DC, Goldblatt A, Kreidberg MB. Exogenous hormones and other drug exposures of children with congenital heart disease. Am J Epidemiol 1979; 109:433-439.
- Boston Collaborative Drug Surveillance Program: Oral contraceptives and venous thromboembolic disease, surgically confirmed gallbladder disease, and breast tumors. Lancet 1973; 1:1399-1404.
- Royal College of General Practitioners: Oral contraceptives and health. New York, Pittman 1974.
- Layde PM, Vessey MP, Yeates D. Risk of gallbladder disease: a cohort study of young women attending family planning clinics. J Epidemiol Community Health 1982; 36:274-278.
- Rome Group for Epidemiology and Prevention of Cholelithiasis (GREPCO): Prevalence of gallstone disease in an Italian adult female population. Am J Epidemiol 1984; 119:796-805.
- Strom BL, Tamragouri RT, Morse ML, Lazar EL, West SL, Stolley PD, Jones JK. Oral contraceptives and other risk factors for gallbladder disease. Clin Pharmacol Ther 1986; 39:335-341.
- Wynn V, Adams PW, Godsland IF, Melrose J, Niththyananthan R, Oakley NW, Seedj A. Comparison of effects of different combined oral contraceptive formulations on carbohydrate and lipid metabolism. Lancet 1979; 1:1045-1049.
- Wynn V. Effect of progesterone and progestins on carbohydrate metabolism. In: Progesterone and Progestin. Bardin CW, Milgrom E, Mauvis-Jarvis P. eds. New York, Raven Press 1983; pp. 395-410.
- Perlman JA, Roussell-Briefel RG, Ezzati TM, Lieberknecht G. Oral glucose tolerance and the potency of oral contraceptive progestogens. J Chronic Dis 1985;38:857-864.
- Royal College of General Practitioners' Oral Contraception Study: Effect on hypertension and benign breast disease of progestogen component in combined oral contraceptives. Lancet 1977; 1:624.
- Fisch IR, Frank J. Oral contraceptives and blood pressure. JAMA 1977; 237:2499-2503.
- Laragh AJ. Oral contraceptive induced hypertension-nine years later. Am J Obstet Gynecol 1976; 126:141-147.
- Ramcharan S, Peritz E, Pellegrin FA, Williams WT. Incidence of hypertension in the Walnut Creek Contraceptive Drug Study cohort: In: Pharmacology of steroid contraceptive drugs. Garattini S, Berendes HW. Eds. New York, Raven Press, 1977; pp. 277-288, (Monographs of the Mario Negri Institute for Pharmacological Research Milan.)
- Stockley I. Interactions with oral contraceptives. J Pharm 1976;216:140-143.
- The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development: Oral contraceptive use and the risk of ovarian cancer. JAMA 1983; 249:1596-1599.
- The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development: Combination oral contraceptive use and the risk of endometrial cancer. JAMA 1987; 257:796-800.
- Ory HW. Functional ovarian cysts and oral contraceptives: negative association confirmed surgically. JAMA 1974; 228:68-69.
- Ory HW, Cole P, MacMahon B, Hoover R. Oral contraceptives and reduced risk of benign breast disease. N Engl J Med 1976; 294:419-422.
- Ory HW. The noncontraceptive health benefits from oral contraceptive use. Fam Plann Perspect 1982; 14:182-184.
- Ory HW, Forrest JD, Lincoln R. Making choices: Evaluating the health risks and benefits of birth control methods. New York, The Alan Guttmacher Institute, 1983; p.1.
- Schlesselman J, Stadel BV, Murray P, Lai S. Breast cancer in relation to early use of oral contraceptives. JAMA 1988; 259:1828-1833.
- Hennekens CH, Speizer FE, Lipnick RJ, Rosner B, Bain C, Belanger C, Stampfer MJ, Willett W, Peto R. A case-control study of oral contraceptive use and breast cancer. JNCI 1984; 72:39-42.
- LaVecchia C, Decarli A, Fasoli M, Franceschi S, Gentile A, Negri E, Parazzini F, Tognoni G. Oral contraceptives and cancers of the breast and of the female genital tract. Interim results from a case-control study. Br J Cancer 1986; 54:311-317.
- Meirik O, Lund E, Adami H, Bergstrom R, Christoffersen T, Bergsjo P. Oral contraceptive use and breast cancer in young women. A Joint National Case-control study in Sweden and Norway. Lancet 1986; 11:650-654.
- Kay CR, Hannaford PC. Breast cancer and the pill-A further report from the Royal College of General Practitioners' oral contraception study. Br J Cancer 1988;58:675-680.
- Stadel BV, Lai S, Schlesselman JJ, Murray P. Oral contraceptives and premenopausal breast cancer in nulliparous women. Contraception 1988; 38:287-299.
- Miller DR, Rosenberg L, Kaufman DW, Stolley P, Warshauer ME, Shapiro S. Breast cancer before age 45 and oral contraceptive use: New Findings. Am J Epidemiol 1989; 129:269-280.
- The UK National Case-Control Study Group, Oral contraceptive use and breast cancer risk in young women. Lancet 1989; 1:973-982.
- Schlesselman JJ. Cancer of the breast and reproductive tract in relation to use of oral contraceptives. Contraception 1989; 40:1-38.
- Vessey MP, McPherson K, Villard-Mackintosh L, Yeates D. Oral contraceptives and breast cancer: latest findings in a large cohort study. Br J Cancer 1989; 59:613-617.
- Jick SS, Walker AM, Stergachis A, Jick H. Oral contraceptives and breast cancer. Br J Cancer 1989; 59:618-621.
- Anderson FD, Selectivity and minimal androgenicity of norgestimate in monophasic and triphasic oral contraceptives. Acta Obstet Gynecol Scand 1992; 156 (Supplement):15-21.
- Chapdelaine A, Desmaris J-L, Derman RJ. Clinical evidence of minimal androgenic activity of norgestimate. Int J Fertil 1989; 34(51):347-352.
- Phillips A, Demarest K, Hahn DW, Wong F, McGuire JL. Progestational and androgenic receptor binding affinities and in vivo activities of norgestimate and other progestins. Contraception 1989; 41(4):399-409.
- Phillips A, Hahn DW, Klimek S, McGuire JL. A comparison of the potencies and activities of progestogens used in contraceptives. Contraception 1987; 36(2):181-192.
- Janaud A, Rouffy J, Upmalis D, Dain M-P. A comparison study of lipid and androgen metabolism with triphasic oral contraceptive formulations containing norgestimate or levonorgestrel Acta Obstet Gynecol Scand 1992; 156 (Supplement):34-38.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347:1713-1727.
- Palmer JR, Rosenberg L, Kaufman DW, Warshauer ME, Stolley P, Shapiro S. Oral Contraceptive Use and Liver Cancer. Am J Epidemiol 1989;130:878-882.
- Lewis M, Spitzer WO, Heinemann LAJ, MacRae KD, Bruppacher R, Thorogood M on behalf of Transnational Research Group on Oral Contraceptives and Health of Young Women. Third generation oral contraceptives and risk of myocardial infarction: an international case-control study. Br Med J, 1996;312:88-90.
- Vessey MP, Smith MA, Yeates D. Return of fertility after discontinuation of oral contraceptives: influence of age and parity. Brit J Fam Plan; 1986; 11:120-124.
- Back DJ, Orme M.L'E. Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokinet 1990; 18:472-484.
- Rosenfeld WE, Doose DR, Walker SA, Nayak RK. Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy. Epilepsia 1997 Mar;38(3):317-323.
- Shenfield GM. Oral Contraceptives. Are drug interaction of clinical significance? Drug Saf 1993 Jul;9(1):21-37.
- Ouellet D, Hsu A, Qian J, Locke CS, Eason CJ, Cavanaugh JH, Leonard JM, Granneman GR. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br J Clin Pharmacol 1998;46(2):111-116.
- Improving access to quality care in family planning: Medical eligibility criteria for contraceptive use. Geneva, WHO, Family and Reproductive Health, 1996 (WHO/FRH/FPP/96.9).
- Skolnick JL, Stoler BS, Katz DG, Anderson WH. Rifampicin, oral contraceptives and pregnancy. J Am Med Assoc 1976;236-1382.
- Henney JE. Risk of drug interactions with St. John's Wort. JAMA 2000;283(13).
- Lahteennmaki P et al, Coagulation factors in women using oral contraceptives or intrauterine devices immediately after abortion. American Journal of Obstetrics and Gynecology, (1981); 141: 175-179.
DETAILED PATIENT LABELING
ORTHO EVRA® (norelgestromin/ethinyl estradiol transdermal system)
Rx only
This product is intended to prevent pregnancy. It does not protect against HIV (AIDS) or other sexually transmitted diseases.
DESCRIPTION
The contraceptive patch ORTHO EVRA® is a thin, beige, plastic patch that sticks to the skin. The sticky part of the patch contains the following hormones: norelgestromin (progestin) and ethinyl estradiol (estrogen). These hormones are absorbed continuously through the skin and into the bloodstream. Each patch delivers 150 micrograms of norelgestromin and 20 micrograms of ethinyl estradiol to the bloodstream per 24 hours. This amount of estrogen delivered through the skin, results in estrogen blood levels that are greater than the amount of estrogen you would be exposed to when taking a birth control pill containing 20 micrograms ethinyl estradiol. Each patch is sealed in a pouch that protects it until you are ready to wear it.
INTRODUCTION
Any woman who considers using the contraceptive patch ORTHO EVRA® should understand the benefits and risks of using this form of birth control. This leaflet will give you much of the information you will need to make this decision and will also help you determine if you are at risk of developing any serious side effects. It will tell you how to use the contraceptive patch properly so that it will be as effective as possible. However, this leaflet is not a replacement for a careful discussion between you and your health care professional. You should discuss the information provided in this leaflet with him or her, both when you first start using the contraceptive patch ORTHO EVRA® and during your revisits. You should also follow your health care professional's advice with regard to regular check-ups while you are using the contraceptive patch.
EFFECTIVENESS OF HORMONAL CONTRACEPTIVE METHODS
Hormonal contraceptives, including ORTHO EVRA®, are used to prevent pregnancy and are more effective than most other non-surgical methods of birth control. When ORTHO EVRA® is used correctly, the chance of becoming pregnant is approximately 1% (1 pregnancy per 100 women per year of use when used correctly), which is comparable to that of the pill. The chance of becoming pregnant increases with incorrect use.
Clinical trials suggested that ORTHO EVRA® may be less effective in women weighing more than 198 lbs. (90 kg). If you weigh more than 198 lbs. (90 kg) you should talk to your health care professional about which method of birth control may be best for you.
Typical failure rates for other methods of birth control during the first year of use are as follows:
Implant: <1%
Injection: <1%
IUD: <1-2%
Diaphragm with spermicides: 20%
Spermicides alone: 26%
Female sterilization: <1%
Male sterilization: <1%
Cervical Cap with spermicide: 20 to 40%
Condom alone (male): 14%
Condom alone (female): 21%
Periodic abstinence: 25%
No birth control method: 85%
Withdrawal: 19%
WHO SHOULD NOT USE ORTHO EVRA®
Hormonal contraceptives include birth control pills, injectables, implants, the vaginal ring, and the contraceptive patch. The following information is derived primarily from studies of birth control pills. The contraceptive patch is expected to be associated with similar risks:
Cigarette smoking increases the risk of serious cardiovascular side effects from hormonal contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use hormonal contraceptives, including ORTHO EVRA®, should be strongly advised not to smoke. Some women should not use the ORTHO EVRA® contra-ceptive patch. For example, you should not use ORTHO EVRA® if you are pregnant or think you may be pregnant. You should also not use ORTHO EVRA® if you have any of the following conditions:
- A history of heart attack or stroke
- Blood clots in the legs (thrombophlebitis), lungs (pulmonary embolism), or eyes
- A history of blood clots in the deep veins of your legs
- Chest pain (angina pectoris)
- Known or suspected breast cancer or cancer of the lining of the uterus, cervix or vagina.
- Unexplained vaginal bleeding (until your doctor reaches a diagnosis)
- Hepatitis or yellowing of the whites of your eyes or of the skin (jaundice) during pregnancy or during previous use of hormonal contraceptives such as ORTHO EVRA®, NORPLANT, or the birth control pill
- Liver tumor (benign or cancerous)
- Known or suspected pregnancy
- Severe high blood pressure
- Diabetes with complications of the kidneys, eyes, nerves, or blood vessels
- Headaches with neurological symptoms
- Use of oral contraceptives (birth control pills)
- Disease of heart valves with complications
- Need for a prolonged period of bed rest following major surgery
- An allergic reaction to any of the components of ORTHO EVRA®
Tell your health care professional if you have ever had any of these conditions. Your health care professional can recommend a non-hormonal method of birth control.
OTHER CONSIDERATIONS BEFORE USING ORTHO EVRA®
Talk to your health care professional about using ORTHO EVRA® if
- you smoke
- you are recovering from the birth of a baby
- you are recovering from a second trimester miscarriage or abortion
- you are breast feeding
- you weigh 198 pounds or more
- you are taking any other medications
Also, tell your health care professional if you have or have had:
- Breast nodules, fibrocystic disease of the breast, an abnormal breast x-ray or mammogram
- A family history of breast cancer
- Diabetes
- Elevated cholesterol or triglycerides
- High blood pressure
- Migraine or other headaches or epilepsy
- Depression
- Gallbladder disease
- Liver disease
- Heart disease
- Kidney disease
- Scanty or irregular menstrual periods
If you have any of these conditions you should be checked often by your health care professional if you use the contraceptive patch.
RISKS OF USING HORMONAL CONTRACEPTIVES, INCLUDING ORTHO EVRA®
The following information is derived primarily from studies of birth control pills. Since ORTHO EVRA® contains hormones similar to those found in birth control pills, it is expected to be associated with similar risks:
-
Risk of Developing Blood Clots
Blood clots and blockage of blood vessels that can cause death or serious disability are some of the most serious side effects of using hormonal contraceptives, including the ORTHO EVRA® contraceptive patch. In particular, a clot in the legs can cause thrombophlebitis, and a clot that travels to the lungs can cause sudden blocking of the vessel carrying blood to the lungs. Rarely, clots occur in the blood vessels of the eye and may cause blindness, double vision, or impaired vision.
If you use ORTHO EVRA® and need elective surgery, need to stay in bed for a prolonged illness or injury or have recently delivered a baby, you may be at risk of developing blood clots. You should consult your doctor about stopping ORTHO EVRA® four weeks before surgery and not using it for two weeks after surgery or during bed rest. You should also not use ORTHO EVRA® soon after delivery of a baby. It is advisable to wait for at least four weeks after delivery if you are not breast-feeding. If you are breast-feeding, you should wait until you have weaned your child before using ORTHO EVRA®. (See also the section on Breast Feeding in General Precautions .) -
Heart Attacks and Strokes
Hormonal contraceptives, including ORTHO EVRA®, may increase the risk of developing strokes (blockage or rupture of blood vessels in the brain) and angina pectoris and heart attacks (blockage of blood vessels in the heart). Any of these conditions can cause death or serious disability.
Smoking and the use of hormonal contraceptives including ORTHO EVRA® greatly increase the chances of developing and dying of heart disease. Smoking also greatly increases the possibility of suffering heart attacks and strokes. -
Gallbladder Disease
Women who use hormonal contraceptives, including ORTHO EVRA®, probably have a greater risk than nonusers of having gallbladder disease. -
Liver Tumors
In rare cases, combination oral contraceptives can cause benign but dangerous liver tumors. Since ORTHO EVRA® contains hormones similar to those in birth control pills, this association may also exist with ORTHO EVRA®. These benign liver tumors can rupture and cause fatal internal bleeding. In addition, some studies report an increased risk of developing liver cancer. However, liver cancers are rare. -
Cancer of the Reproductive Organs and Breasts
Various studies give conflicting reports on the relationship between breast cancer and hormonal contraceptive use. Combination hormonal contraceptives, including ORTHO EVRA®, may slightly increase your chance of having breast cancer diagnosed, particularly after using hormonal contraceptives at a younger age. After you stop using hormonal contraceptives, the chances of having breast cancer diagnosed begin to go back down. You should have regular breast examinations by a health care professional and examine your own breasts monthly. Tell your health care professional if you have a family history of breast cancer or if you have had breast nodules or an abnormal mammogram.
Women who currently have or have had breast cancer should not use oral contraceptives because breast cancer is usually a hormone-sensitive tumor.
Some studies have found an increase in the incidence of cancer of the cervix in women who use oral contraceptives, although this finding may be related to factors other than the use of oral contraceptives. However, there is insufficient evidence to rule out the possibility that oral contraceptives may cause such cancers.
ESTIMATED RISK OF DEATH FROM A BIRTH CONTROL METHOD OR PREGNANCY
All methods of birth control and pregnancy are associated with a risk of developing certain diseases that may lead to disability or death. An estimate of the number of deaths associated with different methods of birth control and pregnancy has been calculated and is shown in the following table.
ORTHO EVRA® is expected to be associated with similar risks as oral contraceptives:
Annual Number of Birth-Related or Method-Related Deaths Associated With Control of Fertility Per 100,000 Nonsterile Women
by Fertility Control Method According to AgeMethod of control
and outcome15-19 20-24 25-29 30-34 35-39 40-44 No fertility control
methods *7.0 7.4 9.1 14.8 25.7 28.2 Oral contraceptives
non-smoker **0.3 0.5 0.9 1.9 13.8 31.6 Oral contraceptives
smoker **2.2 3.4 6.6 13.5 51.1 117.2 IUD **0.8 0.8 1.0 1.0 11.4 1.4 Condom *1.1 1.6 0.7 0.2 0.3 0.4 Diaphragm/spermicide *1.9 1.2 1.2 1.3 2.2 2.8 Periodic abstinence *2.5 1.6 1.6 1.7 2.9 3.6 *Deaths are birth-related**Deaths are method-related
Adapted from H.W. Ory, ref. # 35.
In the above table, the risk of death from any birth control method is less than the risk of childbirth, except for oral contraceptive users over the age of 35 who smoke and pill users over the age of 40 even if they do not smoke. It can be seen in the table that for women aged 15 to 39, the risk of death was highest with pregnancy (7-26 deaths per 100,000 women, depending on age). Among pill users who do not smoke, the risk of death is always lower than that associated with pregnancy for any age group, although over the age of 40, the risk increases to 32 deaths per 100,000 women, compared to 28 associated with pregnancy at that age. However, for pill users who smoke and are over the age of 35, the estimated number of deaths exceeds those for other methods of birth control. If a woman is over the age of 40 and smokes, her estimated risk of death is four times higher (117/100,000 women) than the estimated risk associated with pregnancy (28/100,000 women) in that age group.
In 1989 an Advisory Committee of the FDA concluded that the benefits of low-dose hormonal contraceptive use by healthy, non-smoking women over 40 years of age may outweigh the possible risks.
WARNING SIGNALS
If any of these adverse effects occur while you are using ORTHO EVRA®, call your doctor immediately:
- Sharp chest pain, coughing of blood, or sudden shortness of breath (indicating a possible clot in the lung)
- Pain in the calf (indicating a possible clot in the leg)
- Crushing chest pain or tightness in the chest (indicating a possible heart attack)
- Sudden severe headache or vomiting, dizziness or fainting, disturbances of vision or speech, weakness, or numbness in an arm or leg (indicating a possible stroke)
- Sudden partial or complete loss of vision (indicating a possible clot in the eye)
- Breast lumps (indicating possible breast cancer or fibrocystic disease of the breast; ask your doctor or health care professional to show you how to examine your breasts)
- Severe pain or tenderness in the stomach area (indicating a possibly ruptured liver tumor)
- Severe problems with sleeping, weakness, lack of energy, fatigue, or change in mood (possibly indicating severe depression)
- Jaundice or a yellowing of the skin or eyeballs accompanied frequently by fever, fatigue, loss of appetite, dark colored urine, or light colored bowel movements (indicating possible liver problems)
SIDE EFFECTS OF ORTHO EVRA®
-
Skin Irritation
Skin irritation, redness or rash may occur at the site of application. If this occurs, the patch may be removed and a new patch may be applied to a new location until the next Change Day. Single replacement patches are available from pharmacies. -
Vaginal Bleeding
Irregular vaginal bleeding or spotting may occur while you are using ORTHO EVRA®. Irregular bleeding may vary from slight staining between menstrual periods to breakthrough bleeding which is a flow much like a regular period. Irregular bleeding may occur during the first few months of contraceptive patch use but may also occur after you have been using the contraceptive patch for some time. Such bleeding may be temporary and usually does not indicate any serious problems. It is important to continue using your contraceptive patches on schedule. If the bleeding occurs in more than a few cycles or lasts for more than a few days, talk to your health care professional. -
Problems Wearing Contact Lenses
If you wear contact lenses and notice a change in vision or an inability to wear your lenses, contact your health care professional. -
Fluid Retention or Raised Blood Pressure
Hormonal contraceptives, including the contraceptive patch, may cause edema (fluid retention) with swelling of the fingers or ankles and may raise your blood pressure. If you experience fluid retention, contact your health care professional. -
Melasma
A spotty darkening of the skin is possible, particularly of the face. This may persist after use of hormonal contraceptives is discontinued. -
Other Side Effects
The most common side effects of ORTHO EVRA® include nausea and vomiting, breast symptoms, headache, menstrual cramps, and abdominal pain. In addition, change in appetite, nervousness, depression, dizziness, loss of scalp hair, rash, and vaginal infections may occur.
GENERAL PRECAUTIONS
-
Weight >/= 198 lbs. (90 kg)
Clinical trials suggest that ORTHO EVRA® may be less effective in women weighing 198 lbs. (90 kg) or more compared with its effectiveness in women with lower body weights. If you weigh 198 lbs. (90 kg) or more you should talk to your health care professional about which method of birth control may be best for you. -
Missed Periods and Use of ORTHO EVRA® Before or During Early Pregnancy
There may be times when you may not menstruate regularly during your patch-free week. If you have used ORTHO EVRA® correctly and miss one menstrual period, continue using your contraceptive patches for the next cycle but be sure to inform your health care professional before doing so. If you have not used ORTHO EVRA® as instructed and missed a menstrual period, or if you missed two menstrual periods in a row, you could be pregnant. Check with your health care professional immediately to determine whether you are pregnant. Stop using ORTHO EVRA® if you are pregnant.
There is no conclusive evidence that hormonal contraceptive use causes birth defects when taken accidentally during early pregnancy. Previously, a few studies had reported that oral contraceptives might be associated with birth defects, but these findings have not been seen in more recent studies. Nevertheless, hormonal contraceptives, including ORTHO EVRA®, should not be used during pregnancy. You should check with your health care professional about risks to your unborn child from any medication taken during pregnancy. -
While Breast-Feeding
If you are breast-feeding, consult your health care professional before starting ORTHO EVRA®. Hormonal contraceptives are passed on to the child in the milk. A few adverse effects on the child have been reported, including yellowing of the skin (jaundice) and breast enlargement. In addition, combination hormonal contraceptives may decrease the amount and quality of your milk. If possible, do not use combination hormonal contraceptives such as ORTHO EVRA® while breast-feeding. You should use a barrier method of contraception since breast-feeding provides only partial protection from becoming pregnant and this partial protection decreases significantly as you breast-feed for longer periods of time. You should consider starting ORTHO EVRA® only after you have weaned your child completely. -
Laboratory Tests
If you are scheduled for any laboratory tests, tell your doctor you are using ORTHO EVRA® since certain blood tests may be affected by hormonal contraceptives. -
Drug Interactions
Certain drugs may interact with hormonal contraceptives, including ORTHO EVRA®, to make them less effective in preventing pregnancy or cause an increase in breakthrough bleeding. Such drugs include rifampin, drugs used for epilepsy such as barbiturates (for example, phenobarbital), anticonvulsants such as topiramate (TOPAMAX), carbamazepine (Tegretol is one brand of this drug), phenytoin (Dilantin is one brand of this drug), phenylbutazone (Butazolidin is one brand), certain drugs used in the treatment of HIV or AIDS, and possibly certain antibiotics. Tetracycline has been shown not to interact with ORTHO EVRA®. Pregnancies and breakthrough bleeding have been reported by users of combined hormonal contraceptives who also used some form of St. John's Wort.
As with all prescription products, you should notify your health care professional of any other medications you are taking. You may need to use a barrier contraceptive when you take drugs that can make ORTHO EVRA® less effective. -
Sexually Transmitted Diseases
ORTHO EVRA® is intended to prevent pregnancy. It does not protect against HIV (AIDS) or other sexually transmitted diseases such as chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
HOW TO USE ORTHO EVRA®
Instructions for Use
ORTHO EVRA® keeps you from becoming pregnant by transferring hormones to your body through your skin. The patch must stick securely to your skin in order for it to work properly.
This method uses a 28 day (four week) cycle. You should apply a new patch each week for three weeks (21 total days). You should not apply a patch during the fourth week. Your menstrual period should start during this patch-free week.
Every new patch should be applied on the same day of the week. This day will be your 'Patch Change Day.' For example, if you apply your first patch on a Monday, all of your patches should be applied on a Monday. You should wear only one patch at a time.
On the day after week four ends, you should begin a new four week cycle by applying a new patch.
Save these instructions.
1
If this is the first time you are using ORTHO EVRA®, wait until the day you get your menstrual period . The day you apply your first patch will be Day 1. Your 'Patch Change Day' will be on this day every week.

2
You may choose a first day start or Sunday start
· for First Day start: apply your first patch during the first 24 hours of your menstrual period.
OR
· for Sunday start: apply your first patch on the first Sunday after your menstrual period starts. You must use back-up contraception, such as a condom, spermicide, or diaphragm for the first week of your first cycle.
· The day you apply your first patch will be Day 1. Your 'Patch Change Day' will be on this day every week.
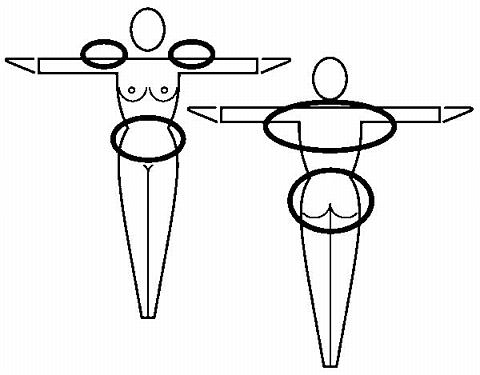
3
Choose a place on your body to put the patch. Put the patch on your buttock, abdomen, upper outer arm or upper torso, in a place where it won't be rubbed by tight clothing. Never put the patch on your breasts. To avoid irritation, apply each new patch to a different place on your skin.
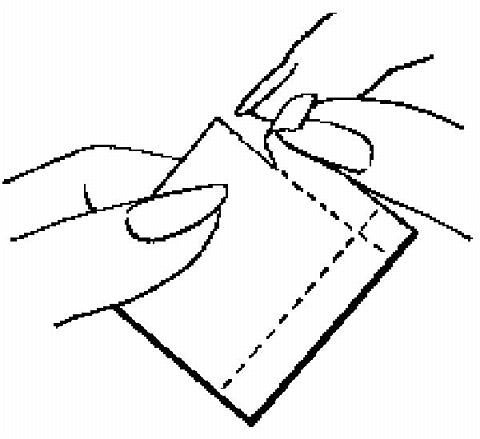
4
Open the foil pouch by tearing it along the top edge and one side edge.
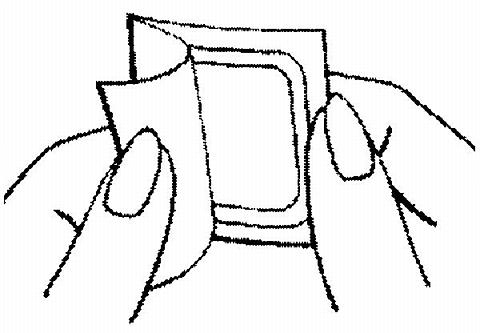
Peel the foil pouch apart and open it flat.
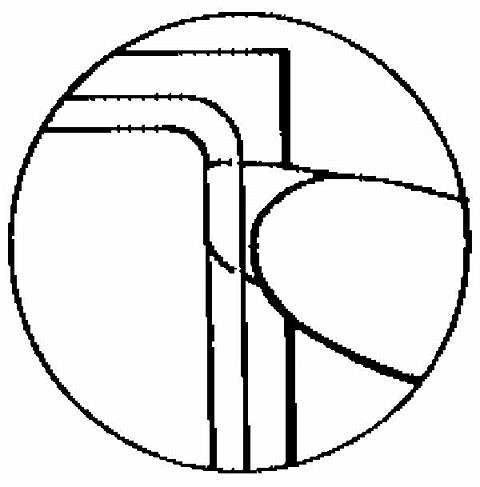
5
You will see that the patch is covered by a layer of clear plastic. It is important to remove the patch and the plastic together from the foil pouch.
Using your fingernail, lift one corner of the patch and peel the patch and the plastic off the foil liner.
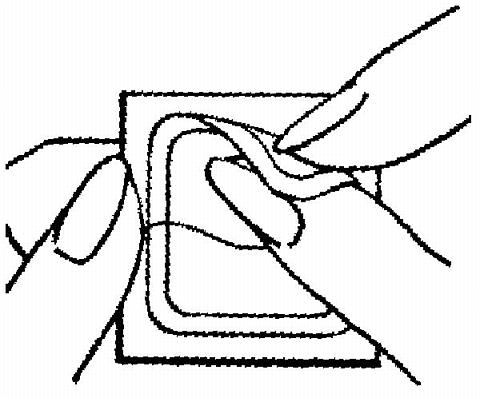
Sometimes patches can stick to the inside of the pouch - be careful not to accidentally remove the clear liner as you remove the patch.
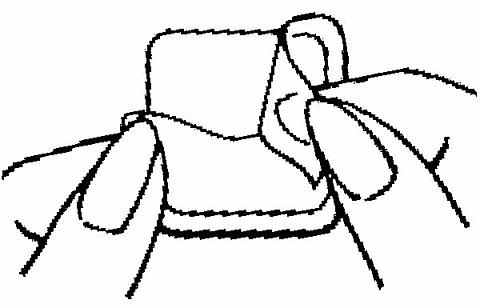
6
Peel away half of the clear plastic and be careful not to touch the exposed sticky surface of the patch with your fingers.
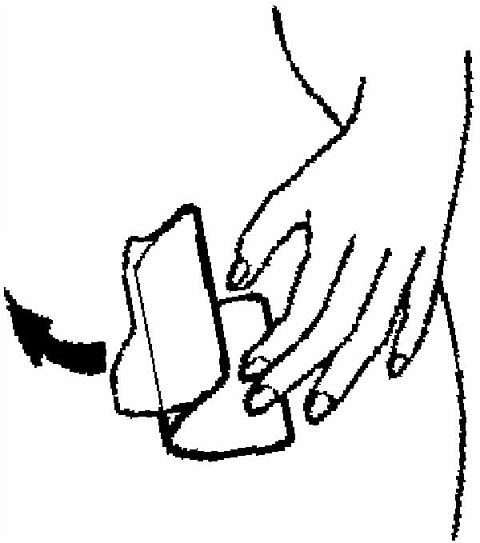
7
Apply the sticky side of the patch to the skin you've cleaned and dried, then remove the other half of the clear plastic.
Press firmly on the patch with the palm of your hand for 10 seconds, making sure the edges stick well. Run your finger around the edge of the patch to make sure it is sticking properly.
Check your patch every day to make sure all the edges are sticking.
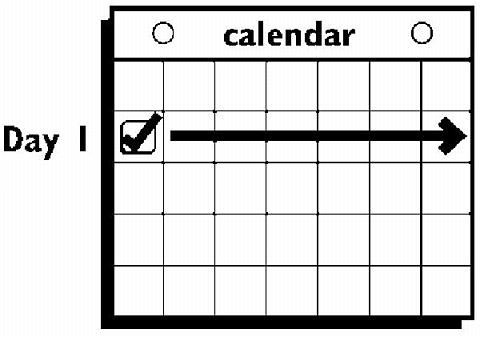
8
Wear the patch for seven days (one week). On your 'Patch Change Day,' Day 8, remove the used patch. Apply a new patch immediately. The used patch still contains some medicine - carefully fold it in half so that it sticks to itself before safely disposing of it in the trash. Used patches should not be flushed down the toilet.
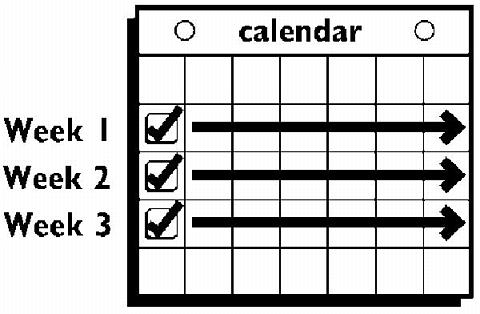
9
Apply a new patch for week two (on Day 8) and for week three (on Day 15), on your 'Patch Change Day.' To avoid irritation, do not apply the new patch to the same exact place on your skin.
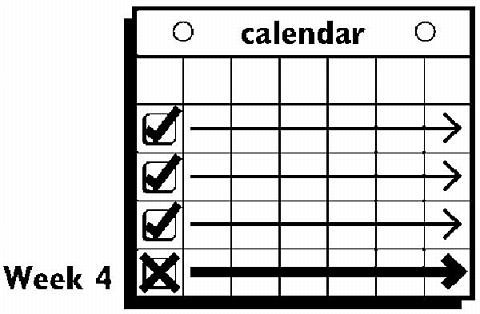
10
Do not wear a patch on week four (Day 22 through Day 28). Your period should start during this week.
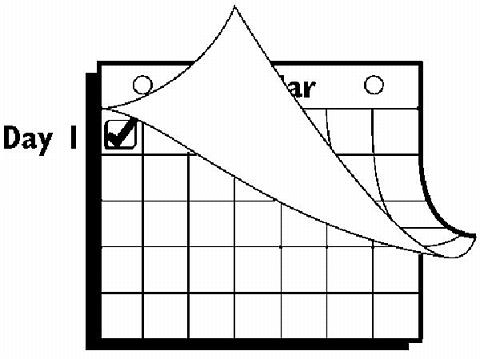
11
Begin your next four week cycle by applying a new patch on your normal 'Patch Change Day,' the day after Day 28 - no matter when your period begins or ends.
If your patch has become loose or has fallen off...
- for less than one day , try to re-apply it or apply a new patch immediately. No back-up contraception is needed. Your 'Patch Change Day' will remain the same.
- for more than one day OR if you are not sure for how long , YOU MAY BECOME PREGNANT - Start a new four week cycle immediately by putting on a new patch. You now have a new Day 1 and a new 'Patch Change Day.' You must use back-up contraception, such as a condom, spermicide, or diaphragm for the first week of your new cycle.
- do not try to re-apply a patch if it's no longer sticky, if it has become stuck to itself or another surface, if it has other material stuck to it or if it has previously become loose or fallen off. No tapes or wraps should be used to keep the patch in place. If you cannot re-apply a patch, apply a new patch immediately.
If you forget to change your patch...
-
at the start of any patch cycle,
Week one (Day 1): If you forget to apply your patch, YOU COULD BECOME PREGNANT - you must use back-up contraception for one week. Apply the first patch of your new cycle as soon as you remember. You now have a new 'Patch Change Day' and new Day 1 . -
in the middle of your patch cycle,
Week two or week three: If you forget to change your patch for one or two days , apply a new patch as soon as you remember. Apply your next patch on your normal 'Patch Change Day.' No back-up contraception is needed.
Week two or week three: If you forget to change your patch for more than two days , YOU COULD BECOME PREGNANT - start a new four week cycle as soon as you remember by putting on a new patch . You now have a different 'Patch Change Day' and a new Day 1. You must use back-up contraception for the first week of your new cycle. -
at the end of your patch cycle,
Week four: If you forget to remove your patch, take it off as soon as you remember. Start your next cycle on your normal 'Patch Change Day,' the day after Day 28. No back-up contraception is needed. -
at the start of your next patch cycle,
Day 1 (week one): If you forget to apply your patch, YOU COULD BECOME PREGNANT - apply the first patch of your new cycle as soon as you remember. You now have a new 'Patch Change Day' and new Day 1. You must use back-up contraception for the first week of your new cycle. - you should never have the patch off for more than seven days.
Other information...
- Always apply your patch to clean, dry skin. Avoid skin that is red, irritated or cut. Do not use creams, oils, powder or makeup on your skin where you will put a patch or near a patch you are wearing. It may cause the patch to become loose.
- If patch use results in uncomfortable irritation, the patch may be removed and a new patch may be applied to a new location until the next Change Day. Only one patch should be worn at a time.
- Some medicines may change the way ORTHO EVRA® works. If you are taking any medication, you must talk to your health care professional BEFORE you use the patch. You may need to use back-up contraception.
- Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
- Single replacement patches are available through your pharmacist.
- For further information log on to www.orthoevra.com or call toll free 1 877 EVRA 888
WHEN YOU SWITCH FROM THE PILL TO ORTHO EVRA®:
If you are switching from the pill to ORTHO EVRA®, wait until you get your menstrual period. If you do not get your period within five days of taking the last active pill, check with your health care professional to be sure that you are not pregnant.
IMPORTANT POINTS TO REMEMBER
- IT IS IMPORTANT TO USE ORTHO EVRA® exactly as directed in this leaflet. Incorrect use increases your chances of becoming pregnant. This includes starting your contraceptive cycle late or missing your scheduled CHANGE DAYS.
- You should wear one patch per week for three weeks, followed by one week off. You should never have the patch off for more than seven days in a row . If you have the patch off for more than seven days in a row and you have had sex during this time, YOU COULD BECOME PREGNANT.
-
IF YOU ARE NOT SURE WHAT TO DO ABOUT MISTAKES WITH PATCH USE:
- Use a BACK-UP METHOD, such as a condom, spermicide, or diaphragm anytime you have sex.
- Contact your health care professional for instructions.
- Do not skip patches even if you do not have sex very often.
- SOME WOMEN HAVE SPOTTING OR LIGHT BLEEDING, BREAST TENDERNESS OR MAY FEEL SICK TO THEIR STOMACH DURING ORTHO EVRA® USE. If these symptoms occur, do not stop using the contraceptive patch. The problem will usually go away. If it doesn't go away, check with your health care professional.
- MISTAKES IN USING YOUR PATCHES CAN ALSO CAUSE SPOTTING OR LIGHT BLEEDING.
- If you miss TWO PERIODS IN A ROW contact your health care professional because you might be pregnant.
- The amount of drug you get from the ORTHO EVRA® patch should not be affected by VOMITING OR DIARRHEA.
- IF YOU TAKE CERTAIN MEDICINES, ORTHO EVRA® may not work as well. Use a non-hormonal back-up method (such as condoms, spermicide, or diaphragm) until you check with your health care professional.
- IF YOU WANT TO MOVE YOUR PATCH CHANGE DAY to a different day of the week, finish your current cycle, removing your third ORTHO EVRA® patch on the correct day. During week four , the "patch-free week" (Day 22 through Day 28), you may choose an earlier Patch Change Day by applying a new patch on the day you prefer. You now have a new Day 1 and a new Patch Change Day. You should never have the patch off for more than seven days in a row.
-
BE SURE YOU HAVE READY AT ALL TIMES:
- A NON-HORMONAL BIRTH CONTROL method (such as condoms, spermicide, or diaphragm) to use as a back-up in case of dosing errors.
- IF YOU HAVE TROUBLE REMEMBERING TO CHANGE YOUR CONTRACEPTIVE PATCH, talk to your health care professional about how to make patch-changing easier or about using another method of birth control.
- Single replacement patches are available through your pharmacist.
- For Patch replacement, see "How to use ORTHO EVRA®" section.
IF YOU HAVE ANY QUESTIONS OR ARE UNSURE ABOUT THE INFORMATION IN THIS LEAFLET, call your health care professional.
PREGNANCY DUE TO ORTHO EVRA® FAILURE
The incidence of pregnancy from hormonal contraceptive failure is approximately one percent (i.e., one pregnancy per 100 women per year) if used correctly. The chance of becoming pregnant increases with incorrect use. If contraceptive patch failure does occur, the risk to the fetus is minimal.
PREGNANCY AFTER STOPPING ORTHO EVRA®
There may be some delay in becoming pregnant after you stop using ORTHO EVRA®, especially if you had irregular menstrual cycles before you used hormonal contraceptives. It may be best to postpone conception until you begin menstruation regularly once you have stopped using ORTHO EVRA® and want to become pregnant.
There does not appear to be any increase in birth defects in newborn babies when pregnancy occurs soon after stopping hormonal contraceptives.
OVERDOSAGE
ORTHO EVRA® is unlikely to cause an overdose because the patch releases a steady amount of the hormones. Do not use more than one patch at a time. Serious ill effects have not been reported when large doses of oral contraceptives were accidentally taken by young children. Overdosage may cause nausea and vomiting. Vaginal bleeding may occur in females. In case of overdosage, contact your health care professional or pharmacist.
OTHER INFORMATION
Your health care professional will take a medical and family history before prescribing ORTHO EVRA® and will examine you. The physical examination may be delayed to another time if you request it and the health care professional believes that it is a good medical practice to postpone it. You should be reexamined at least once a year. Be sure to inform your health care professional if there is a family history of any of the conditions listed previously in this leaflet. Be sure to keep all appointments with your health care professional, because this is a time to determine if there are early signs of side effects of hormonal contraceptive use.
Do not use the drug for any condition other than the one for which it was prescribed. This drug has been prescribed specifically for you; do not give it to others who may want birth control.
If you want more information about ORTHO EVRA®, ask your health care professional or pharmacist. They have a more technical leaflet called the Prescribing Information that you may wish to read.
Special Precautions for Storage and Disposal
Store at room temperature.
Store patches in their protective pouches. Apply to the skin immediately upon removal from the protective pouch.
Do not store in the refrigerator or freezer.
Used patches still contain some active hormones. Fold each patch in half so that it sticks to itself before safely disposing of it in the trash. Used patches should not be flushed down the toilet.
Ortho-Mcneil PHARMACEUTICAL, INC.
Raritan, New Jersey 08869
© OMP 2001 Revised May 2005 631-10-662-4
Subscribe to the "News" RSS Feed 
TOP ۞
