-
Phenytek Capsules (Mylan Bertek)
DESCRIPTION
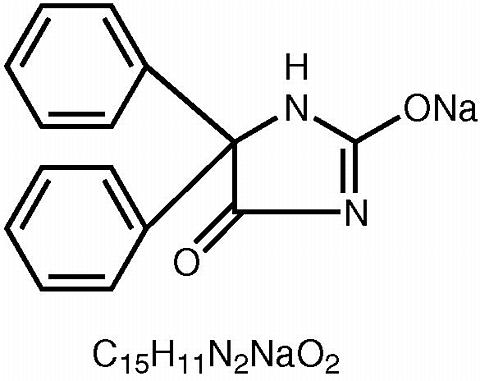
PHENYTEK® (phenytoin sodium) is an antiepileptic drug. Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is 5,5-Diphenylhydantoin sodium salt, having a molecular weight of 274.25 and having the following structural formula and molecular formula:
Each PHENYTEK® CAPSULE (extended phenytoin sodium capsule, USP) for oral administration, contains 200 mg or 300 mg of phenytoin sodium. Each capsule also contains the following inactive ingredients: black iron oxide, colloidal silicon dioxide, D&C yellow no. 10 aluminum lake, FD&C blue #1, FD&C blue no. 1 aluminum lake, FD&C blue no. 2 aluminum lake, FD&C red no. 40 aluminum lake, gelatin, hydroxyethyl cellulose, magnesium oxide, magnesium stearate, microcrystalline cellulose, pharmaceutical glaze, povidone, propylene glycol, silicon dioxide, sodium lauryl sulfate and titanium dioxide. Product in vivo performance is characterized by a slow and extended rate of absorption with peak blood concentrations expected in 4 to 12 hours as contrasted to prompt phenytoin sodium capsules, USP with a rapid rate of absorption with peak blood concentration expected in 1 ½ to 3 hours.
PHENYTEK® CAPSULES, 200 mg and 300 mg meet USP Dissolution Test 3.
CLINICAL PHARMACOLOGY
Phenytoin is an antiepileptic drug which can be useful in the treatment of epilepsy. The primary site of action appears to be the motor cortex where spread of seizure activity is inhibited. Possibly by promoting sodium efflux from neurons, phenytoin tends to stabilize the threshold against hyperexcitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient. This includes the reduction of posttetanic potentiation at synapses. Loss of posttetanic potentiation prevents cortical seizure foci from detonating adjacent cortical areas. Phenytoin reduces the maximal activity of brain stem centers responsible for the tonic phase of tonic-clonic (grand mal) seizures.
The plasma half-life in man after oral administration of phenytoin averages 22 hours, with a range of 7 to 42 hours. Steady-state therapeutic levels are achieved at least 7 to 10 days (5 to 7 half-lives) after initiation of therapy with recommended doses of 300 mg/day.
When serum level determinations are necessary, they should be obtained at least 5 to 7 half-lives after treatment initiation, dosage change, or addition or subtraction of another drug to the regimen so that equilibrium or steady-state will have been achieved. Trough levels provide information about clinically effective serum level range and confirm patient compliance and are obtained just prior to the patient's next scheduled dose. Peak levels indicate an individual's threshold for emergence of dose-related side effects and are obtained at the time of expected peak concentration. For extended phenytoin sodium capsules peak serum levels occur 4 to 12 hours after administration.
Optimum control without clinical signs of toxicity occurs more often with serum levels between 10 and 20 mcg/mL, although some mild cases of tonic-clonic (grand mal) epilepsy may be controlled with lower serum levels of phenytoin.
In most patients maintained at a steady dosage, stable phenytoin serum levels are achieved. There may be wide interpatient variability in phenytoin serum levels with equivalent dosages. Patients with unusually low levels may be noncompliant or hypermetabolizers of phenytoin. Unusually high levels result from liver disease, congenital enzyme deficiency, or drug interactions which result in metabolic interference. The patient with large variations in phenytoin plasma levels, despite standard doses, presents a difficult clinical problem. Serum level determinations in such patients may be particularly helpful. As phenytoin is highly protein bound, free phenytoin levels may be altered in patients whose protein binding characteristics differ from normal.
Most of the drug is excreted in the bile as inactive metabolites which are then reabsorbed from the intestinal tract and excreted in the urine. Urinary excretion of phenytoin and its metabolites occurs partly with glomerular filtration but more importantly by tubular secretion. Because phenytoin is hydroxylated in the liver by an enzyme system which is saturable at high plasma levels, small incremental doses may increase the half-life and produce very substantial increases in serum levels, when these are in the upper range. The steady-state level may be disproportionately increased, with resultant intoxication, from an increase in dosage of 10% or more.
INDICATIONS AND USAGE
PHENYTEK® CAPSULES (extended phenytoin sodium capsules, USP) are indicated for the control of generalized tonic-clonic (grand mal) and complex partial (psychomotor, temporal lobe) seizures and prevention and treatment of seizures occurring during or following neurosurgery.
Phenytoin serum level determinations may be necessary for optimal dosage adjustments (see DOSAGE AND ADMINISTRATION and CLINICAL PHARMACOLOGY ).
CONTRAINDICATIONS
Phenytoin is contraindicated in those patients with a history of hypersensitivity to phenytoin or other hydantoins.
WARNINGS
Abrupt withdrawal of phenytoin in epileptic patients may precipitate status epilepticus. When, in the judgment of the clinician, the need for dosage reduction, discontinuation, or substitution of alternative antiepileptic medication arises, this should be done gradually. However, in the event of an allergic or hypersensitivity reaction, more rapid substitution of alternative therapy may be necessary. In this case, alternative therapy should be an anticonvulsant drug not belonging to the hydantoin chemical class.
There have been a number of reports suggesting a relationship between phenytoin and the development of lymphadenopathy (local or generalized) including benign lymph node hyperplasia, pseudolymphoma, lymphoma, and Hodgkin's Disease. Although a cause and effect relationship has not been established, the occurrence of lymphadenopathy indicates the need to differentiate such a condition from other types of lymph node pathology. Lymph node involvement may occur with or without symptoms and signs resembling serum sickness, e.g., fever, rash, and liver involvement.
In all cases of lymphadenopathy, follow-up observation for an extended period is indicated and every effort should be made to achieve seizure control using alternative antiepileptic drugs.
Acute alcoholic intake may increase phenytoin serum levels while chronic alcoholic use may decrease serum levels.
In view of isolated reports associating phenytoin with exacerbation of porphyria, caution should be exercised in using this medication in patients suffering from this disease.
Usage in Pregnancy: A number of reports suggests an association between the use of antiepileptic drugs by women with epilepsy and a higher incidence of birth defects in children born to these women. Data are more extensive with respect to phenytoin and phenobarbital, but these are also the most commonly prescribed antiepileptic drugs; less systematic or anecdotal reports suggest a possible similar association with the use of all known antiepileptic drugs.
The reports suggesting a higher incidence of birth defects in children of drug-treated epileptic women cannot be regarded as adequate to prove a definite cause and effect relationship. There are intrinsic methodologic problems in obtaining adequate data on drug teratogenicity in humans; genetic factors or the epileptic condition itself may be more important than drug therapy in leading to birth defects. The great majority of mothers on antiepileptic medication deliver normal infants. It is important to note that antiepileptic drugs should not be discontinued in patients in whom the drug is administered to prevent major seizures, because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorder are such that the removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy, although it cannot be said with any confidence that even minor seizures do not pose some hazard to the developing embryo or fetus. The prescribing physician will wish to weigh these considerations in treating or counseling epileptic women of childbearing potential.
In addition to the reports of increased incidence of congenital malformation, such as cleft lip/palate and heart malformations in children of women receiving phenytoin and other antiepileptic drugs, there have more recently been reports of a fetal hydantoin syndrome. This consists of prenatal growth deficiency, microcephaly, and mental deficiency in children born to mothers who have received phenytoin, barbiturates, alcohol, or trimethadione. However, these features are all interrelated and are frequently associated with intrauterine growth retardation from other causes.
There have been isolated reports of malignancies, including neuroblastoma, in children whose mothers received phenytoin during pregnancy.
An increase in seizure frequency during pregnancy occurs in a high proportion of patients, because of altered phenytoin absorption or metabolism. Periodic measurement of serum phenytoin levels is particularly valuable in the management of a pregnant epileptic patient as a guide to an appropriate adjustment of dosage. However, postpartum restoration of the original dosage will probably be indicated.
Neonatal coagulation defects have been reported within the first 24 hours in babies born to epileptic mothers receiving phenobarbital and/or phenytoin. Vitamin K has been shown to prevent or correct this defect and has been recommended to be given to the mother before delivery and to the neonate after birth.
PRECAUTIONS
General: The liver is the chief site of biotransformation of phenytoin; patients with impaired liver function, elderly patients, or those who are gravely ill may show early signs of toxicity.
A small percentage of individuals who have been treated with phenytoin have been shown to metabolize the drug slowly. Slow metabolism may be due to limited enzyme availability and lack of induction; it appears to be genetically determined.
Phenytoin should be discontinued if a skin rash appears (see WARNINGS section regarding drug discontinuation). If the rash is exfoliative, purpuric, or bullous, or if lupus erythematosus, Stevens-Johnson syndrome, or toxic epidermal necrolysis is suspected, use of this drug should not be resumed and alternative therapy should be considered. (See ADVERSE REACTIONS ). If the rash is of a milder type (measles-like or scarlatiniform), therapy may be resumed after the rash has completely disappeared. If the rash recurs upon reinstitution of therapy, further phenytoin medication is contraindicated.
Phenytoin and other hydantoins are contraindicated in patients who have experienced phenytoin hypersensitivity. Additionally, caution should be exercised if using structurally similar compounds (e.g., barbiturates, succinamides, oxazolidinediones and other related compounds) in these same patients.
Hyperglycemia, resulting from the drug's inhibitory effects on insulin release, has been reported. Phenytoin may also raise the serum glucose level in diabetic patients.
Osteomalacia has been associated with phenytoin therapy and is considered to be due to phenytoin's interference with Vitamin D metabolism.
Phenytoin is not indicated for seizures due to hypoglycemic or other metabolic causes. Appropriate diagnostic procedures should be performed as indicated.
Phenytoin is not effective for absence (petit mal) seizures. If tonic-clonic (grand mal) and absence (petit mal) seizures are present, combined drug therapy is needed.
Serum levels of phenytoin sustained above the optimal range may produce confusional states referred to as "delirium," "psychosis," or "encephalopathy," or rarely irreversible cerebellar dysfunction. Accordingly, at the first sign of acute toxicity, plasma levels are recommended. Dose reduction of phenytoin therapy is indicated if plasma levels are excessive; if symptoms persist, termination is recommended. (See WARNINGS ).
Information for Patients: Patients taking phenytoin should be advised of the importance of adhering strictly to the prescribed dosage regimen, and of informing the physician of any clinical condition in which it is not possible to take the drug orally as prescribed, e.g., surgery, etc.
Patients should also be cautioned on the use of other drugs or alcoholic beverages without first seeking the physician's advice.
Patients should be instructed to call their physician if skin rash develops.
The importance of good dental hygiene should be stressed in order to minimize the development of gingival hyperplasia and its complications.
Laboratory Tests: Phenytoin serum level determinations may be necessary to achieve optimal dosage adjustments.
Drug Interactions: There are many drugs which may increase or decrease phenytoin levels or which phenytoin may affect. Serum level determinations for phenytoin are especially helpful when possible drug interactions are suspected. The most commonly occurring drug interactions are listed below.
- Drugs which may increase phenytoin serum levels include: acute alcohol intake, amiodarone, chloramphenicol, chlordiazepoxide, diazepam, dicumarol, disulfiram, estrogens, ethosuximide, H 2 -antagonists, halothane, isoniazid, methylphenidate, phenothiazines, phenylbutazone, salicylates, succinamides, sulfonamides, tolbutamide, trazodone.
- Drugs which may decrease phenytoin levels include: carbamazepine, chronic alcohol abuse, reserpine, and sucralfate. Moban® brand of molindone hydrochloride contains calcium ions which interfere with the absorption of phenytoin. Ingestion times of phenytoin and antacid preparations containing calcium should be staggered in patients with low serum phenytoin levels to prevent absorption problems.
- Drugs which may either increase or decrease phenytoin serum levels include: phenobarbital, sodium valproate, and valproic acid. Similarly, the effect of phenytoin on phenobarbital, valproic acid and sodium valproate serum levels is unpredictable.
- Although not a true drug interaction, tricyclic antidepressants may precipitate seizures in susceptible patients and phenytoin dosage may need to be adjusted.
- Drugs whose efficacy is impaired by phenytoin include: corticosteroids, coumarin anticoagulants, digitoxin, doxycycline, estrogens, furosemide, oral contraceptives, quinidine, rifampin, theophylline, vitamin D.
Drug/Laboratory Test Interactions: Phenytoin may cause decreased serum levels of protein-bound iodine (PBI). It may also produce lower than normal values for dexamethasone or metyrapone tests. Phenytoin may cause increased serum levels of glucose, alkaline phosphatase, and gamma glutamyl transpeptidase (GGT).
Carcinogenesis: See WARNINGS section for information on carcinogenesis.
Pregnancy: See WARNINGS ).
Nursing Mothers: Infant breast feeding is not recommended for women taking this drug because phenytoin appears to be secreted in low concentrations in human milk.
ADVERSE REACTIONS
Central Nervous System: The most common manifestations encountered with phenytoin therapy are referable to this system and are usually dose-related. These include nystagmus, ataxia, slurred speech, decreased coordination, and mental confusion. Dizziness, insomnia, transient nervousness, motor twitchings, and headaches have also been observed. There have also been rare reports of phenytoin induced dyskinesias, including chorea, dystonia, tremor and asterixis, similar to those induced by phenothiazine and other neuroleptic drugs.
A predominantly sensory peripheral polyneuropathy has been observed in patients receiving long-term phenytoin therapy.
Gastrointestinal System: Nausea, vomiting, constipation, toxic hepatitis and liver damage.
Integumentary System: Dermatological manifestations sometimes accompanied by fever have included scarlatiniform or morbilliform rashes. A morbilliform rash (measles-like) is the most common; other types of dermatitis are seen more rarely. Other more serious forms which may be fatal have included bullous, exfoliative or purpuric dermatitis, lupus erythematosus, Stevens-Johnson syndrome, and toxic epidermal necrolysis (see PRECAUTIONS ).
Hemopoietic System: Hemopoietic complications, some fatal, have occasionally been reported in association with administration of phenytoin. These have included thrombocytopenia, leukopenia, granulocytopenia, agranulocytosis, and pancytopenia with or without bone marrow suppression. While macrocytosis and megaloblastic anemia have occurred, these conditions usually respond to folic acid therapy. Lymphadenopathy including benign lymph node hyperplasia, pseudolymphoma, lymphoma, and Hodgkin's Disease have been reported (see WARNINGS ).
Connective Tissue System: Coarsening of the facial features, enlargement of the lips, gingival hyperplasia, hypertrichosis, and Peyronie's Disease.
Cardiovascular: Periarteritis nodosa.
Immunologic: Hypersensitivity syndrome (which may include, but is not limited to, symptoms such as arthralgias, eosinophilia, fever, liver dysfunction, lymphadenopathy or rash), systemic lupus erythematosus, and immunoglobulin abnormalities.
OVERDOSAGE
The lethal dose in children is not known. The lethal dose in adults is estimated to be 2 to 5 grams. The initial symptoms are nystagmus, ataxia, and dysarthria. Other signs are tremor, hyperreflexia, lethargy, slurred speech, nausea, vomiting. The patient may become comatose and hypotensive. Death is due to respiratory and circulatory depression.
There are marked variations among individuals with respect to phenytoin plasma levels where toxicity may occur. Nystagmus, on lateral gaze, usually appears at 20 mcg/mL, ataxia at 30 mcg/mL, dysarthria and lethargy appear when the plasma concentration is over 40 mcg/mL, but as high a concentration as 50 mcg/mL has been reported without evidence of toxicity. As much as 25 times the therapeutic dose has been taken to result in a serum concentration over 100 mcg/mL with complete recovery.
Treatment: Treatment is nonspecific since there is no known antidote.
The adequacy of the respiratory and circulatory systems should be carefully observed and appropriate supportive measures employed. Hemodialysis can be considered since phenytoin is not completely bound to plasma proteins. Total exchange transfusion has been used in the treatment of severe intoxication in children.
In acute overdosage, the possibility of other CNS depressants, including alcohol, should be borne in mind.
DOSAGE AND ADMINISTRATION
Serum concentrations should be monitored in changing from extended phenytoin sodium capsules, USP, to prompt phenytoin sodium capsules, USP, and from the sodium salt to the free acid form.
PHENYTEK® CAPSULES (extended phenytoin sodium capsules, USP) are formulated with the sodium salt of phenytoin. Because there is approximately an 8% increase in drug content with the free acid form over that of the sodium salt, dosage adjustments and serum level monitoring may be necessary when switching from a product formulated with the free acid to a product formulated with the sodium salt and vice versa.
General: Dosage should be individualized to provide maximum benefit. In some cases, serum blood level determinations may be necessary for optimal dosage adjustments -- the clinically effective serum level is usually 10 to 20 mcg/mL. With recommended dosage, a period of seven to ten days may be required to achieve steady-state blood levels with phenytoin and changes in dosage (increase or decrease) should not be carried out at intervals shorter than seven to ten days.
Adult Dosage: Divided Daily Dosage: Patients who have received no previous treatment may be started on one 100 mg extended phenytoin sodium capsule three times daily and the dosage then adjusted to suit individual requirements. For most adults, the satisfactory maintenance dosage will be one 100 mg capsule three to four times a day. An increase up to one 200 mg PHENYTEK® three times a day may be made, if necessary.
Once-A-Day Dosage: In adults, if seizure control is established with divided doses of three 100 mg extended phenytoin sodium capsules daily, once-a-day dosage with 300 mg PHENYTEK® may be considered. Studies comparing divided doses of 300 mg with a single daily dose of this quantity indicated absorption, peak plasma levels, biologic half-life, difference between peak and minimum values, and urinary recovery were equivalent. Once-a-day dosage offers a convenience to the individual patient or to nursing personnel for institutionalized patients and is intended to be used only for patients requiring this amount of drug daily. A major problem in motivating noncompliant patients may also be lessened when the patient can take this drug once a day. However, patients should be cautioned not to miss a dose, inadvertently.
Only extended phenytoin sodium capsules are recommended for once-a-day dosing. Inherent differences in dissolution characteristics and resultant absorption rates of phenytoin due to different manufacturing procedures and/or dosage forms preclude such recommendation for other phenytoin products. When a change in the dosage form or brand is prescribed, careful monitoring of phenytoin serum levels should be carried out.
Loading Dose: Some authorities have advocated use of an oral loading dose of phenytoin in adults who require rapid steady-state serum levels and where intravenous administration is not desirable. This dosing regimen should be reserved for patients in a clinic or hospital setting where phenytoin serum levels can be closely monitored. Patients with a history of renal or liver disease should not receive the oral loading regimen.
Initially, one gram of phenytoin capsules is divided into 3 doses (400 mg, 300 mg, 300 mg) and administered at two-hour intervals. Normal maintenance dosage is then instituted 24 hours after the loading dose, with frequent serum level determinations.
Pediatric Dosage: Initially, 5 mg/kg/day in two or three equally divided doses, with subsequent dosage individualized to a maximum of 300 mg daily. A recommended daily maintenance dosage is usually 4 to 8 mg/kg. Children over 6 years old may require the minimum adult dose (300 mg/day).
HOW SUPPLIED
PHENYTEK® CAPSULES (extended phenytoin sodium capsules, USP) are available containing 200 mg or 300 mg of phenytoin sodium.
The 200 mg capsule has a dark blue opaque cap and a blue opaque body. The hard-shell gelatin capsule is filled with two white to off-white round, beveled edge tablets. The capsule is rectified radially printed with BERTEK over 670 in black ink on both the cap and the body. They are available as follows:
NDC 62794-670-93
bottles of 30 capsules
NDC 62794-670-01
bottles of 100 capsules
The 300 mg capsule has a blue opaque cap and a blue opaque body. The hard-shell gelatin capsule is filled with three white to off-white round, beveled edge tablets. The capsule is rectified radially printed with BERTEK over 750 in black ink on both the cap and the body. They are available as follows:
NDC 62794-750-93
bottles of 30 capsules
NDC 62794-750-01
bottles of 100 capsules
Store at 20° to 25°C (68° to 77°F). [See USP for Controlled Room Temperature.] Protect from light and moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
U.S. Patent No. 6,274,168
BERTEK PHARMACEUTICALS INC.
Research Triangle Park
NC 27709-4149
REVISED FEBRUARY 2004
BKPHTK:R6
Subscribe to the "News" RSS Feed 
TOP ۞
