-
Prevacid I.V. for Injection (TAP)
DESCRIPTION
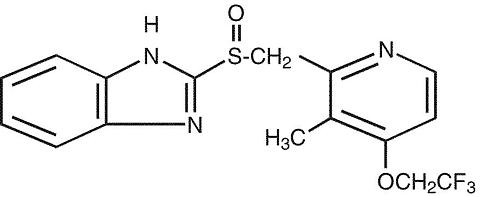
The active ingredient in PREVACID I.V. (lansoprazole) for Injection is a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl] benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C 16 H 14 F 3 N 3 O 2 S with a molecular weight of 369.37. The structural formula is:
Lansoprazole is a white to brownish-white odorless crystalline powder which melts with decomposition at approximately 166°C. Lansoprazole is freely soluble in dimethylformamide; soluble in methanol; sparingly soluble in ethanol; slightly soluble in ethyl acetate, dichloromethane and acetonitrile; very slightly soluble in ether; and practically insoluble in hexane and water.
Lansoprazole is stable when exposed to light for up to two months. The rate of degradation of the compound in aqueous solution increases with decreasing pH.
PREVACID I.V. for Injection contains 30 mg of the active ingredient lansoprazole, 60 mg mannitol, 10 mg meglumine, and 3.45 mg sodium hydroxide and is supplied as a sterile, lyophilized powder for I.V. (intravenous) use. The solution of PREVACID I.V. for Injection has a pH of approximately 11 following the first reconstitution with Sterile Water for Injection, USP, and approximately 10.2, 10.0, or 9.5 after further dilution with either 0.9% Sodium Chloride Injection, USP, Lactated Ringer's Injection, USP, or 5% Dextrose Injection, USP, respectively.
CLINICAL PHARMACOLOGY
Pharmacokinetics and Metabolism
Following the administration of 30 mg of lansoprazole by intravenous infusion over 30 minutes to healthy subjects, plasma concentrations of lansoprazole declined exponentially with a mean (± standard deviation) terminal elimination half-life of 1.3 (± 0.5) hours. The mean peak plasma concentration of lansoprazole (C max ) was 1705 (± 292) ng/mL and the mean area under the plasma concentration versus time curve (AUC) was 3192 (± 1745) ng·h/mL. The absolute bioavailability of lansoprazole following oral administration is over 80%, and C max and AUC of lansoprazole are approximately proportional in doses from 15 mg to 60 mg after single oral administration. The pharmacokinetics of lansoprazole did not change with time after 7-day once daily repeated oral or intravenous administration of 30 mg lansoprazole.
Distribution
The apparent volume of distribution of lansoprazole is approximately 15.7 (± 1.9) L, distributing mainly in extracellular fluid. Lansoprazole is 97% bound to plasma proteins. Plasma protein binding is constant over the concentration range of 0.05 to 5.0 µg/mL.
Metabolism
Lansoprazole is extensively metabolized in the liver. Two metabolites have been identified in measurable quantities in plasma (the hydroxylated sulfinyl and sulfone derivatives of lansoprazole). These metabolites have very little or no antisecretory activity. Lansoprazole is thought to be transformed into two active species which inhibit acid secretion by (H + ,K + )-ATPase within the parietal cell canaliculus, but are not present in the systemic circulation. The plasma elimination half-life of lansoprazole does not reflect its duration of suppression of gastric acid secretion. Thus, the plasma elimination half-life is less than two hours, while the acid inhibitory effect lasts more than 24 hours.
Elimination
Following an intravenous dose of lansoprazole, the mean clearance was 11.1 (± 3.8) L/h. Following single-dose oral administration of lansoprazole, virtually no unchanged lansoprazole was excreted in the urine. In one study, after a single oral dose of 14 C-lansoprazole, approximately one-third of the administered radiation was excreted in the urine and two-thirds was recovered in the feces. This implies a significant biliary excretion of the metabolites of lansoprazole.
Special Populations
Geriatric
Following oral administration, the clearance of lansoprazole is decreased in the elderly, with elimination half-life increased approximately 50% to 100%. Because the mean half-life in the elderly remains between 1.9 to 2.9 hours, repeated once daily dosing does not result in accumulation of lansoprazole. Peak plasma levels were not increased in the elderly. No intravenous dosage adjustment is needed.
Pediatric
The pharmacokinetics of intravenous lansoprazole have not been studied in pediatric patients. For further information, please see the PREVACID package insert for the oral formulations.
Gender
The pharmacokinetic data of intravenous lansoprazole in females is limited; however, in a study with oral lansoprazole comparing 12 male and 6 female human subjects, no gender differences were found in pharmacokinetics and intragastric pH results. No intravenous dosage adjustment is needed. (Also refer to Use in Women .)
Renal Insufficiency
In patients with severe renal insufficiency, plasma protein binding decreased by 1.0%-1.5% after oral administration of 60 mg of lansoprazole. Patients with renal insufficiency had a shortened elimination half-life and decreased total AUC (free and bound). AUC for free lansoprazole in plasma, however, was not related to the degree of renal impairment, and C max and T max were not different from subjects with healthy kidneys. No intravenous dosage adjustment is necessary in patients with renal insufficiency.
Hepatic Insufficiency
In patients with various degrees of chronic hepatic disease, the mean plasma half-life of the drug was prolonged from 1.5 hours to 3.2-7.2 hours after oral administration. An increase in mean AUC of up to 500% was observed at steady state in hepatically-impaired patients compared to healthy subjects. Intravenous dose reduction in patients with severe hepatic disease should be considered.
Race
The pooled mean pharmacokinetic parameters of orally administered lansoprazole from twelve U.S. Phase 1 studies (N=513) were compared to the mean pharmacokinetic parameters from two Asian studies (N=20). The mean AUCs of lansoprazole in Asian subjects were approximately twice those seen in pooled U.S. data; however, the inter-individual variability was high. The C max values were comparable. Information for intravenous dosing is not available.
Pharmacodynamics
Mechanism of Action
Lansoprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that do not exhibit anticholinergic or histamine H 2 -receptor antagonist properties, but that suppress gastric acid secretion by specific inhibition of the (H + ,K + )-ATPase enzyme system at the secretory surface of the gastric parietal cell. Because this enzyme system is regarded as the acid (proton) pump within the parietal cell, lansoprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-related and leads to inhibition of both basal and stimulated gastric acid secretion for at least 24 hours irrespective of the stimulus.
Antisecretory Activity
Acid Output
An open-label, single-center, two period study was conducted to evaluate the pharmacodynamics of 30 mg of intravenous lansoprazole and 30 mg of oral lansoprazole in 29 healthy subjects. The primary pharmacodynamic endpoints were pentagastrin stimulated maximum acid output (MAO) and basal acid output (BAO). Subjects received oral lansoprazole for 7 days in Period 1 and then were immediately switched to intravenous lansoprazole for 7 days in Period 2. MAO and BAO were measured at baseline and 21 hours following the last oral dose and the last intravenous dose of lansoprazole. This study demonstrated that 7 days of oral lansoprazole followed by 7 days of intravenous lansoprazole administration significantly suppressed gastric acid output as compared with baseline. Seven days of 30 mg of intravenous lansoprazole was equivalent to 30 mg of oral lansoprazole in the ability to maintain gastric acid output suppression.
Acid Output (mEq/hr) PREVACID 30 mg Baseline After 7 Days of Oral Dosing After 7 Days of I.V. Dosing Maximum Acid
Output (Median)11.26
n=274.76 *
n=285.13 *
n=28Basal Acid
Output (Median)1.42
n=280.42 *
n=280.27 *
n=28* Significantly (p </= 0.05) less acid output as compared to baseline.
24-Hour Intragastric pH
A multiple-dose study was conducted in 36 healthy subjects comparing the pharmacokinetics and pharmacodynamics of lansoprazole after intravenous administration and oral administration. During the first-hour post-dosing interval, intravenous lansoprazole resulted in significantly higher mean intragastric pH than did oral lansoprazole. There were no statistically significant differences between oral and intravenous regimens in 24-hour mean intragastric pH for the percentage of time that the intragastric pH was above 3 and 4 after 1-day or 5-day once daily repeated administration of 30 mg lansoprazole. Gastric acid suppression was maintained throughout each treatment period. The pharmacodynamic results are summarized in the table below.
Mean Antisecretory Effects after Single and Multiple Daily Dosing ParameterBaseline Value PREVACID 30 mg q.d. Orally x 5 days 30 mg I.V. Infusion q.d. x 5 Days Day 1 Day 5 Day 1 Day 5 Mean 24-Hour pH3.33 4.75 5.25 4.86 5.36 Mean first hour pH4.44 2.74 4.79 4.64 * 5.91 * % Time Gastric pH>345.27 74.08 83.92 78.36 85.54 % Time Gastric pH>431.07 67.18 77.61 70.51 79.68 *Significantly (p </= 0.05) higher than the oral lansoprazole
Refer to CLINICAL PHARMACOLOGY for pharmacokinetic results.
Enterochromaffin-like (ECL) Cell Effects
During lifetime exposure of rats with up to 150 mg/kg/day of lansoprazole dosed orally seven days per week, marked hypergastrinemia was observed followed by ECL cell proliferation and formation of carcinoid tumors, especially in female rats. (Refer to PRECAUTIONS , Carcinogenesis, Mutagenesis, Impairment of Fertility .)
Gastric biopsy specimens from the body of the stomach from approximately 150 patients treated continuously with lansoprazole for at least one year did not show evidence of ECL cell effects similar to those seen in rat studies. Longer term data are needed to rule out the possibility of an increased risk of the development of gastric tumors in patients receiving long-term therapy with lansoprazole.
Other Gastric Effects In Humans
Lansoprazole did not significantly affect mucosal blood flow in the fundus of the stomach. Due to the normal physiologic effect caused by the inhibition of gastric acid secretion, a decrease of about 17% in blood flow in the antrum, pylorus, and duodenal bulb was seen. Lansoprazole signifi-cantly slowed the gastric emptying of digestible solids. Lansoprazole increased serum pepsinogen levels and decreased pepsin activity under basal conditions and in response to meal stimulation or insulin injection. As with other agents that elevate intragastric pH, increases in gastric pH were associated with increases in nitrate-reducing bacteria and elevation of nitrite concentration in gastric juice in patients with gastric ulcer. No significant increase in nitrosamine concentrations was observed.
Serum Gastrin Effects
In over 2,100 patients, median fasting serum gastrin levels increased 50% to 100% from baseline but remained within normal range after treatment with lansoprazole given orally in doses of 15 mg to 60 mg. These elevations reached a plateau within two months of therapy and returned to pretreatment levels within four weeks after discontinuation of therapy.
Endocrine Effects
Human studies for up to one year have not detected any clinically significant effects on the endocrine system. Hormones studied include testosterone, luteinizing hormone (LH), follicle stimulating hormone (FSH), sex hormone binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEA-S), prolactin, cortisol, estradiol, insulin, aldosterone, parathormone, glucagon, thyroid stimulating hormone (TSH), triiodothyronine (T 3 ), thyroxine (T 4 ), and somatotropic hormone (STH). Lansoprazole in oral doses of 15 to 60 mg for up to one year had no clinically significant effect on sexual function. In addition, lansoprazole in oral doses of 15 to 60 mg for two to eight weeks had no clinically significant effect on thyroid function.
In 24-month carcinogenicity studies in Sprague-Dawley rats with daily dosages up to 150 mg/kg, proliferative changes in the Leydig cells of the testes, including benign neoplasm, were increased compared to control rates; these findings are rat specific.
Other Effects
No systemic effects of lansoprazole on the central nervous system, lymphoid, hematopoietic, renal, hepatic, cardiovascular or respiratory systems have been found in humans. No visual toxicity was observed among 56 patients who had extensive baseline eye evaluations, were treated with up to 180 mg/day of lansoprazole and were observed for up to 58 months.
Other rat-specific findings after lifetime exposure included focal pancreatic atrophy, diffuse lymphoid hyperplasia in the thymus and spontaneous retinal atrophy.
CLINICAL STUDIES
Erosive Esophagitis
A multicenter, double-blind, two-period placebo-controlled, pharmacodynamic study was conducted to assess the ability of PREVACID I.V. for Injection to maintain gastric acid suppression in patients switched from the oral dosage form of lansoprazole to the intravenous dosage form. Erosive esophagitis patients (n=87; 18 to 78 years of age; 28 female; 69 Caucasian/non-Hispanic, 14 Hispanic, 3 African-American, and 1 Native American) received 30 mg of oral lansoprazole for 7 days in Period 1. Patients were then immedi-ately switched to receive either 30 mg of intravenous lansoprazole or intravenous placebo (normal saline) for 7 days in Period 2. MAO and BAO were determined 21 hours following the last dose of oral medication and the last dose of intravenous administration. MAO was calculated from two hours of continuous collection of gastric contents following a subcutaneous injection of 6.0 µg/kg of pentagastrin. BAO was calculated from one hour of continuous collection of gastric contents.
This study demonstrated that, after seven days of repeated oral administration followed by 7 days of intravenous administration, the oral and intravenous dosage forms of PREVACID were similar in their ability to suppress MAO and BAO in patients with erosive esophagitis (refer to the table below). Also, patients receiving oral PREVACID, who were switched to intravenous placebo, experienced a significant increase in acid output within 48 hours of their last oral dose.
Acid Output (mEq/h) in Erosive Esophagitis Patients PREVACID Oral
(last oral dose)PREVACID I.V.
(last I.V. dose)Placebo I.V.
(last I.V. dose)Maximum Acid
Output (Median)7.16
n=807.64
n=5626.90 **
n=17Basal Acid Output
(Median)0.77
n=810.51
n=553.19 *
n=16*, ** Significantly different from PREVACID I.V. at p=0.005 and p<0.001 levels, respectivelyINDICATIONS AND USAGE
When patients are unable to take the oral formulations, PREVACID I.V. for Injection is indicated as an alternative for the short-term treatment (up to 7 days) of all grades of erosive esophagitis. Once the patient is able to take medications orally, therapy can be switched to an oral formulation of PREVACID for a total of 6 to 8 weeks. The safety and efficacy of PREVACID I.V. for Injection as an initial treatment of erosive esophagitis have not been demonstrated. Refer to full prescribing information for the oral formulations of PREVACID.
CONTRAINDICATIONS
PREVACID I.V. for Injection is contraindicated in patients with known hypersensitivity to any component of the formulation.
PRECAUTIONS
General
Symptomatic response to therapy with lansoprazole does not preclude the presence of gastric malignancy.
Treatment with PREVACID I.V. for Injection should be discontinued as soon as the patient is able to resume treatment with PREVACID oral formulations.
Drug Interactions
Lansoprazole is metabolized through the cytochrome P 450 system, specifically through the CYP3A and CYP2C19 isozymes. Studies have shown that lansoprazole does not have clinically significant interactions with other drugs metabolized by the cytochrome P 450 system, such as warfarin, antipyrine, indomethacin, ibuprofen, phenytoin, propranolol, prednisone, diazepam, or clarithromycin in healthy subjects. These compounds are metabolized through various cytochrome P 450 isozymes including CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A. When lansoprazole was administered concomitantly with theophylline (CYP1A2, CYP3A), a minor increase (10%) in the clearance of theophylline was seen. Because of the small magnitude and the direction of the effect on theophylline clearance, this interaction is unlikely to be of clinical concern. Nonetheless, individual patients may require additional titration of their theophylline dosage when lansoprazole is started or stopped to ensure clinically effective blood levels.
In a study of healthy subjects neither the pharmacokin-etics of warfarin enantiomers nor prothrombin time were affected following single or multiple 60 mg doses of lansoprazole. However, there have been reports of increased International Normalized Ratio (INR) and prothrombin time in patients receiving proton pump inhibitors, including lansoprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with proton pump inhibitors and warfarin concomitantly may need to be monitored for increases in INR and prothrombin time.
Lansoprazole causes a profound and long-lasting inhibition of gastric acid secretion; therefore, it is theoretically possible that lansoprazole may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability (eg, ketoconazole, ampicillin esters, iron salts, digoxin).
Carcinogenesis, Mutagenesis, Impairment of Fertility
In two 24-month carcinogenicity studies, Sprague-Dawley rats were treated orally with doses of 5 to 150 mg/kg/day, about 1 to 40 times the exposure on a body surface (mg/m 2 ) basis, of a 50-kg person of average height (1.46 m 2 body surface area) given the recommended human dose of 30 mg/day (22.2 mg/m 2 ). Lansoprazole produced dose-related gastric enterochromaffin-like (ECL) cell hyperplasia and ECL cell carcinoids in both male and female rats. It also increased the incidence of intestinal metaplasia of the gastric epithelium in both sexes. In male rats, lansoprazole produced a dose-related increase of testicular interstitial cell adenomas. The incidence of these adenomas in rats receiving doses of 15 to 150 mg/kg/day (4 to 40 times the recommended human dose based on body surface area) exceeded the low background incidence (range = 1.4 to 10%) for this strain of rat. Testicular interstitial cell adenoma also occurred in 1 of 30 rats treated with 50 mg/kg/day (13 times the recommended human dose based on body surface area) in a 1-year toxicity study.
In a 24-month carcinogenicity study, CD-1 mice were treated orally with doses of 15 to 600 mg/kg/day, 2 to 80 times the recommended human dose based on body surface area. Lansoprazole produced a dose-related increased incidence of gastric ECL cell hyperplasia. It also produced an increased incidence of liver tumors (hepatocellular adenoma plus carcinoma). The tumor incidences in male mice treated with 300 and 600 mg/kg/day (40 to 80 times the recommended human dose based on body surface area) and female mice treated with 150 to 600 mg/kg/day (20 to 80 times the recommended human dose based on body surface area) exceeded the ranges of background incidences in historical controls for this strain of mice. Lansoprazole treatment produced adenoma of rete testis in male mice receiving 75 to 600 mg/kg/day (10 to 80 times the recommended human dose based on body surface area).
Lansoprazole was not genotoxic in the Ames test, the ex vivo rat hepatocyte unscheduled DNA synthesis (UDS) test, the in vivo mouse micronucleus test or the rat bone marrow cell chromosomal aberration test. It was positive in in vitro human lymphocyte chromosomal aberration assays.
Lansoprazole at intravenous doses of up to 30 mg/kg/day (approximately 8 times the recommended human dose based on body surface area) was found to have no effect on fertility and reproductive performance in male and female rats.
Pregnancy: Teratogenic Effects
Pregnancy Category B
Teratology studies have been conducted in rats and rabbits using intravenous doses of up to 30 mg/kg/day (approximately 8 times in rats and 16 times in rabbits of the recommended human dose based on body surface area). Treatment with lansoprazole did not result in any impairment of fertility or harm to the fetus.
However, there are no adequate and well-controlled studies in pregnant women using the intravenous route. Because animal reproduction studies are not always predicative of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Lansoprazole or its metabolites are excreted in the milk of rats. It is not known whether lansoprazole is excreted in human milk. Because many drugs are excreted in human milk, because of the potential for serious adverse reactions in nursing infants from lansoprazole, and because of the potential for tumorigenicity shown for lansoprazole in rodent carcinogenicity studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of PREVACID I.V. for Injection have not been established for pediatric patients. For further information, please see the PREVACID package insert for the oral formulations.
Use in Women
Among intravenous lansoprazole treated subjects, similar percentages of adverse events were reported in males and females.
Over 4,000 women were treated with oral lansoprazole. Ulcer healing rates in females were similar to those in males. The incidence rates of adverse events were also similar to those seen in males.
Use in Geriatric Patients
Data in elderly patients administered intravenous lansoprazole is limited; however, with oral lansoprazole, ulcer healing rates in elderly patients are similar to those in a younger age group. The incidence rates of adverse events and laboratory test abnormalities are also similar to those seen in younger patients. For elderly patients, dosage and administration of lansoprazole need not be altered for a particular indication.
ADVERSE REACTIONS
Clinical Safety Experience with PREVACID I.V. for Injection
More than 1,000 patients and subjects have participated in domestic and foreign clinical trials. Treatment with PREVACID I.V. for Injection was well tolerated.
In four U.S. trials involving 161 subjects exposed to PREVACID I.V. for Injection, the following treatment-related adverse events were reported in >/=1% of subjects: headache (1.0%), injection site pain (1.0%), injection site reaction (1.0%) and nausea (1.3%). Treatment-related adverse events occurring in <1% of subjects included abdominal pain, vasodilatation, diarrhea, dyspepsia, vomiting, dizziness, paresthesia, rash, and taste perversion. No additional adverse drug reactions were reported with the intravenous formulation that had not been reported previously with the oral formulations.
Clinical Safety Experience with Oral Formulations of PREVACID
Worldwide, over 10,000 patients have been treated with oral lansoprazole in Phase 2-3 clinical trials involving various dosages and durations of treatment. In general, lansoprazole treatment has been well-tolerated in both short-term and long-term trials.
The following adverse events were reported by the treating physician to have a possible or probable relationship to drug in 1% or more of PREVACID-treated patients and occurred at a greater rate in PREVACID-treated patients than placebo-treated patients:
Incidence of Possibly or Probably Treatment-Related
Adverse Events in Short-Term, Placebo-Controlled StudiesBody System/
Adverse EventPREVACID
Oral
(N= 2768)
%Placebo
(N= 1023)
%Body as a WholeAbdominal Pain2.1 1.2 Digestive SystemConstipation1.0 0.4 Diarrhea3.8 2.3 Nausea1.3 1.2
Headache was also seen at greater than 1% incidence but was more common on placebo. The incidence of diarrhea was similar between patients who received placebo and patients who received lansoprazole 15 mg and 30 mg, but higher in the patients who received lansoprazole 60 mg (2.9%, 1.4%, 4.2%, and 7.4%, respectively).
Additional adverse experiences occurring in <1% of patients or subjects in domestic trials are shown below. Refer to Postmarketing for adverse reactions occurring since the drug was marketed.
Body as a Whole - abdomen enlarged, allergic reaction, asthenia, back pain, candidiasis, carcinoma, chest pain (not otherwise specified), chills, edema, fever, flu syndrome, halitosis, infection (not otherwise specified), malaise, neck pain, neck rigidity, pain, pelvic pain; Cardiovascular System - angina, arrhythmia, bradycardia, cerebrovascular accident/cerebral infarction, hypertension/ hypotension, migraine, myocardial infarction, palpitations, shock (circulatory failure), syncope, tachycardia, vasodilation; Digestive System - abnormal stools, anorexia, bezoar, cardiospasm, cholelithiasis, colitis, dry mouth, dyspepsia, dysphagia, enteritis, eructation, esophageal stenosis, esophageal ulcer, esophagitis, fecal discoloration, flatulence, gastric nodules/fundic gland polyps, gastritis, gastroenteritis, gastrointestinal anomaly, gastrointestinal disorder, gastrointestinal hemorrhage, glossitis, gum hemorrhage, hematemesis, increased appetite, increased salivation, melena, mouth ulceration, nausea and vomiting, nausea and vomiting and diarrhea, oral moniliasis, rectal disorder, rectal hemorrhage, stomatitis, tenesmus, thirst, tongue disorder, ulcerative colitis, ulcerative stomatitis; Endocrine System - diabetes mellitus, goiter, hypothyroidism; Hemic and Lymphatic System - anemia, hemolysis, lymphadenopathy; Metabolic and Nutritional Disorders - gout, dehydration, hyperglycemia/hypoglycemia, peripheral edema, weight gain/loss; Musculoskeletal System - arthralgia, arthritis, bone disorder, joint disorder, leg cramps, musculoskeletal pain, myalgia, myasthenia, synovitis; Nervous System - abnormal dreams, agitation, amnesia, anxiety, apathy, confusion, convulsion, depersonalization, depression, diplopia, dizziness, emotional lability, hallucinations, hemiplegia, hostility aggravated, hyperkinesia, hypertonia, hypesthesia, insomnia, libido decreased/increased, nervousness, neurosis, paresthesia, sleep disorder, somnolence, thinking abnormality, tremor, vertigo; Respiratory System - asthma, bronchitis, cough increased, dyspnea, epistaxis, hemoptysis, hiccup, laryngeal neoplasia, pharyngitis, pleural disorder, pneumonia, respiratory disorder, upper respiratory inflammation/infection, rhinitis, sinusitis, stridor; Skin and Appendages - acne, alopecia, contact dermatitis, dry skin, fixed eruption, hair disorder, maculopapular rash, nail disorder, pruritus, rash, skin carcinoma, skin disorder, sweating, urticaria; Special Senses - abnormal vision, blurred vision, conjunctivitis, deafness, dry eyes, ear disorder, eye pain, otitis media, parosmia, photophobia, retinal degeneration, taste loss, taste perversion, tinnitus, visual field defect; Urogenital System - abnormal menses, breast enlargement, breast pain, breast tenderness, dysmenorrhea, dysuria, gynecomastia, impotence, kidney calculus, kidney pain, leukorrhea, menorrhagia, menstrual disorder, penis disorder, polyuria, testis disorder, urethral pain, urinary frequency, urinary tract infection, urinary urgency, urination impaired, vaginitis.
Postmarketing
On-going Safety Surveillance: Additional adverse experiences have been reported since oral lansoprazole has been marketed. The majority of these cases are foreign-sourced and a relationship to lansoprazole has not been established. Because these events were reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events are listed below by COSTART body system.
Body as a Whole - anaphylactoid-like reaction; Digestive System - hepatotoxicity, pancreatitis, vomiting; Hemic and Lymphatic System - agranulocytosis, aplastic anemia, hemolytic anemia, leukopenia, neutropenia, pancytopenia, thrombocytopenia, and thrombotic thrombocytopenic purpura; Skin and Appendages - severe dermatologic reactions including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis (some fatal); Special Senses - speech disorder; Urogenital System - urinary retention.
Laboratory Values
There were no clinically important changes identified in any laboratory parameter with PREVACID I.V. for Injection.
The following changes in laboratory parameters for oral lansoprazole were reported as adverse events:
Abnormal liver function tests, increased SGOT (AST), increased SGPT (ALT), increased creatinine, increased alkaline phosphatase, increased globulins, increased GGTP, increased/decreased/abnormal WBC, abnormal AG ratio, abnormal RBC, bilirubinemia, eosinophilia, hyperlipemia, increased/decreased electrolytes, increased/decreased cholesterol, increased glucocorticoids, increased LDH, increased/decreased/abnormal platelets, and increased gastrin levels. Urine abnormalities such as albuminuria, glycosuria, and hematuria were also reported. Additional isolated laboratory abnormalities were reported.
In the placebo controlled studies, when SGOT (AST) and SGPT (ALT) were evaluated, 0.4% (4/978) placebo patients and 0.4% (11/2677) lansoprazole patients had enzyme elevations greater than three times the upper limit of normal range at the final treatment visit. None of these lansoprazole patients reported jaundice at any time during the study.
OVERDOSAGE
Single intravenous doses of lansoprazole at 218 mg/kg in mice (approximately 30 times the recommended human dose based on body surface area) and 167 mg/kg in rats (approximately 46 times the recommended human dose based on body surface area) were lethal. The symptoms of acute toxicity were decreased locomotor response, ataxia, ptosis and tonic convulsions.
Lansoprazole is not removed from the circulation by hemodialysis.
DOSAGE AND ADMINISTRATION
PREVACID I.V. for Injection admixtures should be administered intravenously using the in-line filter provided. The filter must be used to remove precipitate that may form when the reconstituted drug product is mixed with I.V. solutions. Studies have shown that filtration does not alter the amount of drug that is available for administration. (See instructions below).
There are two methods for preparing PREVACID I.V. for Injection:
-
Reconstitution in Vial and Preparation of Admixture.
OR - Direct reconstitution with Baxter's MINI-BAG Plus Container.
-
Reconstitution in Vial and Preparation of Admixture
There are two steps for preparing Prevacid I.V. for Injection.
STEP ONE - Reconstitution in Vial
- First Prevacid I.V. MUST be reconstituted with Sterile Water for Injection, USP.
- Inject 5 mL of ONLY Sterile Water for Injection, USP into a 30 mg vial of PREVACID I.V. for Injection. The resulting solution will contain lansoprazole 6 mg/mL (30 mg/5 mL).
- Failure to reconstitute with Sterile Water may result in formation of precipitation/particulates.
-
Mix gently until the powder is dissolved.
The pH of this reconstituted solution is approximately 11. The reconstituted solution can be held for 1 hour when stored at 25[ordm ]C (77[ordm ]F) prior to further dilution.
STEP TWO - Preparation of Admixture
- Dilute the reconstituted solution in either 50 mL of 0.9% Sodium Chloride Injection, USP, Lactated Ringer's Injection, USP, or 5% Dextrose Injection, USP.
-
The admixture should be stored at 25[ordm ]C (77[ordm ]F) and should be administered within the designated time period as listed in the Table below. No refrigeration is required.
DiluentpH Administer within: 0.9% Sodium Chloride Injection, USPApproximately 10.2 24 hours Lactated Ringer's Injection, USPApproximately 10.0 24 hours 5% Dextrose Injection, USPApproximately 9.5 12 hours
- Once the admixture is prepared, proceed to Instructions for Priming and Use of Filter .
-
Reconstitution with Baxter's MINI-BAG Plus Container
- PREVACID I.V. for Injection can be reconstituted directly into 50 mL of 0.9% Sodium Chloride for Injection, USP or 5% Dextrose Injection, USP utilizing Baxter's MINI-BAG Plus Container.
- Refer to separate instructions that are provided with Baxter's MINI-BAG Plus Container.
-
Once the admixture is prepared, proceed to
Instructions for Priming and Use of Filter
.
The admixture should be stored at 25[ordm ]C (77[ordm ]F) and should be administered within the designated time period as listed in the Table below. No refrigeration is required.
DiluentpH Administer within: 0.9% Sodium Chloride Injection, USPApproximately 10.2 24 hours 5% Dextrose Injection, USPApproximately 9.5 8 hours
Instructions for Priming and Use of Filter
TO PRIME FILTER
- Prime administration set in usual manner and close administration set clamp.
- Connect luer adapter of administration set to filter inlet using a twisting motion. Over-tightening should be avoided.
- Hold filter below the level of solution container.
- Open administration set clamp and slowly prime filter.
- Close administration set clamp. Verify no air bubbles are present on patient side of filter.
- If air bubbles are observed, open set clamp slightly to re-establish flow then gently tap filter housing. Observe that no air bubbles are present and close clamp.
- Connect to patient and regulate flow. Filter may be primed using a syringe and saline.
- The administration set can then be connected to inlet of filter.
PRECAUTIONS WITH USE OF FILTER
Follow instructions carefully:
- Use Aseptic technique. For single use only. Do not resterilize or reuse. Do not use if package is damaged.
- If repositioning of filter is required, loosen luer locking collar, reposition, then retighten locking collar firmly.
- Maximum working pressure is 1500 mmHg (30 psi, 2 bar). When the working limits of the filter are exceeded, causes of the added resistance should be investigated and corrected.
- The internal volume of the filter is approx. 0.7 mL.
- The administration set clamp should be closed during solution container change.
- It is recommended that this filter is changed at 24 hours.
- Pumps should not be used downstream of filter.
Administration
- IN-LINE FILTER MUST BE USED.
- Administer intravenously over 30 minutes.
- A dedicated line is not required; however, the intravenous line should be flushed before and after administration of PREVACID I.V. for Injection with either 0.9% Sodium Chloride Injection, USP, Lactated Ringer's Injection, USP, or 5% Dextrose Injection, USP.
- Do not administer with other drugs or diluents as this may cause incompatibilities.
Treatment of Erosive Esophagitis
The recommended adult dose (when patients are unable to take the oral therapy) is 30 mg of lansoprazole (1 vial of PREVACID I.V. for Injection) per day administered by I.V. infusion over 30 minutes for up to 7 days. Once the patient is able to take medications orally, therapy can be switched to an oral PREVACID formulation for a total of 6 to 8 weeks. Refer to full prescribing information for the oral formulations of PREVACID.
No dosage adjustment is necessary in patients with renal insufficiency or the elderly. For patients with severe liver disease, dosage adjustment should be considered.
HOW SUPPLIED
PREVACID I.V. for Injection contains 30 mg of lansoprazole as white to pale yellow friable masses and powder in a vial and is available as follows:
NDC 0300-3954-25Tray containing 10 single dose vial packs: Each pack containing one 30-mg single dose vial of PREVACID I.V. for Injection and 1 required in-line filter (1.2 µm pore size).
Store PREVACID I.V. for Injection at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). Protect from light. Use carton to protect contents from light.
U.S. Patent No. 4,628,098.
Distributed by
TAP Pharmaceuticals Inc.
Lake Forest, IL 60045, U.S.A.
MINI-BAG is a trademark of Baxter International Inc. (List 3954)
102-0001
R2, Rev. December 2004
© 2004 Tap Pharmaceutical Products Inc.
-
Reconstitution in Vial and Preparation of Admixture.
Subscribe to the "News" RSS Feed 
TOP ۞
