-
Purinethol Tablets (Gate)
CAUTION
PURINETHOL (mercaptopurine) is a potent drug. It should not be used unless a diagnosis of acute lymphatic leukemia has been adequately established and the responsible physician is knowledgeable in assessing response to chemotherapy.
DESCRIPTION
PURINETHOL (mercaptopurine) was synthesized and developed by Hitchings, Elion, and associates at the Wellcome Research Laboratories. It is one of a large series of purine analogues which interfere with nucleic acid biosynthesis and has been found active against human leukemias.
Mercaptopurine, known chemically as 1,7-dihydro-6 H -purine-6-thione monohydrate, is an analogue of the purine bases adenine and hypoxanthine. Its structural formula is:
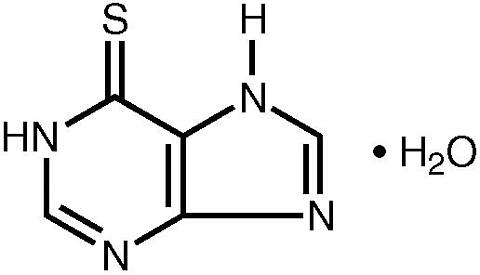
PURINETHOL is available in tablet form for oral administration. Each scored tablet contains 50 mg mercaptopurine and the inactive ingredients corn and potato starch, lactose, magnesium stearate, and stearic acid.
CLINICAL PHARMACOLOGY
Clinical studies have shown that the absorption of an oral dose of mercaptopurine in humans is incomplete and variable, averaging approximately 50% of the administered dose. The factors influencing absorption are unknown. Intravenous administration of an investigational preparation of mercaptopurine revealed a plasma half-disappearance time of 21 minutes in pediatric patients and 47 minutes in adults. The volume of distribution usually exceeded that of the total body water.
Following the oral administration of 35 S-6-mercaptopurine in one subject, a total of 46% of the dose could be accounted for in the urine (as parent drug and metabolites) in the first 24 hours. Metabolites of mercaptopurine were found in urine within the first 2 hours after administration. Radioactivity (in the form of sulfate) could be found in the urine for weeks afterwards.
There is negligible entry of mercaptopurine into cerebrospinal fluid.
Plasma protein binding averages 19% over the concentration range 10 to 50 mcg/mL (a concentration only achieved by intravenous administration of mercaptopurine at doses exceeding 5 to 10 mg/kg).
Monitoring of plasma levels of mercaptopurine during therapy is of questionable value. There is technical difficulty in determining plasma concentrations which are seldom greater than 1 to 2 mcg/mL after a therapeutic oral dose. More significantly, mercaptopurine enters rapidly into the anabolic and catabolic pathways for purines, and the active intracellular metabolites have appreciably longer half-lives than the parent drug. The biochemical effects of a single dose of mercaptopurine are evident long after the parent drug has disappeared from plasma. Because of this rapid metabolism of mercaptopurine to active intracellular derivatives, hemodialysis would not be expected to appreciably reduce toxicity of the drug. There is no known pharmacologic antagonist to the biochemical actions of mercaptopurine in vivo.
Mercaptopurine competes with hypoxanthine and guanine for the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) and is itself converted to thioinosinic acid (TIMP). This intracellular nucleotide inhibits several reactions involving inosinic acid (IMP), including the conversion of IMP to xanthylic acid (XMP) and the conversion of IMP to adenylic acid (AMP) via adenylosuccinate (SAMP). In addition, 6-methylthioinosinate (MTIMP) is formed by the methylation of TIMP. Both TIMP and MTIMP have been reported to inhibit glutamine-5-phosphoribosylpyrophosphate amidotransferase, the first enzyme unique to the de novo pathway for purine ribonucleotide synthesis.
Experiments indicate that radiolabeled mercaptopurine may be recovered from the DNA in the form of deoxythioguanosine. Some mercaptopurine is converted to nucleotide derivatives of 6-thioguanine (6-TG) by the sequential actions of inosinate (IMP) dehydrogenase and xanthylate (XMP) aminase, converting TIMP to thioguanylic acid (TGMP).
Animal tumors that are resistant to mercaptopurine often have lost the ability to convert mercaptopurine to TIMP. However, it is clear that resistance to mercaptopurine may be acquired by other means as well, particularly in human leukemias.
It is not known exactly which of any one or more of the biochemical effects of mercaptopurine and its metabolites are directly or predominantly responsible for cell death.
The catabolism of mercaptopurine and its metabolites is complex. In humans, after oral administration of 35 S-6-mercaptopurine, urine contains intact mercaptopurine, thiouric acid (formed by direct oxidation by xanthine oxidase, probably via 6-mercapto-8-hydroxypurine), and a number of 6-methylated thiopurines. The methylthiopurines yield appreciable amounts of inorganic sulfate. The importance of the metabolism by xanthine oxidase relates to the fact that ZYLOPRIM® (allopurinol) inhibits this enzyme and retards the catabolism of mercaptopurine and its active metabolites. A significant reduction in mercaptopurine dosage is mandatory if a potent xanthine oxidase inhibitor and mercaptopurine are used simultaneously in a patient (see PRECAUTIONS ).
INDICATIONS AND USAGE
PURINETHOL (mercaptopurine) is indicated for remission induction and maintenance therapy of acute lymphatic leukemia. The response to this agent depends upon the particular subclassification of acute lymphatic leukemia and the age of the patient (pediatric patient or adult).
Acute Lymphatic (Lymphocytic, Lymphoblastic) Leukemia: Given as a single agent for remission induction, PURINETHOL induces complete remission in approximately 25% of pediatric patients and 10% of adults. However, reliance upon PURINETHOL alone is not justified for initial remission induction of acute lymphatic leukemia since combination chemotherapy with vincristine, prednisone, and L-asparaginase results in more frequent complete remission induction than with PURINETHOL alone or in combination. The duration of complete remission induced in acute lymphatic leukemia is so brief without the use of maintenance therapy that some form of drug therapy is considered essential. PURINETHOL, as a single agent, is capable of significantly prolonging complete remission duration; however, combination therapy has produced remission duration longer than that achieved with PURINETHOL alone.
Acute Myelogenous (and Acute Myelomonocytic) Leukemia: As a single agent, PURINETHOL will induce complete remission in approximately 10% of pediatric patients and adults with acute myelogenous leukemia or its subclassifications. These results are inferior to those achieved with combination chemotherapy employing optimum treatment schedules.
Central Nervous System Leukemia: PURINETHOL is not effective for prophylaxis or treatment of central nervous system leukemia.
Other Neoplasms: PURINETHOL is not effective in chronic lymphatic leukemia, the lymphomas (including Hodgkins Disease), or solid tumors.
CONTRAINDICATIONS
PURINETHOL should not be used unless a diagnosis of acute lymphatic leukemia has been adequately established and the responsible physician is knowledgeable in assessing response to chemotherapy.
PURINETHOL should not be used in patients whose disease has demonstrated prior resistance to this drug. In animals and humans, there is usually complete cross-resistance between mercaptopurine and thioguanine.
PURINETHOL should not be used in patients who have a hypersensitivity to mercaptopurine or any component of the formulation.
WARNINGS
SINCE DRUGS USED IN CANCER CHEMOTHERAPY ARE POTENTIALLY HAZARDOUS, IT IS RECOMMENDED THAT ONLY PHYSICIANS EXPERIENCED WITH THE RISKS OF PURINETHOL AND KNOWLEDGEABLE IN THE NATURAL HISTORY OF ACUTE LEUKEMIAS ADMINISTER THIS DRUG.
Bone Marrow Toxicity: The most consistent, dose-related toxicity is bone marrow suppression. This may be manifest by anemia, leukopenia, thrombocytopenia, or any combination of these. Any of these findings may also reflect progression of the underlying disease. Since mercaptopurine may have a delayed effect, it is important to withdraw the medication temporarily at the first sign of an abnormally large fall in any of the formed elements of the blood.
There are individuals with an inherited deficiency of the enzyme thiopurine methyltransferase (TPMT) who may be unusually sensitive to the myelosuppressive effects of mercaptopurine and prone to developing rapid bone marrow suppression following the initiation of treatment. Substantial dosage reductions may be required to avoid the development of life-threatening bone marrow suppression in these patients. This toxicity may be more profound in patients treated with concomitant allopurinol (see PRECAUTIONS : Drug Interactions ). This problem could be exacerbated by coadministration with drugs that inhibit TPMT, such as olsalazine, mesalazine, or sulphasalazine.
Hepatotoxicity: Mercaptopurine is hepatotoxic in animals and humans. A small number of deaths have been reported that may have been attributed to hepatic necrosis due to administration of mercaptopurine. Hepatic injury can occur with any dosage, but seems to occur with more frequency when doses of 2.5 mg/kg/day are exceeded. The histologic pattern of mercaptopurine hepatotoxicity includes features of both intrahepatic cholestasis and parenchymal cell necrosis, either of which may predominate. It is not clear how much of the hepatic damage is due to direct toxicity from the drug and how much may be due to a hypersensitivity reaction. In some patients jaundice has cleared following withdrawal of mercaptopurine and reappeared with its reintroduction.
Published reports have cited widely varying incidences of overt hepatotoxicity. In a large series of patients with various neoplastic diseases, mercaptopurine was administered orally in doses ranging from 2.5 mg/kg to 5.0 mg/kg without any evidence of hepatotoxicity. It was noted by the authors that no definite clinical evidence of liver damage could be ascribed to the drug, although an occasional case of serum hepatitis did occur in patients receiving 6-MP who previously had transfusions. In reports of smaller cohorts of adult and pediatric leukemic patients, the incidence of hepatotoxicity ranged from 0% to 6%. In an isolated report by Einhorn and Davidsohn, jaundice was observed more frequently (40%), especially when doses exceeded 2.5 mg/kg. Usually, clinically detectable jaundice appears early in the course of treatment (1 to 2 months). However, jaundice has been reported as early as 1 week and as late as 8 years after the start of treatment with mercaptopurine.
Monitoring of serum transaminase levels, alkaline phosphatase, and bilirubin levels may allow early detection of hepatotoxicity. It is advisable to monitor these liver function tests at weekly intervals when first beginning therapy and at monthly intervals thereafter. Liver function tests may be advisable more frequently in patients who are receiving mercaptopurine with other hepatotoxic drugs or with known pre-existing liver disease.
The concomitant administration of mercaptopurine with other hepatotoxic agents requires especially careful clinical and biochemical monitoring of hepatic function. Combination therapy involving mercaptopurine with other drugs not felt to be hepatotoxic should nevertheless be approached with caution. The combination of mercaptopurine with doxorubicin was reported to be hepatotoxic in 19 of 20 patients undergoing remission-induction therapy for leukemia resistant to previous therapy.
The hepatotoxicity has been associated in some cases with anorexia, diarrhea, jaundice, and ascites. Hepatic encephalopathy has occurred.
The onset of clinical jaundice, hepatomegaly, or anorexia with tenderness in the right hypochondrium are immediate indications for withholding mercaptopurine until the exact etiology can be identified. Likewise, any evidence of deterioration in liver function studies, toxic hepatitis, or biliary stasis should prompt discontinuation of the drug and a search for an etiology of the hepatotoxicity.
Immunosuppression: Mercaptopurine recipients may manifest decreased cellular hypersensitivities and impaired allograft rejection. Induction of immunity to infectious agents or vaccines will be subnormal in these patients; the degree of immunosuppression will depend on antigen dose and temporal relationship to drug. This immunosuppressive effect should be carefully considered with regard to intercurrent infections and risk of subsequent neoplasia.
Pregnancy: Pregnancy Category D. Mercaptopurine can cause fetal harm when administered to a pregnant woman. Women receiving mercaptopurine in the first trimester of pregnancy have an increased incidence of abortion; the risk of malformation in offspring surviving first trimester exposure is not accurately known. In a series of 28 women receiving mercaptopurine after the first trimester of pregnancy, 3 mothers died undelivered, 1 delivered a stillborn child, and 1 aborted; there were no cases of macroscopically abnormal fetuses. Since such experience cannot exclude the possibility of fetal damage, mercaptopurine should be used during pregnancy only if the benefit clearly justifies the possible risk to the fetus, and particular caution should be given to the use of mercaptopurine in the first trimester of pregnancy.
There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy or if the patient becomes pregnant while taking the drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
PRECAUTIONS
General: The safe and effective use of PURINETHOL demands a thorough knowledge of the natural history of the condition being treated. After selection of an initial dosage schedule, therapy will frequently need to be modified depending upon the patient's response and manifestations of toxicity.
The most frequent, serious, toxic effect of PURINETHOL is myelosuppression resulting in leukopenia, thrombocytopenia, and anemia. These toxic effects are often unavoidable during the induction phase of adult acute leukemia if remission induction is to be successful. Whether or not these manifestations demand modification or cessation of dosage depends both upon the response of the underlying disease and a careful consideration of supportive facilities (granulocyte and platelet transfusions) which may be available. Life-threatening infections and bleeding have been observed as a consequence of mercaptopurine-induced granulocytopenia and thrombocytopenia. Severe hematologic toxicity may require supportive therapy with platelet transfusions for bleeding, and antibiotics and granulocyte transfusions if sepsis is documented.
If it is not the intent to deliberately induce bone marrow hypoplasia, it is important to discontinue the drug temporarily at the first evidence of an abnormally large fall in white blood cell count, platelet count, or hemoglobin concentration. In many patients with severe depression of the formed elements of the blood due to PURINETHOL, the bone marrow appears hypoplastic on aspiration or biopsy, whereas in other cases it may appear normocellular. The qualitative changes in the erythroid elements toward the megaloblastic series, characteristically seen with the folic acid antagonists and some other antimetabolites, are not seen with this drug.
It is probably advisable to start with smaller dosages in patients with impaired renal function, since the latter might result in slower elimination of the drug and metabolites and a greater cumulative effect.
Information for Patients: Patients should be informed that the major toxicities of PURINETHOL are related to myelosuppression, hepatotoxicity, and gastrointestinal toxicity. Patients should never be allowed to take the drug without medical supervision and should be advised to consult their physician if they experience fever, sore throat, jaundice, nausea, vomiting, signs of local infection, bleeding from any site, or symptoms suggestive of anemia. Women of childbearing potential should be advised to avoid becoming pregnant.
Laboratory Tests: It is recommended that evaluation of the hemoglobin or hematocrit, total white blood cell count and differential count, and quantitative platelet count be obtained weekly while the patient is on therapy with PURINETHOL. In cases where the cause of fluctuations in the formed elements in the peripheral blood is obscure, bone marrow examination may be useful for the evaluation of marrow status. The decision to increase, decrease, continue, or discontinue a given dosage of PURINETHOL must be based not only on the absolute hematologic values, but also upon the rapidity with which changes are occurring. In many instances, particularly during the induction phase of acute leukemia, complete blood counts will need to be done more frequently than once weekly in order to evaluate the effect of the therapy.
Drug Interactions: When allopurinol and mercaptopurine are administered concomitantly, it is imperative that the dose of mercaptopurine be reduced to one third to one quarter of the usual dose. Failure to observe this dosage reduction will result in a delayed catabolism of mercaptopurine and the strong likelihood of inducing severe toxicity.
There is usually complete cross-resistance between mercaptopurine and thioguanine.
The dosage of mercaptopurine may need to be reduced when this agent is combined with other drugs whose primary or secondary toxicity is myelosuppression. Enhanced marrow suppression has been noted in some patients also receiving trimethoprim-sulfamethoxazole.
Inhibition of the anticoagulant effect of warfarin, when given with mercaptopurine, has been reported.
As there is in vitro evidence that aminosalicylate derivatives (e.g., olsalazine, mesalazine, or sulphasalazine) inhibit the TPMT enzyme, they should be administered with caution to patients receiving concurrent mercaptopurine therapy (see WARNINGS ).
Carcinogenesis, Mutagenesis, Impairment of Fertility: Mercaptopurine causes chromosomal aberrations in animals and humans and induces dominant-lethal mutations in male mice. In mice, surviving female offspring of mothers who received chronic low doses of mercaptopurine during pregnancy were found sterile, or if they became pregnant, had smaller litters and more dead fetuses as compared to control animals. Carcinogenic potential exists in humans, but the extent of the risk is unknown.
The effect of mercaptopurine on human fertility is unknown for either males or females.
Pregnancy: Teratogenic Effects: Pregnancy Category D. See WARNINGS section.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from mercaptopurine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use: See DOSAGE AND ADMINISTRATION section.
ADVERSE REACTIONS
The principal and potentially serious toxic effects of PURINETHOL are bone marrow toxicity and hepatotoxicity (see WARNINGS ).
Hematologic: The most frequent adverse reaction to PURINETHOL is myelosuppression. The induction of complete remission of acute lymphatic leukemia frequently is associated with marrow hypoplasia. Maintenance of remission generally involves multiple-drug regimens whose component agents cause myelosuppression. Anemia, leukopenia, and thrombocytopenia are frequently observed. Dosages and schedules are adjusted to prevent life-threatening cytopenias.
Renal: Hyperuricemia and/or hyperuricosuria may occur in patients receiving PURINETHOL as a consequence of rapid cell lysis accompanying the antineoplastic effect. Adverse effects can be minimized by increased hydration, urine alkalinization, and the prophylactic administration of a xanthine oxidase inhibitor such as allopurinol. The dosage of PURINETHOL should be reduced to one third to one quarter of the usual dose if allopurinol is given concurrently.
Gastrointestinal: Intestinal ulceration has been reported. Nausea, vomiting, and anorexia are uncommon during initial administration. Mild diarrhea and sprue-like symptoms have been noted occasionally, but it is difficult at present to attribute these to the medication. Oral lesions are rarely seen, and when they occur they resemble thrush rather than antifolic ulcerations.
An increased risk of pancreatitis may be associated with the investigational use of PURINETHOL in inflammatory bowel disease.
Miscellaneous: While dermatologic reactions can occur as a consequence of disease, the administration of PURINETHOL has been associated with skin rashes and hyperpigmentation. Alopecia has been reported.
Drug fever has been very rarely reported with PURINETHOL. Before attributing fever to PURINETHOL, every attempt should be made to exclude more common causes of pyrexia, such as sepsis, in patients with acute leukemia.
Oligospermia has been reported.
OVERDOSAGE
Signs and symptoms of overdosage may be immediate such as anorexia, nausea, vomiting, and diarrhea; or delayed such as myelosuppression, liver dysfunction, and gastroenteritis. Dialysis cannot be expected to clear mercaptopurine. Hemodialysis is thought to be of marginal use due to the rapid intracellular incorporation of mercaptopurine into active metabolites with long persistence. The oral LD 50 of mercaptopurine was determined to be 480 mg/kg in the mouse and 425 mg/kg in the rat.
There is no known pharmacologic antagonist of mercaptopurine. The drug should be discontinued immediately if unintended toxicity occurs during treatment. If a patient is seen immediately following an accidental overdosage of the drug, it may be useful to induce emesis.
DOSAGE AND ADMINISTRATION
Induction Therapy: PURINETHOL is administered orally. The dosage which will be tolerated and be effective varies from patient to patient, and therefore careful titration is necessary to obtain the optimum therapeutic effect without incurring excessive, unintended toxicity. The usual initial dosage for pediatric patients and adults is 2.5 mg/kg of body weight per day (100 to 200 mg in the average adult and 50 mg in an average 5-year-old child). Pediatric patients with acute leukemia have tolerated this dose without difficulty in most cases; it may be continued daily for several weeks or more in some patients. If, after 4 weeks at this dosage, there is no clinical improvement and no definite evidence of leukocyte or platelet depression, the dosage may be increased up to 5 mg/kg daily. A dosage of 2.5 mg/kg/day may result in a rapid fall in leukocyte count within 1 to 2 weeks in some adults with acute lymphatic leukemia and high total leukocyte counts.
The total daily dosage may be given at one time. It is calculated to the nearest multiple of 25 mg. The dosage of PURINETHOL should be reduced to one third to one quarter of the usual dose if allopurinol is given concurrently. Because the drug may have a delayed action, it should be discontinued at the first sign of an abnormally large or rapid fall in the leukocyte or platelet count. If subsequently the leukocyte count or platelet count remains constant for 2 or 3 days, or rises, treatment may be resumed.
Maintenance Therapy: Once a complete hematologic remission is obtained, maintenance therapy is considered essential. Maintenance doses will vary from patient to patient. A usual daily maintenance dose of PURINETHOL is 1.5 to 2.5 mg/kg/day as a single dose. It is to be emphasized that in pediatric patients with acute lymphatic leukemia in remission, superior results have been obtained when PURINETHOL has been combined with other agents (most frequently with methotrexate) for remission maintenance. PURINETHOL should rarely be relied upon as a single agent for the maintenance of remissions induced in acute leukemia.
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published. 1-8
There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Dosage in Renal Impairment: Consideration should be given to reducing the dosage in patients with impaired renal function.
Dosage in Hepatic Impairment: Consideration should be given to reducing the dosage in patients with impaired hepatic function.
HOW SUPPLIED
Pale yellow to buff, scored tablets containing 50 mg mercaptopurine, imprinted with "PURINETHOL" and "04A"; bottles of 60 (NDC 57844-522-06).
Store at 15° to 25°C (59° to 77°F) in a dry place.
REFERENCES
- ONS Clinical Practice Committee. Cancer Chemotherapy Guidelines and Recommendations for Practice. Pittsburgh, PA: Oncology Nursing Society;1999:32-41.
- Recommendations for the safe handling of parenteral antineoplastic drugs. Washington, DC: Division of Safety; Clinical Center Pharmacy Department and Cancer Nursing Services, National Institutes of Health; 1992. US Dept of Health and Human Services. Public Health Service publication NIH 92-2621.
- AMA Council on Scientific Affairs. Guidelines for handling parenteral antineoplastics. JAMA. 1985;253:1590-1591.
- National Study Commission on Cytotoxic Exposure. Recommendations for handling cytotoxic agents. 1987. Available from Louis P. Jeffrey, Chairman, National Study Commission on Cytotoxic Exposure. Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Avenue, Boston, MA 02115.
- Clinical Oncological Society of Australia. Guidelines and recommendations for safe handling of antineoplastic agents. Med J Australia. 1983;1:426-428.
- Jones RB, Frank R, Mass T. Safe handling of chemotherapeutic agents: a report from the Mount Sinai Medical Center. CA-A Cancer J for Clinicians. 1983;33:258-263.
- American Society of Hospital Pharmacists. ASHP technical assistance bulletin on handling cytotoxic and hazardous drugs. Am J Hosp Pharm. 1990;47:1033-1049.
- Controlling Occupational Exposure to Hazardous Drugs. (OSHA Work-Practice Guidelines.) Am J Health-Syst Pharm. 1996;53:1669-1685.
Manufactured for:
div. of Teva Pharmaceuticals USA
Sellersville, PA 18960
Manufactured by DSM Pharmaceuticals, Inc.
Greenville, NC 27834
Rev. A 10/2003
Subscribe to the "News" RSS Feed 
TOP ۞
