-
Rebetol Capsules, Rebetol Oral Solution (Schering)
- REBETOL monotherapy is not effective for the treatment of chronic hepatitis C virus infection and should not be used alone for this indication. (See WARNINGS ).
- The primary toxicity of ribavirin is hemolytic anemia. The anemia associated with REBETOL therapy may result in worsening of cardiac disease that has led to fatal and nonfatal myocardial infarctions. Patients with a history of significant or unstable cardiac disease should not be treated with REBETOL. (See WARNINGS , ADVERSE REACTIONS , and DOSAGE AND ADMINISTRATION ).
- Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. In addition, ribavirin has a multiple-dose half-life of 12 days, and so it may persist in nonplasma compartments for as long as 6 months. Therefore, REBETOL therapy is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Extreme care must be taken to avoid pregnancy during therapy and for 6 months after completion of treatment in both female patients and in female partners of male patients who are taking REBETOL therapy. At least two reliable forms of effective contraception must be utilized during treatment and during the 6-month posttreatment follow-up period. (See CONTRAINDICATIONS , WARNINGS , PRECAUTIONS -- Information for Patients and Pregnancy Category X ).
DESCRIPTION
REBETOL®
REBETOL is Schering Corporation's brand name for ribavirin, a nucleoside analog. The chemical name of ribavirin is 1-(beta)-D-ribofuranosyl-1 H -1,2,4-triazole-3-carboxamide and has the following structural formula:
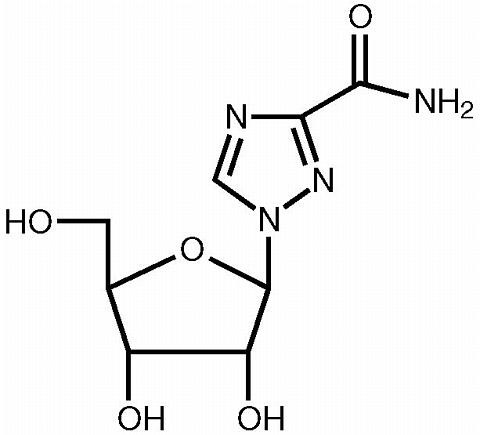
Ribavirin is a white, crystalline powder. It is freely soluble in water and slightly soluble in anhydrous alcohol. The empirical formula is C 8 H 12 N 4 O 5 and the molecular weight is 244.21.
REBETOL Capsules consist of a white powder in a white, opaque, gelatin capsule. Each capsule contains 200 mg ribavirin and the inactive ingredients microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, and magnesium stearate. The capsule shell consists of gelatin, sodium lauryl sulfate, silicon dioxide, and titanium dioxide. The capsule is printed with edible blue pharmaceutical ink which is made of shellac, anhydrous ethyl alcohol, isopropyl alcohol, n-butyl alcohol, propylene glycol, ammonium hydroxide, and FD&C Blue #2 aluminum lake.
REBETOL Oral Solution is a clear, colorless to pale or light yellow bubble gum-flavored liquid. Each milliliter of the solution contains 40 mg of ribavirin and the inactive ingredients sucrose, glycerin, sorbitol, propylene glycol, sodium citrate, citric acid, sodium benzoate, natural and artificial flavor for bubble gum #15864, and water.
Mechanism of Action
The mechanism of inhibition of hepatitis C virus (HCV) RNA by combination therapy with ribavirin and interferon products has not been established.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Ribavirin Single- and multiple-dose pharmacokinetic properties in adults are summarized in TABLE 1 . Ribavirin was rapidly and extensively absorbed following oral administration. However, due to first-pass metabolism, the absolute bioavailability averaged 64% (44%). There was a linear relationship between dose and AUC tf (AUC from time zero to last measurable concentration) following single doses of 200-1200 mg ribavirin. The relationship between dose and C max was curvilinear, tending to asymptote above single doses of 400-600 mg.
Upon multiple oral dosing, based on AUC12 hr , a sixfold accumulation of ribavirin was observed in plasma. Following oral dosing with 600 mg BID, steady-state was reached by approximately 4 weeks, with mean steady-state plasma concentrations of 2200 (37%) ng/mL. Upon discontinuation of dosing, the mean half-life was 298 (30%) hours, which probably reflects slow elimination from nonplasma compartments.
Effect of Food on Absorption of Ribavirin Both AUC tf and C max increased by 70% when REBETOL Capsules were administered with a high-fat meal (841 kcal, 53.8 g fat, 31.6 g protein, and 57.4 g carbohydrate) in a single-dose pharmacokinetic study. There are insufficient data to address the clinical relevance of these results. Clinical efficacy studies with REBETOL/INTRON A were conducted without instructions with respect to food consumption. During clinical studies with REBETOL/PEG-INTRON, all subjects were instructed to take REBETOL Capsules with food. (See DOSAGE AND ADMINISTRATION .)
Effect of Antacid on Absorption of Ribavirin Coadministration of REBETOL Capsules with an antacid containing magnesium, aluminum, and simethicone (Mylanta® 1 ) resulted in a 14% decrease in mean ribavirin AUC tf . The clinical relevance of results from this single-dose study is unknown.
TABLE 1. Mean (% CV) Pharmacokinetic Parameters for
REBETOL When Administered Individually to AdultsParameterREBETOL Single Dose
600 mg
Oral Solution
(N=14)Single Dose
600 mg
Capsules
(N=12)Multiple Dose
600 mg
BID Capsules
(N=12)T max (hr)1.00 (34) 1.7 (46) *** 3 (60) C max *872 (42) 782 (37) 3680 (85) AUC tf **14098 (38) 13400 (48) 228000 (25) T ½ (hr)43.6 (47) 298 (30) Apparent Volume of
Distribution (L)2825 (9) **/* Apparent Clearance
(L/hr)38.2 (40) Absolute
Bioavailability64% (44) # * ng/mL** ng.hr/mL*** N = 11**/* data obtained from a single-dose pharmacokinetic study using 14 C labeled ribavirin; N = 5# N = 6
Ribavirin transport into nonplasma compartments has been most extensively studied in red blood cells, and has been identified to be primarily via an e s -type equilibrative nucleoside transporter. This type of transporter is present on virtually all cell types and may account for the extensive volume of distribution. Ribavirin does not bind to plasma proteins.
Ribavirin has two pathways of metabolism: (i) a reversible phosphorylation pathway in nucleated cells; and (ii) a degradative pathway involving deribosylation and amide hydrolysis to yield a triazole carboxylic acid metabolite. Ribavirin and its triazole carboxamide and triazole carboxylic acid metabolites are excreted renally. After oral administration of 600 mg of 14 C-ribavirin, approximately 61% and 12% of the radioactivity was eliminated in the urine and feces, respectively, in 336 hours. Unchanged ribavirin accounted for 17% of the administered dose.
Results of in vitro studies using both human and rat liver microsome preparations indicated little or no cytochrome P450 enzyme-mediated metabolism of ribavirin, with minimal potential for P450 enzyme-based drug interactions.
No pharmacokinetic interactions were noted between INTRON A Injection and REBETOL Capsules in a multiple-dose pharmacokinetic study.
Drug Interactions Ribavirin has been shown in vitro to inhibit phosphorylation of zidovudine and stavudine which could lead to decreased antiretroviral activity. Exposure to didanosine or its active metabolite (dideoxyadenosine 5'-triphosphate) is increased when didanosine is co-administered with ribavirin, which could cause or worsen clinical toxicities (see PRECAUTIONS : Drug Interactions ).
1 Trademark of Johnson & Johnson-Merck Consumer Pharmaceuticals Co.
Special Populations
Renal Dysfunction The pharmacokinetics of ribavirin were assessed after administration of a single oral dose (400 mg) of ribavirin to non HCV-infected subjects with varying degrees of renal dysfunction. The mean AUC tf value was threefold greater in subjects with creatinine clearance values between 10 to 30 mL/min when compared to control subjects (creatinine clearance >90 mL/min). In subjects with creatinine clearance values between 30 to 60 mL/min, AUC tf was twofold greater when compared to control subjects. The increased AUC tf appears to be due to reduction of renal and non-renal clearance in these patients. Phase III efficacy trials included subjects with creatinine clearance values >50 mL/min. The multiple dose pharmacokinetics of ribavirin cannot be accurately predicted in patients with renal dysfunction. Ribavirin is not effectively removed by hemodialysis. Patients with creatinine clearance <50 mL/min should not be treated with REBETOL (see WARNINGS ).
Hepatic Dysfunction The effect of hepatic dysfunction was assessed after a single oral dose of ribavirin (600 mg). The mean AUC tf values were not significantly different in subjects with mild, moderate, or severe hepatic dysfunction (Child-Pugh Classification A, B, or C) when compared to control subjects. However, the mean C max values increased with severity of hepatic dysfunction and was twofold greater in subjects with severe hepatic dysfunction when compared to control subjects.
Elderly Patients Pharmacokinetic evaluations in elderly subjects have not been performed.
Gender There were no clinically significant pharmacokinetic differences noted in a single-dose study of eighteen male and eighteen female subjects.
Pediatric Patients Multiple-dose pharmacokinetic properties for REBETOL Capsules and INTRON A in pediatric patients with chronic hepatitis C between 5 and 16 years of age are summarized in TABLE 2 . The pharmacokinetics of REBETOL and INTRON A (dose-normalized) are similar in adults and pediatric patients.
Complete pharmacokinetic characteristics of REBETOL Oral Solution have not been determined in pediatric patients. Ribavirin C min values were similar following administration of REBETOL Oral Solution or REBETOL Capsules during 48 weeks of therapy in pediatric patients (3 to 16 years of age).
TABLE 2. Mean (% CV) Multiple-Dose Pharmacokinetic Parameters for INTRON A and REBETOL Capsules
When Administered to Pediatric Patients
With Chronic Hepatitis CParameterREBETOL
15 mg/kg/day as
2 divided doses
(n=17)INTRON A
3 MIU/m 2
TIW (n=54)T max (hr)1.9 (83) 5.9 (36) C max (ng/mL)3275 (25) 51 (48) AUC *29774 (26) 622 (48) Apparent clearance
L/hr/kg0.27 (27) ND *AUC 12 (ng/hr/mL) for REBETOL; AUC 0-24 (IU hr/mL) for INTRON AND=not done
* In this section of the label, numbers in parenthesis indicate % coefficient of variation.INDICATIONS AND USAGE
Adult Use
REBETOL (ribavirin, USP) Capsules and Oral Solution are indicated in combination with INTRON A (interferon alfa-2b, recombinant) Injection for the treatment of chronic hepatitis C in patients 18 years of age and older with compensated liver disease previously untreated with alpha interferon and in patients 18 years of age and older who have relapsed following alpha interferon therapy.
REBETOL Capsules are indicated in combination with PEG-INTRON (peginterferon alfa-2b, recombinant) Injection for the treatment of chronic hepatitis C in patients with compensated liver disease who have not been previously treated with interferon alpha and are at least 18 years of age.
The safety and efficacy of REBETOL Capsules or Oral Solution with interferons other than INTRON A or PEG-INTRON products have not been established.
Pediatric Use
REBETOL (ribavirin, USP) Capsules are indicated in combination with INTRON A (interferon alfa-2b, recombinant) Injection for the treatment of chronic hepatitis C in patients 5 years of age and older with compensated liver disease previously untreated with alpha interferon and in patients who have relapsed following alpha interferon therapy.
REBETOL (ribavirin, USP) Oral Solution is indicated in combination with INTRON A (interferon alfa-2b, recombinant) Injection for the treatment of chronic hepatitis C in patients 3 years of age and older with compensated liver disease previously untreated with alpha interferon and in patients who have relapsed following alpha interferon therapy.
Evidence of disease progression, such as hepatic inflammation and fibrosis, as well as prognostic factors for response, HCV genotype and viral load, should be considered when deciding to treat a pediatric patient. The benefits of treatment should be weighed against the safety findings observed (see PRECAUTIONS Pediatric Use ) for pediatric subjects in the clinical trials.
Description of Clinical Studies
REBETOL/INTRON A Combination Therapy
Adult Patients
Previously Untreated Patients
Adults with compensated chronic hepatitis C and detectable HCV RNA (assessed by a central laboratory using a research-based RT-PCR assay) who were previously untreated with alpha interferon therapy were enrolled into two multicenter, double-blind trials (US and International) and randomized to receive REBETOL Capsules 1200 mg/day (1000 mg/day for patients weighing </=75 kg) plus INTRON A Injection 3 MIU TIW or INTRON A Injection plus placebo for 24 or 48 weeks followed by 24 weeks of off-therapy follow-up. The International study did not contain a 24-week INTRON A plus placebo treatment arm. The US study enrolled 912 patients who, at baseline, were 67% male, 89% Caucasian with a mean Knodell HAI score (I+II+III) of 7.5, and 72% genotype 1. The International study, conducted in Europe, Israel, Canada, and Australia, enrolled 799 patients (65% male, 95% Caucasian, mean Knodell score 6.8, and 58% genotype 1).
Study results are summarized in TABLE 3 .
TABLE 3. Virologic and Histologic Responses: Previously Untreated Patients * US Study International Study 24 weeks
of treatment48 weeks
of treatment24 weeks
of treatment48 weeks
of treatmentINTRON A
plus
REBETOL
(N=228)INTRON A
plus
Placebo
(N=231)INTRON A
plus
REBETOL
(N=228)INTRON A
plus
Placebo
(N=225)INTRON A
plus
REBETOL
(N=265)INTRON A
plus
REBETOL
(N=268)INTRON A
plus
Placebo
(N=266)Virologic
Response-Responder 165 (29) 13 (6) 85 (37) 27 (12) 86 (32) 113 (42) 46 (17) -Nonresponder147 (64) 194 (84) 110 (48) 168 (75) 158 (60) 120 (45) 196 (74) -Missing Data16 (7) 24 (10) 33 (14) 30 (13) 21 (8) 35 (13) 24 (9) Histologic
Response-Improvement 1102 (45) 77 (33) 96 (42) 65 (29) 103 (39) 102 (38) 69 (26) -No
improvement77 (34) 99 (43) 61 (27) 93 (41) 85 (32) 58 (22) 111 (41) -Missing Data49 (21) 55 (24) 71 (31) 67 (30) 77 (29) 108 (40) 86 (32) *Number (%) of patients.1.Defined as HCV RNA below limit of detection using a research-based RT-PCR assay at end of treatment and during follow-up period.2.Defined as posttreatment (end of follow-up) minus pretreatment liver biopsy Knodell HAI score (I+II+III) improvement of >/=2 points.
Of patients who had not achieved HCV RNA below the limit of detection of the research-based assay by week 24 of REBETOL/INTRON A treatment, less than 5% responded to an additional 24 weeks of combination treatment.
Among patients with HCV Genotype 1 treated with REBETOL/INTRON A therapy who achieved HCV RNA below the detection limit of the research-based assay by 24 weeks, those randomized to 48 weeks of treatment had higher virologic responses compared to those in the 24 week treatment group. There was no observed increase in response rates for patients with HCV nongenotype 1 randomized to REBETOL/INTRON A therapy for 48 weeks compared to 24 weeks.
Relapse Patients
Patients with compensated chronic hepatitis C and detectable HCV RNA (assessed by a central laboratory using a research-based RT-PCR assay) who had relapsed following one or two courses of interferon therapy (defined as abnormal serum ALT levels) were enrolled into two multicenter, double-blind trials (US and International) and randomized to receive REBETOL 1200 mg/day (1000 mg/day for patients weighing </=75 kg) plus INTRON A 3 MIU TIW or INTRON A plus placebo for 24 weeks followed by 24 weeks of off-therapy follow-up. The US study enrolled 153 patients who, at baseline, were 67% male, 92% Caucasian with a mean Knodell HAI score (I+II+III) of 6.8, and 58% genotype 1. The International study, conducted in Europe, Israel, Canada, and Australia, enrolled 192 patients (64% male, 95% Caucasian, mean Knodell score 6.6, and 56% genotype 1).
Study results are summarized in TABLE 4 .
TABLE 4. Virologic and Histologic Responses: Relapse Patients * US Study International Study INTRON A
plus
REBETOL
(N=77)INTRON A
plus
Placebo
(N=76)INTRON A
plus
REBETOL
(N=96)INTRON A
plus
Placebo
(N=96)Virologic Response-Responder 133 (43) 3 (4) 46 (48) 5 (5) -Nonresponder36 (47) 66 (87) 45 (47) 91 (95) -Missing Data8 (10) 7 (9) 5 (5) 0 (0) Histologic Response-Improvement 238 (49) 27 (36) 49 (51) 30 (31) -No improvement23 (30) 37 (49) 29 (30) 44 (46) -Missing Data16 (21) 12 (16) 18 (19) 22 (23) * Number (%) of patients.1. Defined as HCV RNA below limit of detection using a research-based RT-PCR assay at end of treatment and during follow-up period.2. Defined as posttreatment (end of follow-up) minus pretreatment liver biopsy Knodell HAI score (I+II+III) improvement of >/=2 points.Virologic and histologic responses were similar among male and female patients in both the previously untreated and relapse studies.
Pediatric Patients
Pediatric patients 3 to 16 years of age with compensated chronic hepatitis C and detectable HCV RNA (assessed by a central laboratory using a research-based RT-PCR assay) were treated with REBETOL 15 mg/kg per day plus INTRON A 3 MIU/m 2 TIW for 48 weeks followed by 24 weeks of off-therapy follow-up. A total of 118 patients received treatment who were 57% male, 80% Caucasian, and 78% genotype 1. Patients <5 years of age received REBETOL Oral Solution and those >/=5 years of age received either REBETOL Oral Solution or Capsules.
Study results are summarized in TABLE 5 .
TABLE 5. Virologic Response: Previously Untreated Pediatric Patients * INTRON A 3 MIU/m 2
TIW Plus REBETOL
15 mg/kg/dayOverall Response 1 (n=118)54 (46) Genotype 1 (n=92)33 (36) Genotype non-1 (n=26)21 (81) *Number (%) of patients. 1. Defined as HCV RNA below limit of detection using a research-based RT-PCR assay at end of treatment and during follow-up period.
Patients with viral genotype 1, regardless of viral load, had a lower response rate to INTRON A/REBETOL combination therapy compared to patients with genotype non-1, 36% versus 81%. Patients with both poor prognostic factors (genotype 1 and high viral load) had a response rate of 26% (13/50).
REBETOL/PEG-INTRON Combination Therapy
A randomized study compared treatment with two PEG-INTRON/REBETOL regimens [PEG-INTRON 1.5 µg/kg SC once weekly (QW)/REBETOL 800 mg PO daily (in divided doses); PEG-INTRON 1.5 µg/kg SC QW for 4 weeks then 0.5 µg/kg SC QW for 44 weeks/REBETOL 1000/1200 mg PO daily (in divided doses)] with INTRON A [3 MIU SC thrice weekly (TIW)/REBETOL 1000/1200 mg PO daily (in divided doses)] in 1530 adults with chronic hepatitis C. Interferon naive patients were treated for 48 weeks and followed for 24 weeks posttreatment. Eligible patients had compensated liver disease, detectable HCV RNA, elevated ALT, and liver histopathology consistent with chronic hepatitis.
Response to treatment was defined as undetectable HCV RNA at 24 weeks posttreatment (see TABLE 6 ).
TABLE 6. Rates of Response to Combination Treatment PEG-INTRON 1.5 µg/kg QW
REBETOL 800 mg QDINTRON A 3 MIU TIW
REBETOL 1000/1200 mg QDOverall 1 , 2
response52% (264/511) 46% (231/505) Genotype 141% (141/348) 33% (112/343) Genotype 2-675% (123/163) 73% (119/162) 1.Serum HCV RNA was measured with a research-based quantitative polymerase chain reaction assay by a central laboratory. 2.Difference in overall treatment response (PEG-INTRON/REBETOL vs. INTRON A/REBETOL) is 6% with 95% confidence interval of (0.18, 11.63) adjusted for viral genotype and presence of cirrhosis at baseline.
The response rate to PEG-INTRON 1.5->0.5µg/kg/REBETOL was essentially the same as the response to INTRON A/REBETOL (data not shown).
Patients with viral genotype 1, regardless of viral load, had a lower response rate to PEG-INTRON (1.5 µg/kg)/REBETOL combination therapy compared to patients with other viral genotypes. Patients with both poor prognostic factors (genotype 1 and high viral load) had a response rate of 30% (78/256) compared to a response rate of 29% (71/247) with INTRON A/REBETOL combination therapy.
Patients with lower body weight tended to have higher adverse event rates (see ADVERSE REACTIONS ) and higher response rates than patients with higher body weights. Differences in response rates between treatment arms did not substantially vary with body weight.
Treatment response rates with PEG-INTRON/REBETOL combination therapy were 49% in men and 56% in women. Response rates were lower in African American and Hispanic patients and higher in Asians compared to Caucasians. Although African Americans had a higher proportion of poor prognostic factors compared to Caucasians the number of non-Caucasians studied (11% of the total) was insufficient to allow meaningful conclusions about differences in response rates after adjusting for prognostic factors.
Liver biopsies were obtained before and after treatment in 68% of patients. Compared to baseline approximately 2 / 3 of patients in all treatment groups were observed to have a modest reduction in inflammation.
CONTRAINDICATIONS
Pregnancy
REBETOL Capsules and Oral Solution may cause birth defects and/or death of the exposed fetus. REBETOL therapy is contraindicated for use in women who are pregnant or in men whose female partners are pregnant. (See WARNINGS , PRECAUTIONS - Information for Patients and Pregnancy Category X .)
REBETOL Capsules and Oral Solution are contraindicated in patients with a history of hypersensitivity to ribavirin or any component of the capsule.
Patients with autoimmune hepatitis must not be treated with combination REBETOL/INTRON A therapy because using these medicines can make the hepatitis worse.
Patients with hemoglobinopathies (eg, thalassemia major, sickle-cell anemia) should not be treated with REBETOL Capsules or Oral Solution.
WARNINGS
Based on results of clinical trials ribavirin monotherapy is not effective for the treatment of chronic hepatitis C virus infection; therefore, REBETOL Capsules or Oral Solution must not be used alone. The safety and efficacy of REBETOL Capsules and Oral Solution have only been established when used together with INTRON A (interferon alfa-2b, recombinant) as REBETRON Combination Therapy or with PEG-INTRON Injection.
There are significant adverse events caused by REBETOL/INTRON A or PEG-INTRON therapy, including severe depression and suicidal ideation, hemolytic anemia, suppression of bone marrow function, autoimmune and infectious disorders, pulmonary dysfunction, pancreatitis, and diabetes. Suicidal ideation or attempts occurred more frequently among pediatric patients, primarily adolescents, compared to adult patients (2.4% versus 1%) during treatment and off-therapy follow-up. The REBETRON Combination Therapy and PEG-INTRON package inserts should be reviewed in their entirety prior to initiation of combination treatment for additional safety information.
Pregnancy
REBETOL Capsules and Oral Solution may cause birth defects and/or death of the exposed fetus. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients. REBETOL has demonstrated significant teratogenic and/or embryocidal effects in all animal species in which adequate studies have been conducted. These effects occurred at doses as low as one twentieth of the recommended human dose of ribavirin. REBETOL THERAPY SHOULD NOT BE STARTED UNTIL A REPORT OF A NEGATIVE PREGNANCY TEST HAS BEEN OBTAINED IMMEDIATELY PRIOR TO PLANNED INITIATION OF THERAPY. Patients should be instructed to use at least two forms of effective contraception during treatment and during the six month period after treatment has been stopped based on multiple dose half-life of ribavirin of 12 days. Pregnancy testing should occur monthly during REBETOL therapy and for six months after therapy has stopped (see CONTRAINDICATIONS and PRECAUTIONS : Information for Patients and Pregnancy Category X ).
Anemia
The primary toxicity of ribavirin is hemolytic anemia, which was observed in approximately 10% of REBETOL/INTRON A-treated patients in clinical trials (see adverse reactions laboratory values - hemoglobin ). The anemia associated with REBETOL capsules occurs within 1-2 weeks of initiation of therapy. BECAUSE THE INITIAL DROP IN HEMOGLOBIN MAY BE SIGNIFICANT, IT IS ADVISED THAT HEMOGLOBIN OR HEMATOCRIT BE OBTAINED PRETREATMENT AND AT WEEK 2 AND WEEK 4 OF THERAPY, OR MORE FREQUENTLY IF CLINICALLY INDICATED. Patients should then be followed as clinically appropriate.
Fatal and nonfatal myocardial infarctions have been reported in patients with anemia caused by REBETOL. Patients should be assessed for underlying cardiac disease before initiation of ribavirin therapy. Patients with pre-existing cardiac disease should have electrocardiograms administered before treatment, and should be appropriately monitored during therapy. If there is any deterioration of cardiovascular status, therapy should be suspended or discontinued. (See DOSAGE AND ADMINISTRATION : Guidelines for Dose Modification.) Because cardiac disease may be worsened by drug induced anemia, patients with a history of significant or unstable cardiac disease should not use REBETOL. (See ADVERSE REACTIONS .)
REBETOL and INTRON A or PEG-INTRON therapy should be suspended in patients with signs and symptoms of pancreatitis and discontinued in patients with confirmed pancreatitis.
REBETOL should not be used in patients with creatinine clearance <50 mL/min. (See Clinical Pharmacology , Special Populations .)
Pulmonary
Pulmonary symptoms, including dyspnea, pulmonary infiltrates, pneumonitis and pneumonia, have been reported during therapy with REBETOL/INTRON A; occasional cases of fatal pneumonia have occurred. In addition, sarcoidosis or the exacerbation of sarcoidosis has been reported. If there is evidence of pulmonary infiltrates or pulmonary function impairment, the patient should be closely monitored, and if appropriate, combination REBETOL/INTRON A treatment should be discontinued.
PRECAUTIONS
The safety and efficacy of REBETOL/INTRON A and PEG-INTRON therapy for the treatment of HIV infection, adenovirus, RSV, parainfluenza, or influenza infections have not been established. REBETOL Capsules should not be used for these indications. Ribavirin for inhalation has a separate package insert, which should be consulted if ribavirin inhalation therapy is being considered.
The safety and efficacy of REBETOL/INTRON A therapy has not been established in liver or other organ transplant patients, patients with decompensated liver disease due to hepatitis C infection, patients who are nonresponders to interferon therapy, or patients coinfected with HBV or HIV.
Information for Patients
Patients must be informed that REBETOL Capsules and Oral Solution may cause birth defects and/or death of the exposed fetus. REBETOL must not be used by women who are pregnant or by men whose female partners are pregnant. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients taking REBETOL. REBETOL should not be initiated until a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy. Patients must perform a pregnancy test monthly during therapy and for 6 months posttherapy. Women of childbearing potential must be counseled about use of effective contraception (two reliable forms) prior to initiating therapy. Patients (male and female) must be advised of the teratogenic/embryocidal risks and must be instructed to practice effective contraception during REBETOL and for 6 months posttherapy. Patients (male and female) should be advised to notify the physician immediately in the event of a pregnancy. (See CONTRAINDICATIONS and WARNINGS .)
If pregnancy does occur during treatment or during 6 months posttherapy, the patient must be advised of the teratogenic risk of REBETOL therapy to the fetus. Patients, or partners of patients, should immediately report any pregnancy that occurs during treatment or within 6 months after treatment cessation to their physician. Physicians should report such cases by calling 1-800-593-2214.
Patients receiving REBETOL Capsules should be informed of the benefits and risks associated with treatment, directed in its appropriate use, and referred to the patient MEDICATION GUIDE . Patients should be informed that the effect of treatment of hepatitis C infection on transmission is not known, and that appropriate precautions to prevent transmission of the hepatitis C virus should be taken.
The most common adverse experience occurring with REBETOL Capsules is anemia, which may be severe. (See ADVERSE REACTIONS .) Patients should be advised that laboratory evaluations are required prior to starting therapy and periodically thereafter. (See Laboratory Tests .) It is advised that patients be well hydrated, especially during the initial stages of treatment.
Laboratory Tests The following laboratory tests are recommended for all patients treated with REBETOL Capsules, prior to beginning treatment and then periodically thereafter.
- Standard hematologic tests--including hemoglobin (pretreatment, week 2 and week 4 of therapy, and as clinically appropriate [see WARNINGS ]), complete and differential white blood cell counts, and platelet count.
- Blood chemistries--liver function tests and TSH.
- Pregnancy--including monthly monitoring for women of childbearing potential.
- ECG (See WARNINGS .)
Carcinogenesis and Mutagenesis Ribavirin did not cause an increase in any tumor type when administered for 6 months in the transgenic p53 deficient mouse model at doses up to 300 mg/kg (estimated human equivalent of 25 mg/kg based on body surface area adjustment for a 60 kg adult; approximately 1.9 times the maximum recommended human daily dose). Ribavirin was non-carcinogenic when administered for 2 years to rats at doses up to 40 mg/kg (estimated human equivalent of 5.71 mg/kg based on body surface area adjustment for a 60 kg adult). However, this dose was less than the maximum tolerated dose, and therefore the study was not adequate to fully characterize the carcinogenic potential of ribavirin.
Ribavirin demonstrated increased incidences of mutation and cell transformation in multiple genotoxicity assays. Ribavirin was active in the Balb/3T3 In Vitro Cell Transformation Assay. Mutagenic activity was observed in the mouse lymphoma assay, and at doses of 20-200 mg/kg (estimated human equivalent of 1.67-16.7 mg/kg, based on body surface area adjustment for a 60 kg adult; 0.1-1 X the maximum recommended human 24-hour dose of ribavirin) in a mouse micronucleus assay. A dominant lethal assay in rats was negative, indicating that if mutations occurred in rats they were not transmitted through male gametes.
Impairment of Fertility Ribavirin demonstrated significant embryocidal and/or teratogenic effects at doses well below the recommended human dose in all animal species in which adequate studies have been conducted.
Fertile women and partners of fertile women should not receive REBETOL unless the patient and his/her partner are using effective contraception (two reliable forms). Based on a multiple dose half-life (t 1/2 ) of ribavirin of 12 days, effective contraception must be utilized for 6 months posttherapy (eg, 15 half-lives of clearance for ribavirin).
REBETOL should be used with caution in fertile men. In studies in mice to evaluate the time course and reversibility of ribavirin-induced testicular degeneration at doses of 15 to 150 mg/kg/day (estimated human equivalent of 1.25-12.5 mg/kg/day, based on body surface area adjustment for a 60 kg adult; 0.1-0.8 X the maximum human 24-hour dose of ribavirin) administered for 3 or 6 months, abnormalities in sperm occurred. Upon cessation of treatment, essentially total recovery from ribavirin-induced testicular toxicity was apparent within 1 or 2 spermatogenesis cycles.
Animal Toxicology Long-term studies in the mouse and rat (18-24 months; doses of 20-75 and 10-40 mg/kg/day, respectively {estimated human equivalent doses of 1.67-6.25 and 1.43-5.71 mg/kg/day, respectively, based on body surface area adjustment for a 60 kg adult; approximately 0.1-0.4 X the maximum human 24-hour dose of ribavirin}) have demonstrated a relationship between chronic ribavirin exposure and increased incidences of vascular lesions (microscopic hemorrhages) in mice. In rats, retinal degeneration occurred in controls, but the incidence was increased in ribavirin-treated rats.
Pregnancy Category X (see CONTRAINDICATIONS )
Ribavirin produced significant embryocidal and/or teratogenic effects in all animal species in which adequate studies have been conducted. Malformations of the skull, palate, eye, jaw, limbs, skeleton, and gastrointestinal tract were noted. The incidence and severity of teratogenic effects increased with escalation of the drug dose. Survival of fetuses and offspring was reduced. In conventional embryotoxicity/teratogenicity studies in rats and rabbits, observed no effect dose levels were well below those for proposed clinical use (0.3 mg/kg/day for both the rat and rabbit; approximately 0.06 X the recommended human 24-hour dose of ribavirin). No maternal toxicity or effects on offspring were observed in a peri/postnatal toxicity study in rats dosed orally at up to 1 mg/kg/day (estimated human equivalent dose of 0.17 mg/kg based on body surface area adjustment for a 60 kg adult; approximately 0.01 X the maximum recommended human 24-hour dose of ribavirin).
Treatment and Posttreatment : Potential Risk to the Fetus Ribavirin is known to accumulate in intracellular components from where it is cleared very slowly. It is not known whether ribavirin contained in sperm will exert a potential teratogenic effect upon fertilization of the ova. In a study in rats, it was concluded that dominant lethality was not induced by ribavirin at doses up to 200 mg/kg for 5 days (estimated human equivalent doses of 7.14-28.6 mg/kg, based on body surface area adjustment for a 60 kg adult; up to 1.7 X the maximum recommended human dose of ribavirin). However, because of the potential human teratogenic effects of ribavirin, male patients should be advised to take every precaution to avoid risk of pregnancy for their female partners.
Women of childbearing potential should not receive REBETOL unless they are using effective contraception (two reliable forms) during the therapy period. In addition, effective contraception should be utilized for 6 months posttherapy based on a multiple-dose half-life (t 1/2 ) of ribavirin of 12 days.
Male patients and their female partners must practice effective contraception (two reliable forms) during treatment with REBETOL and for the 6-month posttherapy period (eg, 15 half-lives for ribavirin clearance from the body).
Ribavirin Pregnancy Registry: A Ribavirin Pregnancy Registry has been established to monitor maternal-fetal outcomes of pregnancies in female patients and female partners of male patients exposed to ribavirin during treatment and for six months following cessation of treatment. Physicians and patients are encouraged to report such cases by calling 1-800-593-2214.
Nursing Mothers It is not known whether the REBETOL product is excreted in human milk. Because of the potential for serious adverse reactions from the drug in nursing infants, a decision should be made whether to discontinue nursing or to delay or discontinue REBETOL.
Geriatric Use Clinical studies of REBETOL/INTRON A or PEG-INTRON therapy did not include sufficient numbers of subjects aged 65 and over to determine if they respond differently from younger subjects.
REBETOL is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients often have decreased renal function, care should be taken in dose selection. Renal function should be monitored and dosage adjustments should be made accordingly. REBETOL should not be used in patients with creatinine clearance <50 mL/min. (See WARNINGS .)
In general, REBETOL Capsules should be administered to elderly patients cautiously, starting at the lower end of the dosing range, reflecting the greater frequency of decreased hepatic and/or cardiac function, and of concomitant disease or other drug therapy. In clinical trials, elderly subjects had a higher frequency of anemia (67%) than did younger patients (28%). (See WARNINGS .)
Pediatric Use
Suicidal ideation or attempts occurred more frequently among pediatric patients, primarily adolescents, compared to adult patients (2.4% versus 1%) during treatment and off-therapy follow-up (see WARNINGS ). As in adult patients, pediatric patients experienced other psychiatric adverse events (eg, depression, emotional lability, somnolence), anemia, and neutropenia (see WARNINGS ). During a 48-week course of therapy there was a decrease in the rate of linear growth (mean percentile assignment decrease of 9%) and a decrease in the rate of weight gain (mean percentile assignment decrease of 13%). A general reversal of these trends was noted during the 24-week posttreatment period.
Drug Interactions
Didanosine: Coadministration of REBETOL Capsules or Oral Solution and didanosine is not recommended. Reports of fatal hepatic failure, as well as peripheral neuropathy, pancreatitis, and symptomatic hyperlactactemia/lactic acidosis have been reported in clinical trials (see CLINICAL PHARMACOLOGY : Drug Interactions ).
Stavudine and Zidovudine: Ribavirin may antagonize the in vitro antiviral activity of stavudine and zidovudine against HIV. Therefore, concomitant use of ribavirin with either of these drugs should be used with caution (see CLINICAL PHARMACOLOGY : Drug Interactions ).
ADVERSE REACTIONS
The primary toxicity of ribavirin is hemolytic anemia. Reductions in hemoglobin levels occurred within the first 1-2 weeks of oral therapy. (See WARNINGS . ) Cardiac and pulmonary events associated with anemia occurred in approximately 10% of patients. (See WARNINGS .)
REBETOL/INTRON A Combination Therapy
In clinical trials, 19% and 6% of previously untreated and relapse patients, respectively, discontinued therapy due to adverse events in the combination arms compared to 13% and 3% in the interferon arms. Selected treatment-emergent adverse events that occurred in the US studies with >/=5% incidence are provided in TABLE 7 by treatment group. In general, the selected treatment-emergent adverse events were reported with lower incidence in the international studies as compared to the US studies with the exception of asthenia, influenza-like symptoms, nervousness, and pruritus.
Pediatric Patients
In clinical trials of 118 pediatric patients 3 to 16 years of age, 6% discontinued therapy due to adverse events. Dose modifications were required in 30% of patients, most commonly for anemia and neutropenia. In general, the adverse event profile in the pediatric population was similar to that observed in adults. Injection site disorders, fever, anorexia, vomiting, and emotional lability occurred more frequently in pediatric patients compared to adult patients. Conversely, pediatric patients experienced less fatigue, dyspepsia, arthralgia, insomnia, irritability, impaired concentration, dyspnea, and pruritus compared to adult patients. Selected treatment-emergent adverse events that occurred with >/=5% incidence among all pediatric patients who received the recommended dose of REBETOL/INTRON A combination therapy are provided in TABLE 7 .
TABLE 7. Selected Treatment-Emergent Adverse Events:
Previously Untreated and Relapse Adult Patients
and Previously Untreated Pediatric PatientsPercentage of Patients US Previously
Untreated StudyUS Relapse
StudyPediatric
Patients24 weeks
of treatment48 weeks
of treatment24 weeks
of treatment48 weeks
of treatmentPatients
Reporting
Adverse
Events *INTRON A
plus
REBETOL
(N=228)INTRON A
plus
Placebo
(N=231)INTRON A
plus
REBETOL
(N=228)INTRON A
plus
Placebo
(N=225)INTRON A
plus
REBETOL
(N=77)INTRON A
plus
Placebo
(N=76)INTRON A
plus
REBETOL
(N=118)Application Site
DisordersInjection Site
Inflammation13 10 12 14 6 8 14 Injection Site
Reaction7 9 8 9 5 3 19 Body as a Whole
- General DisordersHeadache63 63 66 67 66 68 69 Fatigue68 62 70 72 60 53 58 Rigors40 32 42 39 43 37 25 Fever37 35 41 40 32 36 61 Influenza-Like
Symptoms14 18 18 20 13 13 31 Asthenia9 4 9 9 10 4 5 Chest pain5 4 9 8 6 7 5 Central & Peripheral
Nervous System
DisordersDizziness17 15 23 19 26 21 20 Gastrointestinal
System DisordersNausea38 35 46 33 47 33 33 Anorexia27 16 25 19 21 14 51 Dyspepsia14 6 16 9 16 9 <1 Vomiting11 10 9 13 12 8 42 Musculoskeletal
System DisordersMyalgia61 57 64 63 61 58 32 Arthralgia30 27 33 36 29 29 15 Musculoskeletal Pain20 26 28 32 22 28 21 Psychiatric DisordersInsomnia39 27 39 30 26 25 14 Irritability23 19 32 27 25 20 10 Depression32 25 36 37 23 14 13 Emotional Lability7 6 11 8 12 8 16 Concentration
Impaired11 14 14 14 10 12 5 Nervousness4 2 4 4 5 4 3 Respiratory System
DisordersDyspnea19 9 18 10 17 12 5 Sinusitis9 7 10 14 12 7 <1 Skin and Appendages
DisordersAlopecia28 27 32 28 27 26 23 Rash20 9 28 8 21 5 17 Pruritus21 9 19 8 13 4 12 Special Senses,
Other DisordersTaste Perversion7 4 8 4 6 5 <1 *Patients reporting one or more adverse events. A patient may have reported more than one adverse event within a body system/organ class category.
In addition, the following spontaneous adverse events have been reported during the marketing surveillance of REBETOL/INTRON A therapy: hearing disorder and vertigo.
REBETOL/PEG-INTRON Combination Therapy
Overall, in clinical trials, 14% of patients receiving REBETOL in combination with PEG-INTRON, discontinued therapy compared with 13% treated with REBETOL in combination with INTRON A. The most common reasons for discontinuation of therapy were related to psychiatric, systemic (eg, fatigue, headache), or gastrointestinal adverse events. Adverse events that occurred in clinical trial at >5% incidence are provided in TABLE 8 by treatment group. Safety and effectiveness of REBETOL in combination with PEG-INTRON has not been established in pediatric patients.
TABLE 8. Adverse Events Occurring in > 5% of Patients Percentage of Patients Reporting Adverse Events *Adverse EventsPEG-INTRON
1.5 µg/kg/REBETOL
(N=511)INTRON A/
REBETOL
(N=505)Application SiteInjection Site
Inflammation25 18 Injection Site Reaction58 36 Autonomic Nervous SystemMouth Dry12 8 Sweating Increased11 7 Flushing4 3 Body as a WholeFatigue/Asthenia66 63 Headache62 58 Rigors48 41 Fever46 33 Weight Decrease29 20 RUQ Pain12 6 Chest Pain8 7 Malaise4 6 Central/Peripheral Nervous SystemDizziness21 17 EndocrineHypothyroidism5 4 GastrointestinalNausea43 33 Anorexia32 27 Diarrhea22 17 Vomiting14 12 Abdominal Pain13 13 Dyspepsia9 8 Constipation5 5 Hematologic DisordersNeutropenia26 14 Anemia12 17 Leukopenia6 5 Thrombocytopenia5 2 Liver and Biliary SystemHepatomegaly4 4 MusculoskeletalMyalgia56 50 Arthralgia34 28 Musculoskeletal Pain21 19 PsychiatricInsomnia40 41 Depression31 34 Anxiety/Emotional
Lability/Irritability47 47 Concentration
Impaired17 21 Agitation8 5 Nervousness6 6 Reproductive, FemaleMenstrual Disorder7 6 Resistance MechanismInfection Viral12 12 Infection Fungal6 1 Respiratory SystemDyspnea26 24 Coughing23 16 Pharyngitis12 13 Rhinitis8 6 Sinusitis6 5 Skin and AppendagesAlopecia36 32 Pruritus29 28 Rash24 23 Skin Dry24 23 Special Senses, OtherTaste Perversion9 4 Vision DisordersVision Blurred5 6 Conjunctivitis4 5 *Patients reporting one or more adverse events. A patient may have reported more than one adverse event within a body system/organ class category.Laboratory Values
REBETOL/INTRON A Combination Therapy
Changes in selected hematologic values (hemoglobin, white blood cells, neutrophils, and platelets) during therapy are described below. (See TABLE 9 .)
Hemoglobin Hemoglobin decreases among patients receiving REBETOL therapy began at Week 1, with stabilization by Week 4. In previously untreated patients treated for 48 weeks the mean maximum decrease from baseline was 3.1 g/dL in the US study and 2.9 g/dL in the International study. In relapse patients the mean maximum decrease from baseline was 2.8 g/dL in the US study and 2.6 g/dL in the International study. Hemoglobin values returned to pretreatment levels within 4-8 weeks of cessation of therapy in most patients.
Bilirubin and Uric Acid Increases in both bilirubin and uric acid, associated with hemolysis, were noted in clinical trials. Most were moderate biochemical changes and were reversed within 4 weeks after treatment discontinuation. This observation occurs most frequently in patients with a previous diagnosis of Gilbert's syndrome. This has not been associated with hepatic dysfunction or clinical morbidity.
TABLE 9. Selected Hematologic Values During Treatment With REBETOL plus INTRON A:
Previously Untreated and Relapse Adult Patients and Previously Untreated Pediatric PatientsPercentage of Patients US Previously
Untreated StudyUS Relapse
StudyPediatric
Patients24 weeks
of treatment48 weeks
of treatment24 weeks
of treatment48 weeks
of treatmentINTRON A
plus
REBETOL
(N=228)INTRON A
plus
Placebo
(N=231)INTRON A
plus
REBETOL
(N=228)INTRON A
plus
Placebo
(N=225)INTRON A
plus
REBETOL
(N=77)INTRON A
plus
Placebo
(N=76)INTRON A
plus
REBETOL
(N=118)Hemoglobin (g/dL)9.5-10.924 1 32 1 21 3 24 8.0-9.45 0 4 0 4 0 3 6.5-7.90 0 0 0.4 0 0 0 <6.50 0 0 0 0 0 0 Leukocytes (× 10 9 /L)2.0-2.940 20 38 23 45 26 35 1.5-1.94 1 9 2 5 3 8 1.0-1.40.9 0 2 0 0 0 0 <1.00 0 0 0 0 0 0 Neutrophils (× 10 9 /L)1.0-1.4930 32 31 44 42 34 37 0.75-0.9914 15 14 11 16 18 15 0.5-0.749 9 14 7 8 4 16 <0.511 8 11 5 5 8 3 Platelets (× 10 9 /L)70-999 11 11 14 6 12 0.8 50-692 3 2 3 0 5 2 30-490 0.4 0 0.4 0 0 0 <300.9 0 1 0.9 0 0 0 Total Bilirubin (mg/dL)1.5-3.027 13 32 13 21 7 2 3.1-6.00.9 0.4 2 0 3 0 0 6.1-12.00 0 0.4 0 0 0 0 >12.00 0 0 0 0 0 0 REBETOL/PEG-INTRON Combination Therapy
Changes in selected hematologic values (hemoglobin, white blood cells, neutrophils, and platelets) during therapy are described below. (See TABLE 10 .)
Hemoglobin
REBETOL induced a decrease in hemoglobin levels in approximately two thirds of patients. Hemoglobin levels decreased to <11 g/dL in about 30% of patients. Severe anemia (<8 g/dL) occurred in <1% of patients. Dose modification was required in 9 and 13% of patients in the PEG-INTRON/REBETOL and INTRON A/REBETOL groups.
Bilirubin and Uric Acid
In the REBETOL/PEG-INTRON combination trial 10-14% of patients developed hyperbilirubinemia and 33-38% developed hyperuricemia in association with hemolysis. Six patients developed mild to moderate gout.
TABLE 10. Selected Hematologic Values During
Treatment With REBETOL plus PEG-INTRONNumber (%) of Subjects PEG-INTRON
plus
REBETOL
(N=511)INTRON A
plus
REBETOL
(N=505)Hemoglobin (g/dL)9.5-10.926 27 8.0-9.43 3 6.5-7.90.2 0.2 <6.50 0 Leukocytes ( × 10 9 /L)2.0-2.946 41 1.5-1.924 8 1.0-1.45 1 <1.00 0 Neutrophils ( × 10 9 /L)1.0-1.4933 37 0.75-0.9925 13 0.5-0.7418 7 <0.54 2 Platelets ( × 10 9 /L)70-9915 5 50-693 0.8 30-490.2 0.2 <300 0 Total Bilirubin (mg/dL)1.5-3.010 13 3.1-6.00.6 0.2 6.1-12.00 0.2 >12.00 0 ALT (SGPT)2 × Baseline0.6 0.2 2.1-5 × Baseline3 1 5.1-10 × Baseline0 0 >10 × Baseline0 0 OVERDOSAGE
There is limited experience with overdosage. Acute ingestion of up to 20 grams of REBETOL Capsules, INTRON A ingestion of up to 120 million units, and subcutaneous doses of INTRON A up to 10 times the recommended doses have been reported. Primary effects that have been observed are increased incidence and severity of the adverse events related to the therapeutic use of INTRON A and REBETOL. However, hepatic enzyme abnormalities, renal failure, hemorrhage, and myocardial infarction have been reported with administration of single subcutaneous doses of INTRON A that exceed dosing recommendations.
There is no specific antidote for INTRON A or REBETOL overdose, and hemodialysis and peritoneal dialysis are not effective for treatment of overdose of either agent.
DOSAGE AND ADMINISTRATION (see CLINICAL PHARMACOLOGY , Special Populations ; see WARNINGS )
REBETOL/INTRON A Combination Therapy
Adults The recommended dose of REBETOL Capsules depends on the patient's body weight. The recommended dose of REBETOL is provided in TABLE 11 .
The recommended duration of treatment for patients previously untreated with interferon is 24 to 48 weeks. The duration of treatment should be individualized to the patient depending on baseline disease characteristics, response to therapy, and tolerability of the regimen. (See Description of Clinical Studies and ADVERSE REACTIONS .) After 24 weeks of treatment virologic response should be assessed. Treatment discontinuation should be considered in any patient who has not achieved an HCV RNA below the limit of detection of the assay by 24 weeks. There are no safety and efficacy data on treatment for longer than 48 weeks in the previously untreated patient population.
In patients who relapse following non-pegylated interferon monotherapy, the recommended duration of treatment is 24 weeks. There are no safety and efficacy data on treatment for longer than 24 weeks in the relapse patient population.
TABLE 11. Recommended Dosing Body weight REBETOL Capsules </= 75 kg 2 × 200-mg capsules AM,
3 × 200-mg capsules PM daily p.o.> 75 kg 3 × 200 mg capsules AM,
3 × 200 mg capsules PM daily p.o.
Pediatrics The recommended dose of REBETOL is 15 mg/kg per day orally (divided dose AM and PM). For children weighing </=25 kg or who cannot swallow capsules, REBETOL Oral Solution is supplied in a concentration of 40 mg/mL. For children weighing >25 kg, either the Oral Solution or 200-mg capsule may be administered. Refer to TABLE 12 for dosing recommendations for the 200-mg capsule to achieve the recommended dose.
The recommended duration of treatment is 48 weeks for pediatric patients with genotype 1. After 24 weeks of treatment virologic response should be assessed. Treatment discontinuation should be considered in any patient who has not achieved an HCV RNA below the limit of detection of the assay by this time. The recommended duration of treatment for pediatric patients with genotype 2/3 is 24 weeks. There are no safety and efficacy data on treatment for longer than 48 weeks in pediatrics.
TABLE 12. Pediatric Dosing Body weight REBETOL Capsules INTRON A Injection 25-36 kg 1 × 200-mg
capsules AM,
1 × 200-mg
capsules PM
daily p.o.3 million IU/m 2
3 times weekly
s.c.37-49 kg 1 × 200-mg
capsules AM,
2 × 200-mg
capsules PM
daily p.o.3 million IU/m 2
3 times weekly
s.c.50-61 kg 2 × 200-mg
capsules AM,
2 × 200-mg
capsules PM
daily p.o.3 million IU/m 2
3 times weekly
s.c.>61 kg Refer to adult
dosing table.Refer to adult
dosing table
REBETOL may be administered without regard to food, but should be administered in a consistent manner with respect to food intake. (See CLINICAL PHARMACOLOGY .)
REBETOL/PEG-INTRON Combination Therapy
The recommended dose of REBETOL Capsules is 800 mg/day in 2 divided doses: two capsules (400 mg) in the morning with food and two capsules (400 mg) in the evening with food.
Dose Modifications (TABLE 13)
If severe adverse reactions or laboratory abnormalities develop during combination REBETOL/INTRON A therapy the dose should be modified, or discontinued if appropriate, until the adverse reactions abate. If intolerance persists after dose adjustment, REBETOL/INTRON A therapy should be discontinued.
REBETOL should not be used in patients with creatinine clearance <50 mL/min. (See WARNINGS and CLINICAL PHARMACOLOGY , Special Populations .)
REBETOL should be administered with caution to patients with pre-existing cardiac disease. Patients should be assessed before commencement of therapy and should be appropriately monitored during therapy. If there is any deterioration of cardiovascular status, therapy should be stopped. (See WARNINGS .)
For patients with a history of stable cardiovascular disease, a permanent dose reduction is required if the hemoglobin decreases by >/=2 g/dL during any 4-week period. In addition, for these cardiac history patients, if the hemoglobin remains <12 g/dL after 4 weeks on a reduced dose, the patient should discontinue combination REBETOL/INTRON A therapy.
It is recommended that a patient whose hemoglobin level falls below 10 g/dL have his/her REBETOL dose reduced to 600 mg daily (1 × 200-mg capsule AM, 2 × 200-mg capsules PM) for adults and 7.5 mg/kg per day (divided dose AM and PM) for pediatric patients. A patient whose hemoglobin level falls below 8.5 g/dL should be permanently discontinued from REBETOL therapy. (See WARNINGS .)
TABLE 13. Guidelines for Dose Modifications
and Discontinuation for AnemiaHemoglobinDose Reduction*
REBETOL-
600 mg daily adults 7.5 mg/kg daily for pediatricsPermanent Discontinuation
of REBETOL TreatmentNo Cardiac
History<10 g/dL <8.5 g/dL Cardiac History
Patients>/=2 g/dL decrease
during any 4-week
period during treatment<12 g/dL after
4 weeks
of dose reductionHOW SUPPLIED
REBETOL 200-mg Capsules are white, opaque capsules with REBETOL, 200 mg, and the Schering Corporation logo imprinted on the capsule shell; the capsules are packaged in a bottle containing 42 capsules (NDC 0085-1327-04), 56 capsules (NDC 0085-1351-05), 70 capsules (NDC 0085-1385-07), and 84 capsules (NDC 0085-1194-03).
REBETOL Oral Solution 40 mg/mL is a clear, colorless to pale or light yellow bubble gum-flavored liquid and it is packaged in 4-oz amber glass bottles (100 mL/bottle) with child-resistant closures (NDC 0085-1318-01).
Storage Conditions
The bottle of REBETOL Capsules should be stored at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
REBETOL Oral Solution should be stored between 2° and 8°C (36° and 46°F) or at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
Kenilworth, NJ 07033 USA
U.S. Patents 4,530,901 & 4,211,771
Copyright © 2003, Schering Corporation. All rights reserved.
24819671 Rev. 6/04
27002420T
Subscribe to the "News" RSS Feed 
TOP ۞
