-
ReFacto Vials (Wyeth)
This product's label may have been revised after this insert was used in production. For further product information and current package insert, please visit www.wyeth.com or call our medical communications department toll-free at 1-800-934-5556.
DESCRIPTION
ReFacto ® Antihemophilic Factor (Recombinant) is a purified protein produced by recombinant DNA technology for use in therapy of factor VIII deficiency. ReFacto is a glycoprotein with an approximate molecular mass of 170 kDa consisting of 1438 amino acids. It has an amino acid sequence that is comparable to the 90 + 80 kDa form of factor VIII, and post-translational modifications that are similar to those of the plasma-derived molecule. ReFacto has in vitro functional characteristics comparable to those of endogenous factor VIII.
ReFacto is produced by a genetically engineered Chinese hamster ovary (CHO) cell line. The CHO cell line secretes B-domain deleted recombinant factor VIII into a defined cell culture medium that contains human serum albumin and recombinant insulin, but does not contain any proteins derived from animal sources. The protein is purified by a chromatography purification process that yields a high-purity, active product. The potency expressed in international units (IU) is determined using the European Pharmacopoeial chromogenic assay against the WHO standard. The specific activity of ReFacto is 9110-13700 IU per milligram of protein. ReFacto is not purified from human blood and contains no preservatives or added human components in the final formulation.
ReFacto is formulated as a sterile, nonpyrogenic, lyophilized powder preparation for intravenous (IV) injection. It is available in single-use vials containing the labeled amount of factor VIII activity (IU). Each vial contains nominally 250, 500, 1000 or 2000 IU of ReFacto per vial. The formulated product is a clear colorless solution upon reconstitution and contains sodium chloride, sucrose, L-histidine, calcium chloride, and polysorbate 80.
CLINICAL PHARMACOLOGY
Factor VIII is the specific clotting factor deficient in patients with hemophilia A (classical hemophilia). The administration of ReFacto ® Antihemophilic Factor (Recombinant) increases plasma levels of factor VIII activity and can temporarily correct the in vitro coagulation defect in these patients.
Activated factor VIII acts as a cofactor for activated factor IX accelerating the conversion of factor X to activated factor X. Activated factor X converts prothrombin into thrombin. Thrombin then converts fibrinogen into fibrin and a clot is formed. Factor VIII activity is greatly reduced in patients with hemophilia A and therefore replacement therapy is necessary.
In a crossover pharmacokinetic study of eighteen (18) previously treated patients using the chromogenic assay, the circulating mean half-life for ReFacto was 14.5 ± 5.3 hours (ranged from 7.6-27.7 hours), which was not statistically significantly different from plasma-derived Antihemophilic Factor (Human) (pdAHF), which had a mean half-life of 13.7 ± 3.4 hours (ranged from 8.8-23.7 hours). Mean incremental recovery (K-value) of ReFacto in plasma was 2.4 ± 0.4 IU/dL per IU/kg (ranged from 1.9-3.3 IU/dL per IU/kg). This was comparable to the mean incremental recovery observed in plasma for pdAHF which was 2.3 ± 0.3 IU/dL per IU/kg (ranged from 1.7-2.9 IU/dL per IU/kg). Results obtained from this controlled pharmacokinetic study, which used a central laboratory for the analysis of all plasma samples, showed that the one-stage factor VIII clotting assay gave results which were approximately 50% of the values obtained with the chromogenic assay (see DOSAGE AND ADMINISTRATION ).
In two additional clinical studies, pharmacokinetic parameters were evaluated for previously treated patients [PTPs] and previously untreated patients [PUPs]. In PTPs (n=87) ReFacto had a mean incremental recovery of 2.4 ± 0.4 IU/dL per IU/kg (ranged from 1.1-3.8 IU/dL per IU/kg) and an elimination half-life (n=67) of 10.7 ± 2.8 hours. In PUPs (n=45) ReFacto had a lower mean incremental recovery of 1.7 ± 0.4 IU/dL per IU/kg (ranged from 0.2-2.8 IU/dL per IU/kg) as compared to PTPs. Population pharmacokinetic modeling using data from 44 PUPs led to a mean estimated half-life of ReFacto in PUPs of 8.0 ± 2.2 hours. These parameters did not change over time (12 months) for PTPs or PUPs.
In clinical studies of ReFacto involving a total of 218 patients (117 PTPs including 4 who participated in the surgery study only, and 101 PUPs), more than 84 million IU were administered over a period of up to 54 months. The 117 PTPs were given a median of 230 injections (range of 4-1530 injections) over a median of 1200 days (range of 31-1640 days). The 101 PUPs were given a median of 26 injections (range of 1-490 injections) over a median of 830 days (range of 1-1298 days). One hundred thirteen PTPs and 99 PUPs were evaluated for efficacy in bleeding episodes. The 113 PTPs experienced a median of 54 bleeding episodes and the 99 PUPs experienced a median of 12 bleeding episodes. All were treated successfully on an on-demand basis or for the reduction of bleeding episodes except for one PTP and two PUPs who discontinued ReFacto treatment and switched to another product after the development of inhibitors. Bleeding episodes included hemarthroses, and bleeding in soft tissue, muscle, and other anatomical sites.
One of 113 previously treated patients (PTPs) who were evaluated for efficacy in bleeding episodes developed a high titer inhibitor. The patient was noted initially to have low titer inhibitor (1.2 BU) at a local laboratory at 98 exposure days and 2 BU at the central laboratory at 113 exposure days. After 18 months on continued treatment with ReFacto, the inhibitor level rose to nearly 13 BU and a bleeding episode failed to respond to ReFacto treatment. In this study the incidence of inhibitor development to factor VIII using ReFacto is similar to that reported for other factor VIII products 1-4 .
ReFacto has been studied in short-term routine prophylaxis. In uncontrolled clinical trials, an average dose of 27 ± 10 IU/kg in PTPs (n=77) and an average dose of 57 ± 20 IU/kg in PUPs (n=17) was given repeatedly at variable intervals longer than 2 weeks. In 64 patients who had both on-demand and prophylactic periods during their time on study, the mean rate of spontaneous musculoskeletal bleeding episodes was less during periods of routine prophylaxis. There were an average of 10 bleeding episodes per year during the prophylactic periods compared to an average of 37 bleeding episodes per year during the on-demand periods. The clinical trial experience with routine prophylaxis in PUPs is limited (n=17). These non-randomized trial results should be interpreted with caution, as the investigators exercised their own discretion in deciding when and in whom prophylaxis was to be initiated and terminated.
Management of hemostasis was evaluated in the surgical setting where 28 surgical procedures have been performed in 25 patients. The average preoperative dose in PTPs was 59 IU/kg. Procedures included orthopedic procedures, inguinal hernia repair, epidural hematoma evacuation, transposition ulnar nerve, and other minor procedures (e.g., venous access catheter placement and explantation, toenail removal). Circulatory factor VIII levels targeted to restore and maintain hemostasis were achieved. While the one-stage clotting assay was used most frequently in the surgical setting (24 versus 4 surgeries), hemostasis was maintained throughout the surgical period regardless of which assay was used. Hemostatic efficacy was rated as excellent or good in all procedures.
The occurrence of neutralizing antibody (inhibitors) is well known in the treatment of patients with hemophilia A 5,6,7 . Thirty-two out of 101 PUPs (32%) developed an inhibitor: 16 out of 101 (16%) with a high titer (> 5 BU) (12 of the 16 patients had peak values >/= 10 BU) and 16 out of 101 (16%) with a low titer (</= 5 BU). In this study the incidence of inhibitor development to factor VIII using ReFacto is similar to that reported for other factor VIII products 5-10 .
INDICATIONS AND USAGE
ReFacto ® Antihemophilic Factor (Recombinant) is indicated for the control and prevention of hemorrhagic episodes and for surgical prophylaxis in patients with hemophilia A (congenital factor VIII deficiency or classic hemophilia).
ReFacto is indicated for short-term routine prophylaxis to reduce the frequency of spontaneous bleeding episodes. The effect of regular routine prophylaxis on long-term morbidity and mortality is unknown.
ReFacto can be of a significant therapeutic value for treatment of hemophilia A in certain patients with inhibitors to factor VIII 11 . In clinical studies of ReFacto, patients who developed inhibitors on study continued to manifest a clinical response when inhibitor titers were < 10 BU. When an inhibitor is present, the dosage requirement of factor VIII is variable. The dosage can be determined only by a clinical response and by monitoring of circulating factor VIII levels after treatment (see DOSAGE AND ADMINISTRATION ).
ReFacto does not contain von Willebrand factor and therefore is not indicated in von Willebrand's disease.
CONTRAINDICATIONS
Known hypersensitivity to mouse, hamster, or bovine proteins may be a contraindication to the use of ReFacto ® Antihemophilic Factor (Recombinant).
WARNINGS
As with any intravenous protein product, allergic type hypersensitivity reactions are possible. Patients should be informed of the early signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. Patients should be advised to discontinue use of the product and contact their physicians if these symptoms occur.
PRECAUTIONS
General
Activity-neutralizing antibodies (inhibitors) have been detected in patients receiving factor VIII-containing products. Low titer inhibitors are common in previously untreated patients and in previously treated patients on factor VIII products, as are high titer inhibitors in previously untreated patients. High titer inhibitors, which are generally rare in previously treated patients, have been reported in previously treated patients on ReFacto. As with all coagulation factor VIII products, patients should be monitored for the development of inhibitors that should be titrated in Bethesda Units using appropriate biological testing.
Reports of less than expected or lack of effect following infusion of ReFacto, mainly in prophylaxis patients, have been received during the clinical trials and in the post-marketing setting. The reported less than expected or lack of effect has been described as unexpected bleeding into target joints, bleeding into new joints or a subjective feeling by the patient of new onset bleeding. Less than expected or lack of effect and/or low factor VIII recovery has been reported in patients with inhibitors but also in patients who had no evidence of inhibitors. When switching to ReFacto it is important to closely monitor each patient's clinical hemostatic response and plasma FVIII:C activity following administration of the product and to titrate the dose accordingly in order to ensure an adequate therapeutic response (see DOSAGE AND ADMINISTRATION ). Monitoring plasma FVIII:C activity is particularly important in the setting of surgical prophylaxis and major bleeds.
Formation of Antibodies to Mouse and Hamster Protein
As Antihemophilic Factor (Recombinant), ReFacto contains trace amounts of mouse protein (maximum of 5 ng/1000 IU) and hamster protein (maximum of 30 ng/1000 IU), the remote possibility exists that patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Carcinogenicity, Mutagenicity, Impairment of Fertility
ReFacto ® Antihemophilic Factor (Recombinant) has been shown to be nonmutagenic in the mouse micronucleus assay. No other mutagenicity studies and no investigations on carcinogenesis or impairment of fertility have been conducted.
Pregnancy Category C
Animal reproduction and lactation studies have not been conducted with ReFacto ® Antihemophilic Factor (Recombinant). It is not known whether ReFacto can affect reproductive capacity or cause fetal harm when given to pregnant women. ReFacto should be administered to pregnant and lactating women only if clearly indicated.
Pediatric Use
ReFacto ® Antihemophilic Factor (Recombinant) is appropriate for use in children of all ages, including newborns. Safety and efficacy studies have been performed both in previously treated children and adolescents (N=22, ages 8-15 years) and in previously untreated neonates, infants, and children (N=101, ages 0-52 months) (see CLINICAL PHARMACOLOGY and PRECAUTIONS ).
Geriatric Use
Clinical studies of ReFacto did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. As with any patient receiving ReFacto, dose selection for an elderly patient should be individualized.
ADVERSE REACTIONS
As with the intravenous administration of any protein product, the following reactions may be observed after administration: headache, fever, chills, flushing, nausea, vomiting, lethargy, or manifestations of allergic reactions. During clinical studies with ReFacto ® Antihemophilic Factor (Recombinant), 77 adverse reactions in 43 of 218 patients (20%) probably or possibly-related to therapy were reported for 64,363 infusions (0.12%). These were anaphylaxis (1), dyspnea (6), urticaria (1), nausea (11), headache (4), vasodilation (5), dizziness (4), permanent venous access catheter complications (3), asthenia (3), fever (3), taste perversion [altered taste] (3), bleeding/hematoma (3), infected hematoma (1), anorexia (2), diarrhea (2), injection site reaction (2), somnolence (2), rash (2), pruritus (2), angina pectoris (1), tachycardia (1), perspiration increased (1), chills (1), increased amino transferase (1), increased bilirubin (1), pain in finger (1), muscle weakness (1), CPK increase (1), cold sensation (1), eye disorder-vision abnormal (1), coughing (1), myalgia (1), gastroenteritis (1), abdominal pain (1), acne (1), and forehead bruises (1). If any adverse reaction takes place that is thought to be related to administration of ReFacto, the rate of infusion should be decreased or stopped.
Inhibitor development is a known adverse event associated with the treatment of patients with hemophilia A. In addition to the one report of high titer inhibitors in the clinical study of PTPs (see CLINICAL PHARMACOLOGY ), there have been reports of high titer inhibitors in PTPs in the post-marketing setting. High and low titer inhibitors have been reported in PUPs in both clinical trials and the post-marketing setting (see PRECAUTIONS , General ).
A total of 182 adverse reactions in 54 of 218 patients (25%) who received 32,013 infusions (0.6%) were reported by the investigator to have an "unlikely" or "not assessable" relationship to ReFacto administration. The study sponsor considered that the events may be of possible or of unknown relationship to therapy because of the temporal relationship to the infusion and/or the frequency of the event for a given patient and/or because insufficient information was available to assign another causality. In this category, 25 patients experienced the following 38 events which are different from the events described above: pain (10), rhinitis (10), vomiting (4), insomnia (3), constipation (2), pharyngitis (2), flushing (1), palpitation (1), sinusitis (1), gastritis (1), dyspepsia (1), hypotension (1), and URI (1).
Other adverse experiences that were reported during the clinical trials, but which were assessed by both the investigator and the sponsor as "unlikely" to be related to ReFacto administration included: dyspnea (3), rash (2), pruritus (1), neuropathy (1), arm weakness (1), and thrombophlebitis of upper arm (1).
DOSAGE AND ADMINISTRATION
Treatment with ReFacto ® Antihemophilic Factor (Recombinant) should be initiated under the supervision of a physician experienced in the treatment of hemophilia A.
The labeled potency of ReFacto is based on the European Pharmacopoeial chromogenic substrate assay, whereas other factor VIII products are labeled based on the one-stage clotting assay. With recombinant factor VIII products, the chromogenic assay typically yields results which are higher than the results obtained with the one-stage clotting assay. When switching between products it is important to individually titrate each patient's dose in order to ensure an adequate therapeutic response (see PRECAUTIONS , General ). Results obtained from a controlled pharmacokinetic study, which used one central laboratory for the analysis of all plasma samples, showed that the one-stage factor VIII clotting assay gave results that were approximately 50% of those obtained with the chromogenic substrate assay (see CLINICAL PHARMACOLOGY ). In addition, in clinical trials of ReFacto use in the surgical setting in which multiple laboratories were used for plasma sample analysis, the ratio of factor VIII activity results obtained by the one-stage clotting and chromogenic substrate assays ranged between 20 and 80%.
When monitoring patients' factor VIII activity levels during treatment, the available clinical data suggest that either assay may be used. Most patients in clinical trials were monitored with the one-stage clotting assay (see CLINICAL PHARMACOLOGY ). It is necessary to adhere to the incubation/activation times and other test conditions as specified by the assay manufacturers.
Dosage and duration of treatment depend on the severity of the factor VIII deficiency, the location and extent of bleeding, and the patient's clinical condition. Doses administered should be titrated to the patient's clinical response. In the presence of an inhibitor, higher doses may be required.
Precise monitoring of the replacement therapy by means of coagulation analysis (plasma factor VIII activity) is recommended, particularly for surgical intervention.
One international unit (IU) of factor VIII activity corresponds approximately to the quantity of factor VIII in one mL of normal human plasma. The calculation of the required dosage of factor VIII is based upon the empirical finding that, on average, 1 IU of factor VIII per kg body weight raises the plasma factor VIII activity by approximately 2 IU/dL per IU/kg administered. The required dosage is determined using the following formula:
Required units =body weight (kg) × desired factor VIII rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL) The following chart can be used to guide dosing in bleeding episodes and surgery:
Type of HemorrhageFactor VIII
Level Required
(IU/dL or % of
normal)Frequency of Doses (h)/
Duration of Therapy (d)MinorEarly hemarthrosis, minor muscle or oral bleeds.20-40Repeat every 12 to 24 hours as necessary until resolved. At least 1 day, depending upon the severity of the hemorrhage.ModerateHemorrhages into muscles. Mild trauma capitis. Minor operations including tooth extraction. Hemorrhages into the oral cavity.30-60Repeat infusion every 12-24 hours for 3-4 days or until adequate local hemostasis is achieved. For tooth extraction a single infusion plus oral antifibrinolytic therapy within 1 hour may be sufficient.MajorGastrointestinal bleeding. Intracranial, intra-abdominal or intrathoracic hemorrhages. Fractures. Major operations.60-100Repeat infusion every 8-24 hours until threat is resolved or in the case of surgery, until adequate local hemostasis is achieved.For short-term routine prophylaxis to prevent or reduce the frequency of spontaneous musculoskeletal hemorrhage in patients with hemophilia A, ReFacto should be given at least twice a week. In some cases, especially pediatric patients, shorter dosage intervals or higher doses may be necessary. Pharmacokinetic/pharmacodynamic modeling, based on pharmacokinetic data from 185 infusions in 102 PTPs, predicts that routine prophylactic dosing 3 times per week may be associated with a lower bleeding risk than with dosing twice weekly. No randomized comparison of different doses or frequency regimens of ReFacto for routine prophylaxis has been performed. In clinical studies in PTPs (ages 8-73 years) and PUPs (ages 9-52 months), the mean dose used for routine prophylaxis was 27 ± 10 IU/kg and 57 ± 20 IU/kg, respectively.
Patients using ReFacto should be monitored for the development of factor VIII inhibitors. If expected factor VIII activity plasma levels are not attained, or if bleeding is not controlled with an appropriate dose, an assay should be performed to determine if a factor VIII inhibitor is present. If the inhibitor is present at levels less than 5 Bethesda Units, administration of additional antihemophilic factor may neutralize the inhibitor.
ReFacto is administered by IV infusion after reconstitution of the lyophilized powder with Sodium Chloride Diluent (provided).
ReFacto, when reconstituted, contains polysorbate-80, which is known to increase the rate of di-(2-ethylhexyl)-phthalate (DEHP) extraction from polyvinyl chloride (PVC). This should be considered during the preparation and administration of ReFacto, including storage time elapsed in a PVC container following reconstitution. It is important that the recommendations in DOSAGE AND ADMINISTRATION be followed closely.
INSTRUCTIONS FOR USE
Patients should follow the specific reconstitution and administration procedures provided by their physicians. The procedures below are provided as general guidelines for the reconstitution and administration of ReFacto.
Reconstitution
Always wash your hands before performing the following procedures. Aseptic technique (meaning clean and germ free) should be used during the reconstitution procedure. All components used in the reconstitution and administration of this product should be used as soon as possible after opening their sterile containers to minimize unnecessary exposure to the atmosphere.
ReFacto ® Antihemophilic Factor (Recombinant) is administered by intravenous (IV) infusion after reconstitution with the supplied diluent (0.9% Sodium Chloride Diluent, 4 mL disposable syringe for drug diluent use with ReFacto Antihemophilic Factor [Recombinant]) syringe.
- Allow the vials of lyophilized ReFacto and the pre-filled diluent syringe to reach room temperature.
-
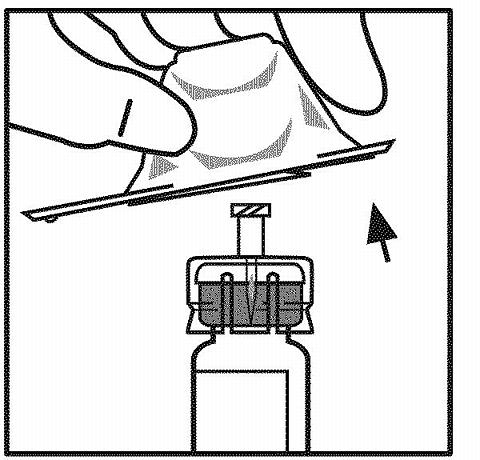
Remove the plastic flip-top cap from the ReFacto vial to expose the central portions of the rubber stopper.
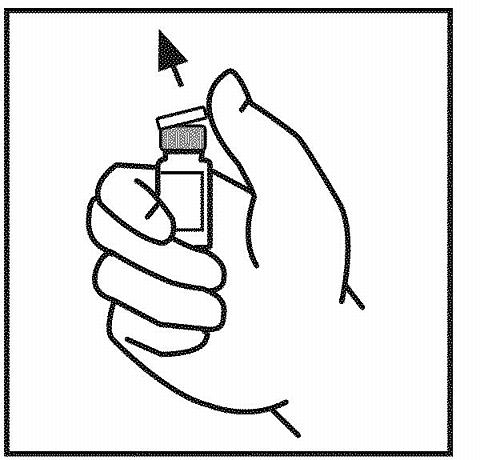
- Wipe the top of the vial with the alcohol swab provided, or use another antiseptic solution, and allow to dry. After cleaning, do not touch the rubber stopper with your hand or allow it to touch any surface.
- Peel back the cover from the clear plastic vial adapter package. Do not remove the adapter from the package.
-
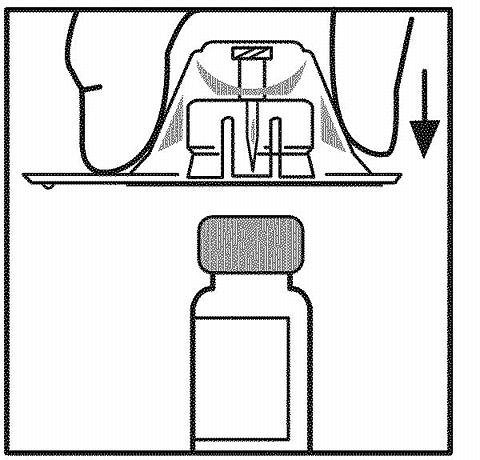
While holding the adapter package, place the vial adapter over the vial and press down firmly on the package until the adapter spike penetrates the vial stopper. Leave the adapter package in place.
-
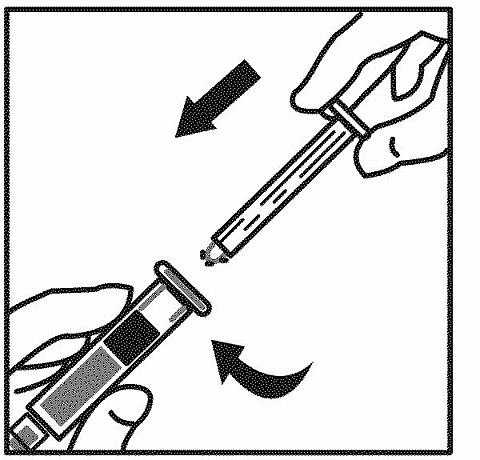
Grasp the plunger rod per the diagram. Avoid contact with the shaft of the plunger rod. Attach the threaded end of the plunger rod to the diluent syringe plunger by pushing and turning firmly.
-
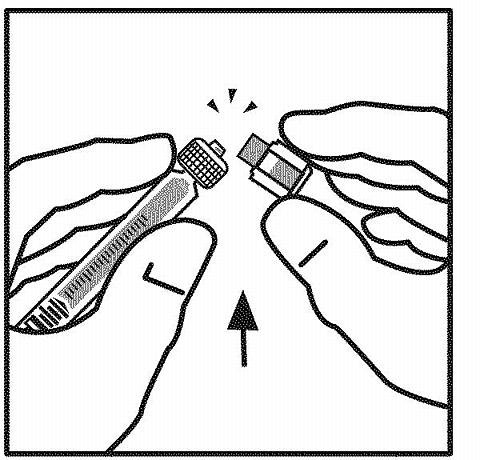
Remove the tamper-resistant plastic tip cap from the diluent syringe by bending down and up to break the perforation. Do not touch the inside of the cap or the syringe tip. Place the cap on its side on a clean surface in a spot where it would be least likely to become environmentally contaminated.
-
Lift the package away from the adapter and discard.
-
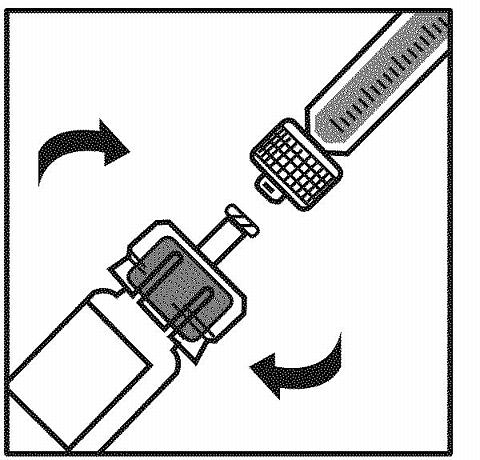
Connect the diluent syringe to the vial adapter by inserting the tip into the adapter opening while firmly pushing and turning the syringe clockwise until secured.
-
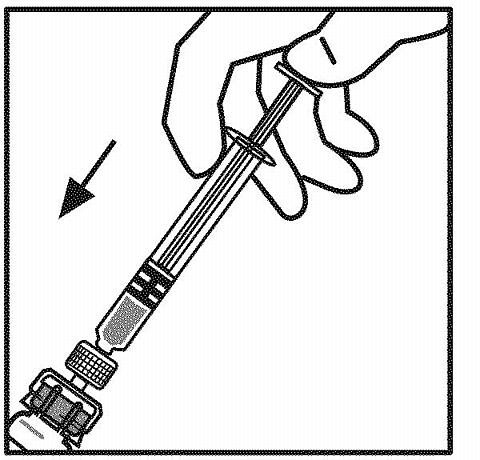
Inject all the diluent into the ReFacto vial.
- Without removing the syringe, gently swirl the contents of the vial until the powder is dissolved.
-
Inspect the final solution for specks before administration. The solution should appear clear and colorless.
Note: If you use more than one vial of ReFacto per infusion, reconstitute each vial as per the previous instructions. -
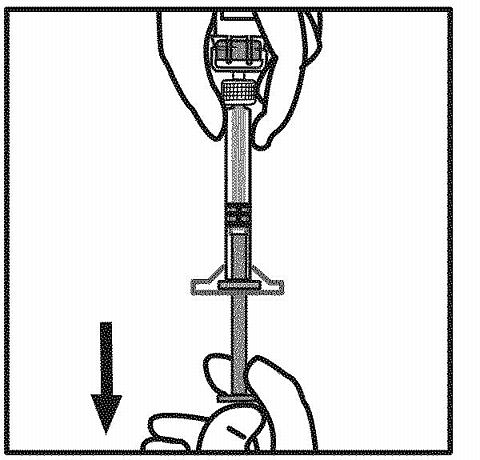
Invert the vial and draw the solution into the syringe.
Note: If you prepared more than one vial of ReFacto, remove the diluent syringe from the vial adapter, leaving the vial adapter attached to the vial. Quickly attach a separate large luer lock syringe and draw back the reconstituted contents as instructed above. Repeat this procedure with each vial in turn. Do not detach the diluent syringes or the large luer lock syringe until you are ready to attach the large luer lock syringe to the next vial adapter.
-
Detach the syringe from the vial adapter by gently pulling and turning the syringe counter-clockwise. Discard the vial with the adapter attached.
Note: If the solution is not to be used immediately, the syringe cap should be carefully replaced. Do not touch the syringe tip or the inside of the cap.
ReFacto should be administered within 3 hours after reconstitution. The reconstituted solution may be stored at room temperature prior to administration.
Administration (Intravenous Injection)
ReFacto ® Antihemophilic Factor (Recombinant) should be administered using the tubing provided in this kit, and the pre-filled diluent syringe provided or a single sterile disposable plastic syringe. In addition, the solution should be withdrawn from the vial using the vial adapter.
- Attach the syringe to the luer end of the infusion set tubing provided and perform venipuncture as instructed by your physician.
After reconstitution, ReFacto should be injected intravenously over several minutes. The rate of administration should be determined by the patient's comfort level.
Following completion of ReFacto treatment, remove the infusion set and discard. The amount of drug product remaining in the infusion set is not clinically significant.
Dispose of all unused solution, the empty vial(s), and the used needles and syringes in an appropriate container for throwing away waste that might hurt others if not handled properly.
Storage
Product as packaged for sale: ReFacto ® Antihemophilic Factor (Recombinant) should be stored under refrigeration at a temperature of 2° to 8°C (36° to 46°F). ReFacto may also be stored at room temperature not to exceed 25°C (77°F) for up to 3 months, until the expiration date. The patient should write in the space provided on the outer carton the date the product was placed at room temperature. At the end of the 3-month period, the product should not be put back into the refrigerator, but should be used immediately or discarded. Freezing should be avoided to prevent damage to the pre-filled diluent syringe. During storage, avoid prolonged exposure of ReFacto ® vial to light. Do not useReFacto after the expiry date on the label.
Product after reconstitution: The product does not contain a preservative and should be used within 3 hours.
HOW SUPPLIED
ReFacto ® Antihemophilic Factor (Recombinant) is supplied in single-use (4mL size, dried) vials which contain nominally 250, 500, 1000 or 2000 IU per vial (NDC 58394-007-02, 58394-006-02, 58394-005-02, 58394-011-02, respectively) with one pre-filled syringe (0.9% Sodium Chloride Diluent, 4 mL disposable syringe for drug diluent use with ReFacto Antihemophilic Factor [Recombinant]) for reconstitution, one vial adapter, one sterile infusion set, and two (2) alcohol swabs. Actual factor VIII activity in IU is stated on the label of each vial.
REFERENCES
- Kessler C, Sachse K. Factor VIII:C inhibitor associated with monoclonal-antibody purified FVIII concentrate. Lancet 1990; 335:1403.
- Schwartz RS, Abildgaard CF, Aledort LM, et al. Human recombinant DNA-derived antihemophilic factor (factor VIII) in the treatment of hemophilia A. N Engl J Med 1990;323:1800-1805.
- White GC II, Courter S, Bray GL, et al. A multicenter study of recombinant factor VIII (recombinate) in previously treated patients with hemophilia A. Thromb Haemost 1997;77(4):660-667.
- Abshire TC, Brackmann HH, Scharrer I, et al. Sucrose formulated recombinant human antihemophilic Factor VIII is safe and efficacious for treatment of hemophilia A in home therapy: Results of a multicenter, international, clinical investigation. Thromb Haemost 2000;83(6):811-816.
- Ehrenforth S, Kreuz W, Scharrer I, et al. Incidence of development of factor VIII and factor IX inhibitors in hemophiliacs. Lancet. 1992;339:594-598.
- Bray GL, Gomperts ED, Courter S, et al. A multicenter study of recombinant factor VIII (Recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. Blood. 1994;83(9):2428-2435.
- Lusher J, Arkin S, Abildgaard CF, Schwartz RS, Group TKPUPS. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. N Engl J Med. 1993;328:453-459.
- Scharrer I, Bray G. Incidence of inhibitors in haemophilia A patients - a review of recent studies of recombinant and plasma-derived factor VIII concentrates. Hemophilia 1999;5:145.
- Gruppo R, Chen H, Schroth P, et al. Safety and immunogenicity of recombinant factor VIII (Recombinate) in previously untreated patients: A 7.3 year update. Haemophilia 1998;4:228 (abstract no. 291, XXIII Congress of the WFH, The Hague).
- Lusher J, Abildgaard C, Arkin S, et al. Human recombinant DNA-derived antihemophilic factor in the treatment of previously untreated patients with hemophilia A: Final report on a hallmark clinical investigation. J Thromb Haemost 2004;2:574-83.
- Kessler CM. An Introduction to Factor VIII Inhibitors: The Detection and Quantitation. American Journal of Medicine 91 1991, (Supplement 5A): 1S-5S.
Wyeth ®
Wyeth Pharmaceuticals Inc. Philadelphia, PA 19101 US Govt. License No. 3 Telephone: 1-800-934-5556 W10403C009ET01Rev 05/05
Subscribe to the "News" RSS Feed 
TOP ۞
