-
Remicade for IV Injection (Centocor)
WARNING
RISK OF INFECTIONS
TUBERCULOSIS (FREQUENTLY DISSEMINATED OR EXTRAPULMONARY AT CLINICAL PRESENTATION), INVASIVE FUNGAL INFECTIONS, AND OTHER OPPORTUNISTIC INFECTIONS, HAVE BEEN OBSERVED IN PATIENTS RECEIVING REMICADE. SOME OF THESE INFECTIONS HAVE BEEN FATAL (SEE WARNINGS ). ANTI-TUBERCULOSIS TREATMENT OF PATIENTS WITH A REACTIVE TUBERCULIN SKIN TEST REDUCES THE RISK OF TB REACTIVATION IN PATIENTS RECEIVING TREATMENT WITH REMICADE. HOWEVER, ACTIVE TUBERCULOSIS HAS DEVELOPED IN PATIENTS RECEIVING REMICADE WHO WERE TUBERCULIN SKIN TEST NEGATIVE PRIOR TO RECEIVING REMICADE.
PATIENTS SHOULD BE EVALUATED FOR LATENT TUBERCULOSIS INFECTION WITH A TUBERCULIN SKIN TEST. 1 TREATMENT OF LATENT TUBERCULOSIS INFECTION SHOULD BE INITIATED PRIOR TO THERAPY WITH REMICADE. PHYSICIANS SHOULD MONITOR PATIENTS RECEIVING REMICADE FOR SIGNS AND SYMPTOMS OF ACTIVE TUBERCULOSIS, INCLUDING PATIENTS WHO ARE TUBERCULIN SKIN TEST NEGATIVE.
DESCRIPTION
REMICADE® is a chimeric IgG1[kgr ] monoclonal antibody with an approximate molecular weight of 149,100 daltons. It is composed of human constant and murine variable regions. Infliximab binds specifically to human tumor necrosis factor alpha (TNF(alpha)) with an association constant of 10 10 M -1 . Infliximab is produced by a recombinant cell line cultured by continuous perfusion and is purified by a series of steps that includes measures to inactivate and remove viruses.
REMICADE is supplied as a sterile, white, lyophilized powder for intravenous infusion. Following reconstitution with 10 mL of Sterile Water for Injection, USP, the resulting pH is approximately 7.2. Each single-use vial contains 100 mg infliximab, 500 mg sucrose, 0.5 mg polysorbate 80, 2.2 mg monobasic sodium phosphate, monohydrate, and 6.1 mg dibasic sodium phosphate, dihydrate. No preservatives are present.
CLINICAL PHARMACOLOGY
General
Infliximab neutralizes the biological activity of TNF(alpha) by binding with high affinity to the soluble and transmembrane forms of TNF(alpha) and inhibits binding of TNF(alpha) with its receptors. 2,3 Infliximab does not neutralize TNF(beta) (lymphotoxin (alpha)), a related cytokine that utilizes the same receptors as TNF(alpha). Biological activities attributed to TNF(alpha) include: induction of pro-inflammatory cytokines such as interleukins (IL) 1 and 6, enhancement of leukocyte migration by increasing endothelial layer permeability and expression of adhesion molecules by endothelial cells and leukocytes, activation of neutrophil and eosinophil functional activity, induction of acute phase reactants and other liver proteins, as well as tissue degrading enzymes produced by synoviocytes and/or chondrocytes. Cells expressing transmembrane TNF(alpha) bound by infliximab can be lysed in vitro 3 or in vivo . 4 Infliximab inhibits the functional activity of TNF(alpha) in a wide variety of in vitro bioassays utilizing human fibroblasts, endothelial cells, neutrophils, B and T lymphocytes and epithelial cells. Anti-TNF(alpha) antibodies reduce disease activity in the cotton-top tamarin colitis model, and decrease synovitis and joint erosions in a murine model of collagen-induced arthritis. Infliximab prevents disease in transgenic mice that develop polyarthritis as a result of constitutive expression of human TNF(alpha), and when administered after disease onset, allows eroded joints to heal.
Pharmacodynamics
Elevated concentrations of TNF(alpha) have been found in involved tissues and fluids of patients with rheumatoid arthritis, Crohn's disease, ankylosing spondylitis and psoriatic arthritis. In rheumatoid arthritis, treatment with REMICADE reduced infiltration of inflammatory cells into inflamed areas of the joint as well as expression of molecules mediating cellular adhesion [E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)], chemoattraction [IL-8 and monocyte chemotactic protein (MCP-1)] and tissue degradation [matrix metalloproteinase (MMP) 1 and 3]. In Crohn's disease, treatment with REMICADE reduced infiltration of inflammatory cells and TNF(alpha) production in inflamed areas of the intestine, and reduced the proportion of mononuclear cells from the lamina propria able to express TNF(alpha) and interferon. After treatment with REMICADE, patients with rheumatoid arthritis or Crohn's disease exhibited decreased levels of serum IL-6 and C-reactive protein (CRP) compared to baseline. Peripheral blood lymphocytes from REMICADE-treated patients showed no significant decrease in number or in proliferative responses to in vitro mitogenic stimulation when compared to cells from untreated patients. In psoriatic arthritis, treatment with REMICADE resulted in a reduction in the number of T-cells and blood vessels in the synovium and psoriatic skin as well as a reduction of macrophages in the synovium. The relationship between these pharmacodynamic activities and the mechanism(s) by which REMICADE exerts its clinical effects is unknown.
Pharmacokinetics
Single intravenous (IV) infusions of 3 mg/kg to 20 mg/kg showed a linear relationship between the dose administered and the maximum serum concentration. The volume of distribution at steady state was independent of dose and indicated that infliximab was distributed primarily within the vascular compartment. Median pharmacokinetic results for doses of 3 mg/kg to 10 mg/kg in rheumatoid arthritis and 5 mg/kg in Crohn's disease indicate that the terminal half-life of infliximab is 8.0 to 9.5 days.
Following an initial dose of REMICADE, repeated infusions at 2 and 6 weeks resulted in predictable concentration-time profiles following each treatment. No systemic accumulation of infliximab occurred upon continued repeated treatment with 3 mg/kg or 10 mg/kg at 4- or 8-week intervals. Development of antibodies to infliximab increased infliximab clearance. At 8 weeks after a maintenance dose of 3 to 10 mg/kg of REMICADE, median infliximab serum concentrations ranged from approximately 0.5 to 6 mcg/mL; however, infliximab concentrations were not detectable (<0.1 mcg/mL) in patients who became positive for antibodies to infliximab. No major differences in clearance or volume of distribution were observed in patient subgroups defined by age, weight, or gender. It is not known if there are differences in clearance or volume of distribution in patients with marked impairment of hepatic or renal function.
A pediatric Crohn's disease pharmacokinetic study was conducted in 21 patients aged 11 to 17 years old. No notable differences in single-dose pharmacokinetic parameters were observed between pediatric and adult Crohn's disease patients (see PRECAUTIONS , Pediatric Use ).
CLINICAL STUDIES
Rheumatoid Arthritis
The safety and efficacy of REMICADE were assessed in two multicenter, randomized, double-blind, pivotal trials: ATTRACT (Study RA I) and ASPIRE (Study RA II). Concurrent use of stable doses of folic acid, oral corticosteroids (</=10 mg/day) and/or non-steroidal anti-inflammatory drugs was permitted.
Study RA I was a placebo-controlled study of 428 patients with active rheumatoid arthritis despite treatment with MTX. Patients enrolled had a median age of 54 years, median disease duration of 8.4 years, median swollen and tender joint count of 20 and 31 respectively, and were on a median dose of 15 mg/wk of MTX. Patients received either placebo + MTX or one of 4 doses/schedules of REMICADE + MTX: 3 mg/kg or 10 mg/kg of REMICADE by IV infusion at weeks 0, 2 and 6 followed by additional infusions every 4 or 8 weeks in combination with MTX.
Study RA II was a placebo-controlled study of three active treatment arms in 1004 MTX naive patients of 3 or fewer years duration active rheumatoid arthritis. Patients enrolled had a median age of 51 years with a median disease duration of 0.6 years, median swollen and tender joint count of 19 and 31, respectively, and >80% of patients had baseline joint erosions. At randomization, all patients received MTX (optimized to 20 mg/wk by week 8) and either placebo, 3mg/kg or 6 mg/kg REMICADE at weeks 0, 2, and 6 and every 8 weeks thereafter.
Data on use of REMICADE without concurrent MTX are limited (see ADVERSE REACTIONS , Immunogenicity ). 5,6
Clinical response
In Study RA I, all doses/schedules of REMICADE + MTX resulted in improvement in signs and symptoms as measured by the American College of Rheumatology response criteria (ACR 20) with a higher percentage of patients achieving an ACR 20, 50 and 70 compared to placebo + MTX (Table 1). This improvement was observed at week 2 and maintained through week 102. Greater effects on each component of the ACR 20 were observed in all patients treated with REMICADE + MTX compared to placebo + MTX (Table 2). More patients treated with REMICADE reached a major clinical response than placebo-treated patients (Table 1).
In Study RA II, after 54 weeks of treatment, both doses of REMICADE + MTX resulted in statistically significantly greater response in signs and symptoms compared to MTX alone as measured by the proportion of patients achieving ACR 20, 50 and 70 responses (Table 1). More patients treated with REMICADE reached a major clinical response than placebo-treated patients (Table 1).
Table 1
ACR RESPONSE (PERCENT OF PATIENTS)Study RA I Study RA II REMICADE + MTX REMICADE + MTX 3 mg/kg 10 mg/kg 3 mg/kg 6 mg/kg ResponsePlacebo
+ MTXq 8
wksq 4
wksq 8
wksq 4
wksPlacebo
+ MTXq 8
wksq 8
wks(n=88) (n=86) (n=86) (n=87) (n=81) (n=274) (n=351) (n=355) ACR 20Week 3020% 50% a 50% a 52% a 58% a N/A N/A N/A Week 5417% 42% a 48% a 59% a 59% a 54% 62% c 66% a ACR 50Week 305% 27% a 29% a 31% a 26% a N/A N/A N/A Week 549% 21% c 34% a 40% a 38% a 32% 46% a 50% a ACR 70Week 300% 8% b 11% b 18% a 11% a N/A N/A N/A Week 542% 11% c 18% a 26% a 19% a 21% 33% b 37% a Major clinical response # 0% 7% c 8% b 15% a 6% c 8% 12% 17% a # A major clinical response was defined as a 70% ACR response for 6 consecutive months (consecutive visits spanning at least 26 weeks) through week 102 for Study RA I and week 54 for Study RA II. a p</= 0.001 b p< 0.01 c p< 0.05 Table 2
COMPONENTS OF ACR 20 AT BASELINE AND 54 WEEKS (Study RA I)Placebo + MTX REMICADE + MTX a (n=88) (n=340) Parameter (medians)Baseline Week 54 Baseline Week 54 No. of Tender Joints24 16 32 8 No. of Swollen Joints19 13 20 7 Pain b6.7 6.1 6.8 3.3 Physician's Global Assessment b6.5 5.2 6.2 2.1 Patient's Global Assessment b6.2 6.2 6.3 3.2 Disability Index (HAQ-DI) c1.8 1.5 1.8 1.3 CRP (mg/dL)3.0 2.3 2.4 0.6 a All doses/schedules of REMICADE + MTX b Visual Analog Scale (0=best, 10=worst) c Health Assessment Questionnaire, measurement of 8 categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities (0=best, 3=worst) Radiographic response
Structural damage in both hands and feet was assessed radiographically at week 54 by the change from baseline in the van der Heijde-modified Sharp (vdH-S) score, a composite score of structural damage that measures the number and size of joint erosions and the degree of joint space narrowing in hands/wrists and feet. 7
In Study RA I, approximately 80% of patients had paired x-ray data at 54 weeks and approximately 70% at 102 weeks. The inhibition of progression of structural damage was observed at 54 weeks (Table 3) and maintained through 102 weeks.
In Study RA II, >90% of patients had at least two evaluable x-rays. Inhibition of progression of structural damage was observed at weeks 30 and 54 (Table 3) in the REMICADE + MTX groups compared to MTX alone. In an exploratory analysis of Study RA II, patients treated with REMICADE + MTX demonstrated less progression of structural damage compared to MTX alone, whether baseline acute phase reactants (ESR and CRP) were normal or elevated: patients with elevated baseline acute phase reactants treated with MTX alone demonstrated a mean progression in vdH-S score of 4.2 units compared to patients treated with REMICADE + MTX who demonstrated 0.5 units of progression; patients with normal baseline acute phase reactants treated with MTX alone demonstrated a mean progression in vdH-S score of 1.8 units compared to REMICADE + MTX who demonstrated 0.2 units of progression. Of patients receiving REMICADE + MTX, 59% had no progression (vdH-S score </= 0 unit) of structural damage compared to 45% patients receiving MTX alone. In a subset of patients who began the study without erosions, REMICADE + MTX maintained an erosion free state at 1 year in a greater proportion of patients than MTX alone, 79% (77/98) vs. 58% (23/40), respectively (p<0.01). Fewer patients in the REMICADE + MTX groups (47%) developed erosions in uninvolved joints compared to MTX alone (59%).
Table 3
RADIOGRAPHIC CHANGE FROM BASELINE TO WEEK 54Study RA I Study RA II REMICADE + MTX REMICADE + MTX 3 mg/kg 10 mg/kg 3 mg/kg 6 mg/kg Placebo
+ MTXq 8
wksq 8
wksPlacebo
+ MTXq 8
wksq 8
wks(n=64) (n=71) (n=77) (n=282) (n=359) (n=363) Total Score BaselineMean79 78 65 11.3 11.6 11.2 Median55 57 56 5.1 5.2 5.3 Change from
baselineMean6.9 1.3 a 0.2 a 3.7 0.4 a 0.5 a Median4.0 0.5 0.5 0.4 0.0 0.0 Erosion ScoreBaselineMean44 44 33 8.3 8.8 8.3 Median25 29 22 3.0 3.8 3.8 Change from
baselineMean4.1 0.2 a 0.2 a 3.0 0.3 a 0.1 a Median2.0 0.0 0.5 0.3 0.0 0.0 JSN ScoreBaselineMean36 34 31 3.0 2.9 2.9 Median26 29 24 1.0 1.0 1.0 Change from
baselineMean2.9 1.1 a 0.0 a 0.6 0.1 a 0.2 Median1.5 0.0 0.0 0.0 0.0 0.0 a P< 0.001 for each outcome against placebo. Physical function response
Physical function and disability were assessed using the Health Assessment Questionnaire (HAQ-DI) and the general health-related quality of life questionnaire SF-36.
In Study RA I, all doses/schedules of REMICADE + MTX showed significantly greater improvement from baseline in HAQ-DI and SF-36 physical component summary score averaged over time through week 54 compared to placebo + MTX, and no worsening in the SF-36 mental component summary score. The median (interquartile range) improvement from baseline to week 54 in HAQ-DI was 0.1 (-0.1, 0.5) for the placebo + MTX group and 0.4 (0.1, 0.9) for REMICADE + MTX (p<0.001). Both HAQ-DI and SF-36 effects were maintained through week 102. Approximately 80% of patients in all doses/schedules of REMICADE + MTX remained in the trial through 102 weeks.
In Study RA II, both REMICADE treatment groups showed greater improvement in HAQ-DI from baseline averaged over time through week 54 compared to MTX alone; 0.7 for REMICADE + MTX vs. 0.6 for MTX alone (p</=0.001). No worsening in the SF-36 mental component summary score was observed.
Active Crohn's Disease
The safety and efficacy of single and multiple doses of REMICADE were assessed in two randomized, double-blind, placebo-controlled clinical studies in 653 patients with moderate to severely active Crohn's disease [Crohn's Disease Activity Index (CDAI) >/=220 and </=400] with an inadequate response to prior conventional therapies. Concomitant stable doses of aminosalicylates, corticosteroids and/or immunomodulatory agents were permitted and 92% of patients continued to receive at least one of these medications.
In the single-dose trial 8 of 108 patients, 16% (4/25) of placebo patients achieved a clinical response (decrease in CDAI >/=70 points) at week 4 vs. 81% (22/27) of patients receiving 5 mg/kg REMICADE (p<0.001, two-sided, Fisher's Exact test). Additionally, 4% (1/25) of placebo patients and 48% (13/27) of patients receiving 5 mg/kg REMICADE achieved clinical remission (CDAI<150) at week 4.
In a multidose trial (ACCENT I [Study Crohn's I]) 9 , 545 patients received 5 mg/kg at week 0 and were then randomized to one of three treatment groups; the placebo maintenance group received placebo at weeks 2 and 6, and then every 8 weeks; the 5 mg/kg maintenance group received 5 mg/kg at weeks 2 and 6, and then every 8 weeks; and the 10 mg/kg maintenance group received 5 mg/kg at weeks 2 and 6, and then 10 mg/kg every 8 weeks. Patients in response at week 2 were randomized and analyzed separately from those not in response at week 2. Corticosteroid taper was permitted after week 6.
At week 2, 57% (311/545) of patients were in clinical response. At week 30, a significantly greater proportion of these patients in the 5 mg/kg and 10 mg/kg maintenance groups achieved clinical remission compared to patients in the placebo maintenance group (Table 4).
Additionally, a significantly greater proportion of patients in the 5 mg/kg and 10 mg/kg REMICADE maintenance groups were in clinical remission and were able to discontinue corticosteroid use compared to patients in the placebo maintenance group at week 54 (Table 4).
Table 4
CLINICAL REMISSION AND STEROID WITHDRAWALSingle 5 mg/kg Dose a
Placebo MaintenanceThree Dose Induction b
REMICADE Maintenance q 8 wks5 mg/kg 10 mg/kg Week 3025/102 41/104 48/105 Clinical remission25% 39% 46% p-value c0.022 0.001 Week 54Patients in remission able to
discontinue corticosteroid use d6/54
11%14/56
25%18/53
34%p-value c0.059 0.005 a REMICADE at week 0 b REMICADE 5 mg/kg administered at weeks 0, 2 and 6 c p-values represent pairwise comparisons to placebo d Of those receiving corticosteroids at baseline Patients in the REMICADE maintenance groups (5 mg/kg and 10 mg/kg) had a longer time to loss of response than patients in the placebo maintenance group (Figure 1). At weeks 30 and 54, significant improvement from baseline was seen among the 5 mg/kg and 10 mg/kg REMICADE-treated groups compared to the placebo group in the disease specific inflammatory bowel disease questionnaire (IBDQ), particularly the bowel and systemic components, and in the physical component summary score of the general health-related quality of life questionnaire SF-36.
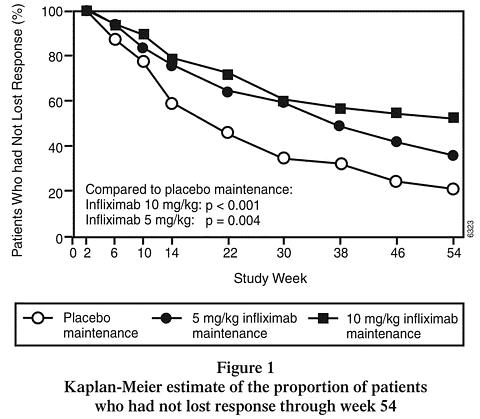
In a subset of 78 patients who had mucosal ulceration at baseline and who participated in an endoscopic substudy, 13 of 43 patients in the REMICADE maintenance group had endoscopic evidence of mucosal healing compared to 1 of 28 patients in the placebo group at week 10. Of the REMICADE-treated patients showing mucosal healing at week 10, 9 of 12 patients also showed mucosal healing at week 54.
Patients who achieved a response and subsequently lost response were eligible to receive REMICADE on an episodic basis at a dose that was 5 mg/kg higher than the dose to which they were randomized. The majority of such patients responded to the higher dose. Among patients who were not in response at week 2, 59% (92/157) of REMICADE maintenance patients responded by week 14 compared to 51% (39/77) of placebo maintenance patients. Among patients who did not respond by week 14, additional therapy did not result in significantly more responses (see DOSAGE AND ADMINISTRATION ).
Fistulizing Crohn's Disease
The safety and efficacy of REMICADE were assessed in 2 randomized, double-blind, placebo-controlled studies in patients with fistulizing Crohn's disease with fistula(s) that were of at least 3 months duration. Concurrent use of stable doses of corticosteroids, 5-aminosalicylates, antibiotics, MTX, 6-mercaptopurine (6-MP) and/or azathioprine (AZA) was permitted.
In the first trial, 10 94 patients received three doses of either placebo or REMICADE at weeks 0, 2 and 6. Fistula response (>/=50% reduction in number of enterocutaneous fistulas draining upon gentle compression on at least two consecutive visits without an increase in medication or surgery for Crohn's disease) was seen in 68% (21/31) of patients in the 5 mg/kg REMICADE group (p=0.002) and 56% (18/32) of patients in the 10 mg/kg REMICADE group (p=0.021) vs. 26% (8/31) of patients in the placebo arm. The median time to onset of response and median duration of response in REMICADE-treated patients was 2 and 12 weeks, respectively. Closure of all fistula was achieved in 52% of REMICADE-treated patients compared with 13% of placebo-treated patients (p<0.001).
In the second trial (ACCENT II [Study Crohn's II]), patients who were enrolled had to have at least one draining enterocutaneous (perianal, abdominal) fistula. All patients received 5 mg/kg REMICADE at weeks 0, 2 and 6. Patients were randomized to placebo or 5 mg/kg REMICADE maintenance at week 14. Patients received maintenance doses at week 14 and then every eight weeks through week 46. Patients who were in fistula response (fistula response was defined the same as in the first trial) at both weeks 10 and 14 were randomized separately from those not in response. The primary endpoint was time from randomization to loss of response among those patients who were in fistula response.
Among the randomized patients (273 of the 296 initially enrolled), 87% had perianal fistulas and 14% had abdominal fistulas. Eight percent also had rectovaginal fistulas. Greater than 90% of the patients had received previous immunosuppressive and antibiotic therapy.
At week 14, 65% (177/273) of patients were in fistula response. Patients randomized to REMICADE maintenance had a longer time to loss of fistula response compared to the placebo maintenance group (Figure 2). At week 54, 38% (33/87) of REMICADE-treated patients had no draining fistulas compared with 22% (20/90) of placebo-treated patients (p=0.02). Compared to placebo maintenance, patients on REMICADE maintenance had a trend toward fewer hospitalizations.
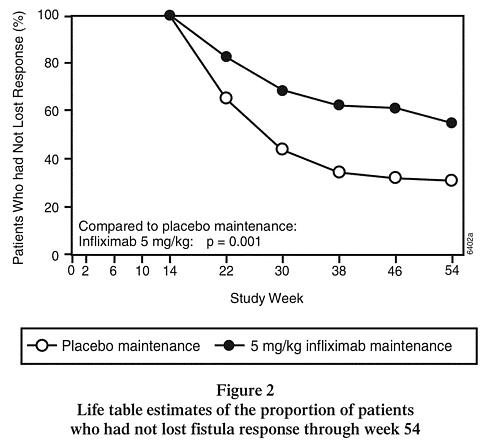
Patients who achieved a fistula response and subsequently lost response were eligible to receive REMICADE maintenance therapy at a dose that was 5 mg/kg higher than the dose to which they were randomized. Of the placebo maintenance patients, 66% (25/38) responded to 5 mg/kg REMICADE, and 57% (12/21) of REMICADE maintenance patients responded to 10 mg/kg.
Patients who had not achieved a response by week 14 were unlikely to respond to additional doses of REMICADE.
Similar proportions of patients in either group developed new fistulas (17% overall) and similar numbers developed abscesses (15% overall).
Ankylosing Spondylitis
The safety and efficacy of REMICADE were assessed in a randomized, multicenter, double-blind, placebo-controlled study in 279 patients with active ankylosing spondylitis. Patients were between 18 and 74 years of age, and had ankylosing spondylitis as defined by the modified New York criteria for Ankylosing Spondylitis. 11 Patients were to have had active disease as evidenced by both a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score >4 (possible range 0-10) and spinal pain >4 (on a Visual Analog Scale [VAS] of 0-10). Patients with complete ankylosis of the spine were excluded from study participation, and the use of Disease Modifying Anti-Rheumatic Drugs (DMARDs) and systemic corticosteroids were prohibited. Doses of REMICADE 5 mg/kg or placebo were administered intravenously at Weeks 0, 2, 6, 12 and 18.
At 24 weeks, improvement in the signs and symptoms of ankylosing spondylitis, as measured by the proportion of patients achieving a 20% improvement in ASAS response criteria (ASAS 20), was seen in 60% of patients in the REMICADE-treated group vs. 18% of patients in the placebo group (p<0.001). Improvement was observed at week 2 and maintained through week 24 (Figure 3 and Table 5).
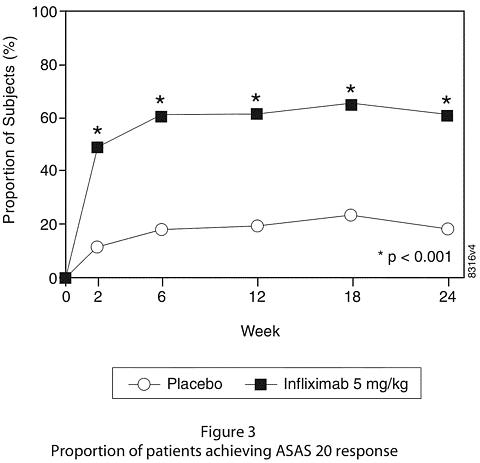
At 24 weeks, the proportions of patients achieving a 50% and a 70% improvement in the signs and symptoms of ankylosing spondylitis, as measured by ASAS response criteria (ASAS 50 and ASAS 70, respectively), were 44% and 28%, respectively, for patients receiving REMICADE, compared to 9% and 4%, respectively, for patients receiving placebo (p<0.001, REMICADE vs. placebo). A low level of disease activity (defined as a value <20 [on a scale of 0-100 mm] in each of the four ASAS response parameters) was achieved in 22% of REMICADE-treated patients vs. 1% in placebo-treated patients (p<0.001).
Table 5
Components of Ankylosing Spondylitis Disease ActivityPlacebo REMICADE 5mg/kg p-value (n=78) (n=201) Baseline 24
WeeksBaseline 24
WeeksASAS 20 responseCriteria (Mean)Patient global assessment a6.6 6.0 6.8 3.8 <0.001 Spinal pain a7.3 6.5 7.6 4.0 <0.001 BASFI b5.8 5.6 5.7 3.6 <0.001 Inflammation c6.9 5.8 6.9 3.4 <0.001 Acute Phase ReactantsMedian CRP d (mg/dL)1.7 1.5 1.5 0.4 <0.001 Spinal Mobility (cm, Mean)Modified Schober's test e4.0 5.0 4.3 4.4 0.75 Chest expansion e3.6 3.7 3.3 3.9 0.04 Tragus to wall e17.3 17.4 16.9 15.7 0.02 Lateral spinal flexion e10.6 11.0 11.4 12.9 0.03 a measured on a VAS with 0="none" and 10="severe" b Bath Ankylosing Spondylitis Functional Index (BASFI), average of 10 questions c Inflammation, average of last 2 questions on the 6 question BASDAI d CRP normal range 0-1.0 mg/dL e Spinal mobility normal values: modified Schober's test: >4 cm; chest expansion:>6 cm; tragus to wall: <15 cm; lateral spinal flexion: >10 cm The median improvement from baseline in the general health-related quality of life questionnaire SF-36 physical component summary score at week 24 was 10.2 for the REMICADE group vs. 0.8 for the placebo group (p<0.001). There was no change in the SF-36 mental component summary score in either the REMICADE group or the placebo group.
Results of this study were similar to those seen in a multicenter double-blind, placebo-controlled study of 70 patients with ankylosing spondylitis.
Psoriatic Arthritis
Safety and efficacy of REMICADE were assessed in a multicenter, double-blind, placebo-controlled study in 200 adult patients with active psoriatic arthritis despite DMARD or NSAID therapy (>/= 5 swollen joints and >/= 5 tender joints) with one or more of the following subtypes: arthritis involving DIP joints (n = 49), arthritis mutilans (n = 3), asymmetric peripheral arthritis (n = 40), polyarticular arthritis (n = 100), and spondylitis with peripheral arthritis (n = 8). Patients also had plaque psoriasis with a qualifying target lesion >/= 2 cm in diameter. Forty-six percent of patients continued on stable doses of methotrexate (</= 25 mg/week). During the 24-week double-blind phase, patients received either 5 mg/kg REMICADE or placebo at weeks 0, 2, 6, 14, and 22 (100 patients in each group). At week 16, placebo patients with < 10% improvement from baseline in both swollen and tender joint counts were switched to REMICADE induction (early escape).
Treatment with REMICADE resulted in improvement in signs and symptoms, as assessed by the ACR criteria, with 58% of REMICADE-treated patients achieving ACR 20 at week 14, compared with 11% of placebo-treated patients (p < 0.001). The response was similar regardless of concomitant use of methotrexate. Improvement was observed as early as week 2. At 6 months, the ACR 20/50/70 responses were achieved by 54%, 41%, and 27%, respectively, of patients receiving REMICADE compared to 16%, 4%, and 2%, respectively, of patients receiving placebo. Similar responses were seen in patients with each of the subtypes of psoriatic arthritis, although few patients were enrolled with the arthritis mutilans and spondylitis with peripheral arthritis subtypes.
Compared to placebo, treatment with REMICADE resulted in improvements in the components of the ACR response criteria, as well as in dactylitis and enthesopathy (Table 6).
The results of this study were similar to those seen in an earlier multicenter, randomized, placebo-controlled study of 104 patients with psoriatic arthritis.
Table 6
COMPONENTS OF ACR 20 AND PERCENTAGE OF PATIENTS WITH 1 OR MORE JOINTS
WITH DACTYLITIS AND PERCENTAGE OF PATIENTS WITH ENTHESOPATHY
AT BASELINE and WEEK 24Placebo
(n=100)REMICADE 5mg/kg a
(n=100)Parameter (medians)Baseline Week 24 Baseline Week 24 No of Tender Joints b24 20 20 6 No. of Swollen Joints c12 9 12 3 Pain d6.4 5.6 5.9 2.6 Physician's Global Assessment d6.0 4.5 5.6 1.5 Patient's Global Assessment d6.1 5.0 5.9 2.5 Disability Index (HAQ-DI) e1.1 1.1 1.1 0.5 CRP (mg/dL) f1.2 0.9 1.0 0.4 % Patients with 1 or more
digits with dactylitis41 33 40 15 % Patients with enthesopathy35 36 42 22 a p<0.001 for percent change from baseline in all components of ACR 20 at week 24, p<0.05 for % of patients with dactylitis, and p=0.004 for % of patients with enthesopathy at week 24 b Scale 0-68 c Scale 0-66 d Visual Analog Scale (0=best, 10=worst) e Health Assessment Questionnaire, measurement of 8 categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities (0=best, 3=worst) f Normal range 0-0.6 mg/dL Improvement in PASI in patients with baseline body surface area (BSA) >/= 3% (n=87 placebo, n=83 REMICADE) was achieved at week 14, regardless of concomitant methotrexate use, with 64% of REMICADE-treated patients achieving at least 75% improvement from baseline vs. 2% of placebo-treated patients; improvement was observed as early as week 2. At 6 months, the PASI 75 and PASI 90 responses were achieved by 60% and 39%, respectively, of patients receiving REMICADE compared to 1% and 0%, respectively, of patients receiving placebo.
INDICATIONS AND USAGE
Rheumatoid Arthritis
REMICADE, in combination with methotrexate, is indicated for reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active rheumatoid arthritis.
Crohn's Disease
REMICADE is indicated for reducing signs and symptoms and inducing and maintaining clinical remission in patients with moderately to severely active Crohn's disease who have had an inadequate response to conventional therapy.
REMICADE is indicated for reducing the number of draining enterocutaneous and rectovaginal fistulas and maintaining fistula closure in patients with fistulizing Crohn's disease.
Ankylosing Spondylitis
REMICADE is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis.
Psoriatic Arthritis
REMICADE is indicated for reducing signs and symptoms of active arthritis in patients with psoriatic arthritis.
CONTRAINDICATIONS
REMICADE at doses >5 mg/kg should not be administered to patients with moderate to severe heart failure. In a randomized study evaluating REMICADE in patients with moderate to severe heart failure (New York Heart Association [NYHA] Functional Class III/IV), REMICADE treatment at 10 mg/kg was associated with an increased incidence of death and hospitalization due to worsening heart failure (see WARNINGS and ADVERSE REACTIONS , Patients with Heart Failure ).
REMICADE should not be administered to patients with known hypersensitivity to any murine proteins or other component of the product.
WARNINGS
RISK OF INFECTIONS
(See boxed WARNING )
SERIOUS INFECTIONS, INCLUDING SEPSIS AND PNEUMONIA, HAVE BEEN REPORTED IN PATIENTS RECEIVING TNF-BLOCKING AGENTS. SOME OF THESE INFECTIONS HAVE BEEN FATAL. MANY OF THE SERIOUS INFECTIONS IN PATIENTS TREATED WITH REMICADE HAVE OCCURRED IN PATIENTS ON CONCOMITANT IMMUNOSUPPRESSIVE THERAPY THAT, IN ADDITION TO THEIR CROHN'S DISEASE OR RHEUMATOID ARTHRITIS, COULD PREDISPOSE THEM TO INFECTIONS.
REMICADE SHOULD NOT BE GIVEN TO PATIENTS WITH A CLINICALLY IMPORTANT, ACTIVE INFECTION. CAUTION SHOULD BE EXERCISED WHEN CONSIDERING THE USE OF REMICADE IN PATIENTS WITH A CHRONIC INFECTION OR A HISTORY OF RECURRENT INFECTION. PATIENTS SHOULD BE MONITORED FOR SIGNS AND SYMPTOMS OF INFECTION WHILE ON OR AFTER TREATMENT WITH REMICADE. NEW INFECTIONS SHOULD BE CLOSELY MONITORED. IF A PATIENT DEVELOPS A SERIOUS INFECTION, REMICADE THERAPY SHOULD BE DISCONTINUED (see ADVERSE REACTIONS , Infections ).
CASES OF TUBERCULOSIS, HISTOPLASMOSIS, COCCIDIOIDOMYCOSIS, LISTERIOSIS, PNEUMOCYSTOSIS, OTHER BACTERIAL, MYCOBACTERIAL AND FUNGAL INFECTIONS HAVE BEEN OBSERVED IN PATIENTS RECEIVING REMICADE. FOR PATIENTS WHO HAVE RESIDED IN REGIONS WHERE HISTOPLASMOSIS OR COCCIDIOIDOMYCOSIS IS ENDEMIC, THE BENEFITS AND RISKS OF REMICADE TREATMENT SHOULD BE CAREFULLY CONSIDERED BEFORE INITIATION OF REMICADE THERAPY.
SERIOUS INFECTIONS WERE SEEN IN CLINICAL STUDIES WITH CONCURRENT USE OF ANAKINRA AND ANOTHER TNF(alpha)-BLOCKING AGENT, ETANERCEPT, WITH NO ADDED CLINICAL BENEFIT COMPARED TO ETANERCEPT ALONE. BECAUSE OF THE NATURE OF THE ADVERSE EVENTS SEEN WITH COMBINATION OF ETANERCEPT AND ANAKINRA THERAPY, SIMILAR TOXICITIES MAY ALSO RESULT FROM THE COMBINATION OF ANAKINRA AND OTHER TNF(alpha)-BLOCKING AGENTS. THEREFORE, THE COMBINATION OF REMICADE AND ANAKINRA IS NOT RECOMMENDED.
Hepatotoxicity
Severe hepatic reactions, including acute liver failure, jaundice, hepatitis and cholestasis have been reported rarely in postmarketing data in patients receiving REMICADE. Autoimmune hepatitis has been diagnosed in some of these cases. Severe hepatic reactions occurred between two weeks to more than a year after initiation of REMICADE; elevations in hepatic aminotransferase levels were not noted prior to discovery of the liver injury in many of these cases. Some of these cases were fatal or necessitated liver transplantation. Patients with symptoms or signs of liver dysfunction should be evaluated for evidence of liver injury. If jaundice and/or marked liver enzyme elevations (e.g., >/=5 times the upper limit of normal) develops, REMICADE should be discontinued, and a thorough investigation of the abnormality should be undertaken. As with other immunosuppressive drugs, use of REMICADE has been associated with reactivation of hepatitis B in patients who are chronic carriers of this virus (i.e., surface antigen positive). Chronic carriers of hepatitis B should be appropriately evaluated and monitored prior to the initiation of and during treatment with REMICADE. In clinical trials, mild or moderate elevations of ALT and AST have been observed in patients receiving REMICADE without progression to severe hepatic injury (see ADVERSE REACTIONS , Hepatotoxicity ).
Patients with Heart Failure
REMICADE has been associated with adverse outcomes in patients with heart failure, and should be used in patients with heart failure only after consideration of other treatment options. The results of a randomized study evaluating the use of REMICADE in patients with heart failure (NYHA Functional Class III/IV) suggested higher mortality in patients who received 10 mg/kg REMICADE, and higher rates of cardiovascular adverse events at doses of 5 mg/kg and 10 mg/kg. There have been post-marketing reports of worsening heart failure, with and without identifiable precipitating factors, in patients taking REMICADE. There have also been rare post-marketing reports of new onset heart failure, including heart failure in patients without known pre-existing cardiovascular disease. Some of these patients have been under 50 years of age. If a decision is made to administer REMICADE to patients with heart failure, they should be closely monitored during therapy, and REMICADE should be discontinued if new or worsening symptoms of heart failure appear. (See CONTRAINDICATIONS and ADVERSE REACTIONS , Patients with Heart Failure .)
Hematologic Events
Cases of leukopenia, neutropenia, thrombocytopenia, and pancytopenia, some with a fatal outcome, have been reported in patients receiving REMICADE. The causal relationship to REMICADE therapy remains unclear. Although no high-risk group(s) has been identified, caution should be exercised in patients being treated with REMICADE who have ongoing or a history of significant hematologic abnormalities. All patients should be advised to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever) while on REMICADE. Discontinuation of REMICADE therapy should be considered in patients who develop significant hematologic abnormalities.
Hypersensitivity
REMICADE has been associated with hypersensitivity reactions that vary in their time of onset and required hospitalization in some cases. Most hypersensitivity reactions, which include urticaria, dyspnea, and/or hypotension, have occurred during or within 2 hours of REMICADE infusion. However, in some cases, serum sickness-like reactions have been observed in Crohn's disease patients 3 to 12 days after REMICADE therapy was reinstituted following an extended period without REMICADE treatment. Symptoms associated with these reactions include fever, rash, headache, sore throat, myalgias, polyarthralgias, hand and facial edema and/or dysphagia. These reactions were associated with marked increase in antibodies to infliximab, loss of detectable serum concentrations of infliximab, and possible loss of drug efficacy. REMICADE should be discontinued for severe reactions. Medications for the treatment of hypersensitivity reactions (e.g., acetaminophen, antihistamines, corticosteroids and/or epinephrine) should be available for immediate use in the event of a reaction (see ADVERSE REACTIONS , Infusion-related Reactions ).
Neurologic Events
REMICADE and other agents that inhibit TNF have been associated in rare cases with optic neuritis, seizure and new onset or exacerbation of clinical symptoms and/or radiographic evidence of central nervous system demyelinating disorders, including multiple sclerosis, and CNS manifestation of systemic vasculitis. Prescribers should exercise caution in considering the use of REMICADE in patients with pre-existing or recent onset of central nervous system demyelinating or seizure disorders. Discontinuation of REMICADE should be considered in patients who develop significant central nervous system adverse reactions.
Malignancies
In the controlled portions of clinical trials of all the TNF(alpha)-blocking agents, more cases of lymphoma have been observed among patients receiving a TNF blocker compared with control patients. During the controlled portions of REMICADE trials in patients with moderately to severely active rheumatoid arthritis and Crohn's disease, 2 patients developed lymphoma among 1964 REMICADE-treated patients versus 0 among 483 control patients (median duration of follow-up 0.9 years). In the controlled and open-label portions of these clinical trials of REMICADE, 4 patients developed lymphomas (2 patients with rheumatoid arthritis and 2 patients with Crohn's disease) among 3469 patients (median duration of follow-up 1.0 years). In rheumatoid arthritis patients, this is approximately 3-fold higher than expected in the general population. In the combined clinical trial population for rheumatoid arthritis and Crohn's disease, this is approximately 5-fold higher than expected in the general population. Rates in clinical trials for REMICADE cannot be compared to rates of clinical trials of other TNF blockers and may not predict rates observed in a broader patient population. Patients with Crohn's disease or rheumatoid arthritis, particularly patients with highly active disease and/or chronic exposure to immunosuppressant therapies, may be at a higher risk (up to several fold) than the general population for the development of lymphoma. The potential role of TNF(alpha)-blocking therapy in the development of malignancies is not known (see ADVERSE REACTIONS , Malignancies ). No studies have been conducted that include patients with a history of malignancy or that continue treatment in patients who develop malignancy while receiving REMICADE; thus additional caution should be exercised in considering REMICADE treatment of these patients.
PRECAUTIONS
Autoimmunity
Treatment with REMICADE may result in the formation of autoantibodies and, rarely, in the development of a lupus-like syndrome. If a patient develops symptoms suggestive of a lupus-like syndrome following treatment with REMICADE, treatment should be discontinued (see ADVERSE REACTIONS , Autoantibodies/Lupus-like Syndrome ).
Vaccinations
No data are available on the response to vaccination with live vaccines or on the secondary transmission of infection by live vaccines in patients receiving anti-TNF therapy. It is recommended that live vaccines not be given concurrently.
Information for Patients
Patients should be provided the REMICADE Patient Information Sheet and provided an opportunity to read it prior to each treatment infusion session. Because caution should be exercised in administering REMICADE to patients with clinically important active infections, it is important that the patient's overall health be assessed at each treatment visit and any questions resulting from the patient's reading of the Patient Information Sheet be discussed.
Drug Interactions
Concurrent administration of etanercept (another TNF(alpha)-blocking agent) and anakinra (an interleukin-1 antagonist) has been associated with an increased risk of serious infections, and increased risk of neutropenia and no additional benefit compared to these medicinal products alone. Other TNF(alpha)-blocking agents (including REMICADE) used in combination with anakinra may also result in similar toxicities (see WARNINGS , RISK OF INFECTIONS ).
Specific drug interaction studies, including interactions with MTX, have not been conducted. The majority of patients in rheumatoid arthritis or Crohn's disease clinical studies received one or more concomitant medications. In rheumatoid arthritis, concomitant medications besides MTX were nonsteroidal anti-inflammatory agents, folic acid, corticosteroids and/or narcotics. Concomitant Crohn's disease medications were antibiotics, antivirals, corticosteroids, 6-MP/AZA and aminosalicylates. In psoriatic arthritis clinical trials, concomitant medications included MTX in approximately half of the patients as well as nonsteroidal anti-inflammatory agents, folic acid and corticosteroids.
Patients with Crohn's disease who received immunosuppressants tended to experience fewer infusion reactions compared to patients on no immunosuppressants (see ADVERSE REACTIONS , Immunogenicity and Infusion-related Reactions ). Serum infliximab concentrations appeared to be unaffected by baseline use of medications for the treatment of Crohn's disease including corticosteroids, antibiotics (metronidazole or ciprofloxacin) and aminosalicylates.
Carcinogenesis, Mutagenesis and Impairment of Fertility
A repeat dose toxicity study was conducted with mice given cV1q anti-mouse TNF(alpha) to evaluate tumorigenicity. CV1q is an analogous antibody that inhibits the function of TNF(alpha) in mice. Animals were assigned to 1 of 3 dose groups: control, 10 mg/kg or 40 mg/kg cV1q given weekly for 6 months. The weekly doses of 10 mg/kg and 40 mg/kg are 2 and 8 times, respectively, the human dose of 5 mg/kg for Crohn's disease. Results indicated that cV1q did not cause tumorigenicity in mice. No clastogenic or mutagenic effects of infliximab were observed in the in vivo mouse micronucleus test or the Salmonella-Escherichia coli (Ames) assay, respectively. Chromosomal aberrations were not observed in an assay performed using human lymphocytes. The significance of these findings for human risk is unknown. It is not known whether infliximab can impair fertility in humans. No impairment of fertility was observed in a fertility and general reproduction toxicity study with the analogous mouse antibody used in the 6-month chronic toxicity study.
Pregnancy Category B
Since infliximab does not cross-react with TNF(alpha) in species other than humans and chimpanzees, animal reproduction studies have not been conducted with REMICADE. No evidence of maternal toxicity, embryotoxicity or teratogenicity was observed in a developmental toxicity study conducted in mice using an analogous antibody that selectively inhibits the functional activity of mouse TNF(alpha). Doses of 10 to 15 mg/kg in pharmacodynamic animal models with the anti-TNF analogous antibody produced maximal pharmacologic effectiveness. Doses up to 40 mg/kg were shown to produce no adverse effects in animal reproduction studies. It is not known whether REMICADE can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. REMICADE should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether REMICADE is excreted in human milk or absorbed systemically after ingestion. Because many drugs and immunoglobulins are excreted in human milk, and because of the potential for adverse reactions in nursing infants from REMICADE, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of REMICADE in patients with juvenile rheumatoid arthritis and in pediatric patients with Crohn's disease have not been established.
Geriatric Use
In rheumatoid arthritis clinical trials, no overall differences were observed in effectiveness or safety in 181 patients aged 65 or older compared to younger patients although the incidence of serious adverse events in patients aged 65 or older was higher in both REMICADE and control groups compared to younger patients. In Crohn's disease, ankylosing spondylitis and psoriatic arthritis studies, there were insufficient numbers of patients aged 65 and over to determine whether they respond differently from patients aged 18 to 65. Because there is a higher incidence of infections in the elderly population in general, caution should be used in treating the elderly (see ADVERSE REACTIONS , Infections ).
ADVERSE REACTIONS
The data described herein reflect exposure to REMICADE in 2779 patients, including 1484 patients exposed beyond 30 weeks and 296 exposed beyond one year. The most common reason for discontinuation of treatment was infusion-related reactions (e.g. dyspnea, flushing, headache and rash). Adverse events have been reported in a higher proportion of rheumatoid arthritis patients receiving the 10 mg/kg dose than the 3 mg/kg dose, however, no differences were observed in the frequency of adverse events between the 5 mg/kg dose and 10 mg/kg dose in patients with Crohn's disease.
Infusion-related Reactions
Acute infusion reactions
An infusion reaction was defined in clinical trials as any adverse event occurring during an infusion or within 1 to 2 hours after an infusion. Approximately 20% of REMICADE-treated patients in all clinical studies experienced an infusion reaction compared to approximately 10% of placebo-treated patients. Among all REMICADE infusions, 3% were accompanied by nonspecific symptoms such as fever or chills, 1% were accompanied by cardiopulmonary reactions (primarily chest pain, hypotension, hypertension or dyspnea), and <1% were accompanied by pruritus, urticaria, or the combined symptoms of pruritus/urticaria and cardiopulmonary reactions. Serious infusion reactions occurred in <1% of patients and included anaphylaxis, convulsions, erythematous rash and hypotension. Approximately 3% of patients discontinued REMICADE because of infusion reactions, and all patients recovered with treatment and/or discontinuation of the infusion. REMICADE infusions beyond the initial infusion were not associated with a higher incidence of reactions.
Patients who became positive for antibodies to infliximab were more likely (approximately 2- to 3-fold) to have an infusion reaction than were those who were negative. Use of concomitant immunosuppressant agents appeared to reduce the frequency of antibodies to infliximab and infusion reactions (see ADVERSE REACTIONS , Immunogenicity and PRECAUTIONS , Drug Interactions ).
In post-marketing experience, cases of anaphylactic-like reactions, including laryngeal/pharyngeal edema and severe bronchospasm, and seizure have been associated with REMICADE administration.
Reactions following readministration
In a study where 37 of 41 patients with Crohn's disease were retreated with infliximab following a 2 to 4 year period without infliximab treatment, 10 patients experienced adverse events manifesting 3 to 12 days following infusion of which 6 were considered serious. Signs and symptoms included myalgia and/or arthralgia with fever and/or rash, with some patients also experiencing pruritus, facial, hand or lip edema, dysphagia, urticaria, sore throat, and headache. Patients experiencing these adverse events had not experienced infusion-related adverse events associated with their initial infliximab therapy. These adverse events occurred in 39% (9/23) of patients who had received liquid formulation which is no longer in use and 7% (1/14) of patients who received lyophilized formulation. The clinical data are not adequate to determine if occurrence of these reactions is due to differences in formulation. Patients' signs and symptoms improved substantially or resolved with treatment in all cases. There are insufficient data on the incidence of these events after drug-free intervals of 1 to 2 years. These events have been observed only infrequently in clinical studies and post-marketing surveillance with retreatment intervals up to 1 year.
Infections
In REMICADE clinical studies, treated infections were reported in 36% of REMICADE-treated patients (average of 51 weeks of follow-up) and in 25% of placebo-treated patients (average of 37 weeks of follow-up). The infections most frequently reported were respiratory tract infections (including sinusitis, pharyngitis, and bronchitis) and urinary tract infections. Among REMICADE-treated patients, serious infections included pneumonia, cellulitis, abscess, skin ulceration, sepsis, and bacterial infection. In all clinical trials, six opportunistic infections were reported; two cases of coccidioidomycosis (one of which resulted in death), and one case each of histoplasmosis, pneumocystosis, nocardiosis and cytomegalovirus. Tuberculosis was reported in thirteen patients, four of whom died due to miliary tuberculosis. Other cases of tuberculosis, including disseminated tuberculosis, also have been reported post-marketing. Most of these cases of tuberculosis occurred within the first two months after initiation of therapy with REMICADE and may reflect recrudescence of latent disease (see WARNINGS , RISK OF INFECTIONS ). In the 1 year placebo-controlled studies RA I and RA II, 5.3% of patients receiving REMICADE every 8 weeks with MTX developed serious infections as compared to 3.4% of placebo patients receiving MTX. Of 924 patients receiving REMICADE, 1.7% developed pneumonia and 0.4% developed TB, when compared to 0.3% and 0.0% in the placebo arm respectively. In a shorter (22-week) placebo-controlled study of 1082 RA patients randomized to receive placebo, 3 mg/kg or 10 mg/kg REMICADE infusions at 0, 2, and 6 weeks, followed by every 8 weeks with MTX, serious infections were more frequent in the 10 mg/kg REMICADE group (5.3%) than the 3 mg/kg or placebo groups (1.7% in both). During the 54 weeks Crohn's II Study, 15% of patients with fistulizing Crohn's disease developed a new fistula-related abscess.
In post-marketing experience, infections have been observed with various pathogens including viral, bacterial, fungal, and protozoal organisms. Infections have been noted in all organ systems and have been reported in patients receiving REMICADE alone or in combination with immunosuppressive agents.
Autoantibodies/Lupus-like Syndrome
Approximately half of REMICADE-treated patients in clinical trials who were antinuclear antibody (ANA) negative at baseline developed a positive ANA during the trial compared with approximately one-fifth of placebo-treated patients. Anti-dsDNA antibodies were newly detected in approximately one-fifth of REMICADE-treated patients compared with 0% of placebo-treated patients. Reports of lupus and lupus-like syndromes, however, remain uncommon.
Malignancies
Among 3469 patients with moderately to severely active rheumatoid arthritis and Crohn's disease treated with REMICADE in clinical trials with a median of 1.0 years of follow-up, 4 patients developed lymphomas, for a rate of 0.08 cases per 100 patient-years of follow-up in patients with rheumatoid arthritis and 0.12 cases per 100 patient-years of follow up in the combined clinical trial data for rheumatoid arthritis and Crohn's disease patients. This is approximately 3-fold higher in the RA clinical trial population and 5-fold higher in the overall clinical trial population than expected in an age-, gender-, and race-matched general population based on the Surveillance, Epidemiology and End Results Database. Rates in clinical trials for REMICADE cannot be compared to rates of clinical trials of other TNF blockers and may not predict rates observed in a broader patient population. An increased rate of lymphoma up to several fold has been reported in the Crohn's disease and rheumatoid arthritis patient populations, and may be further increased in patients with more severe disease activity. Other than lymphoma, 23 patients developed noncutaneous malignancies, which was similar in number to what would be expected in the general population. Of these, the most common malignancies were breast, colorectal, and melanoma. (See WARNINGS , Malignancies .)
Malignancies, including non-Hodgkin's lymphoma and Hodgkin's disease, have also been reported in patients receiving REMICADE during post-approval use.
Patients with Heart Failure
In a randomized study evaluating REMICADE in moderate to severe heart failure (NYHA Class III/IV; left ventricular ejection fraction </=35%), 150 patients were randomized to receive treatment with 3 infusions of REMICADE 10 mg/kg, 5 mg/kg, or placebo, at 0, 2, and 6 weeks. Higher incidences of mortality and hospitalization due to worsening heart failure were observed in patients receiving the 10 mg/kg REMICADE dose. At 1 year, 8 patients in the 10 mg/kg REMICADE group had died compared with 4 deaths each in the 5 mg/kg REMICADE and the placebo groups. There were trends towards increased dyspnea, hypotension, angina, and dizziness in both the 10 mg/kg and 5 mg/kg REMICADE treatment groups, versus placebo. REMICADE has not been studied in patients with mild heart failure (NYHA Class I/II). (See CONTRAINDICATIONS and WARNINGS , Patients with Heart Failure .)
Immunogenicity
Treatment with REMICADE can be associated with the development of antibodies to infliximab. The incidence of antibodies to infliximab in patients given a 3-dose induction regimen followed by maintenance dosing was approximately 10% as assessed through one to two years of REMICADE treatment. A higher incidence of antibodies to infliximab was observed in Crohn's disease patients receiving REMICADE after drug free intervals >16 weeks. The majority of antibody-positive patients had low titers. Patients who were antibody-positive were more likely to have higher rates of clearance, reduced efficacy and to experience an infusion reaction (see ADVERSE REACTIONS , Infusion-related Reactions) than were patients who were antibody negative. Antibody development was lower among rheumatoid arthritis and Crohn's disease patients receiving immunosuppressant therapies such as 6-MP/AZA or MTX.
The data reflect the percentage of patients whose test results were positive for antibodies to infliximab in an ELISA assay, and are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to infliximab with the incidence of antibodies to other products may be misleading.
Hepatotoxicity
Severe liver injury, including acute liver failure and autoimmune hepatitis, has been reported rarely in patients receiving REMICADE (see WARNINGS , Hepatotoxicity ). Reactivation of hepatitis B has occurred in patients receiving REMICADE who are chronic carriers of this virus (i.e., surface antigen positive) (see WARNINGS Hepatotoxicity ).
In clinical trials in RA, Crohn's disease, ankylosing spondylitis and psoriatic arthritis, elevations of aminotransferases were observed (ALT more common than AST) in a greater proportion of patients receiving REMICADE than in controls, both when REMICADE was given as monotherapy and when it was used in combination with other immunosuppressive agents. In general, patients who developed ALT and AST elevations were asymptomatic, and the abnormalities decreased or resolved with either continuation or discontinuation of REMICADE, or modification of concomitant medications. ALT elevations >/=5 times the upper limit of normal were observed in 1% of patients receiving REMICADE.
In rheumatoid arthritis clinical trials, 34% of patients who received REMICADE + MTX experienced transient mild (<2 times the upper limit of normal) or moderate (>/=2 but <3 times the upper limit of normal) elevations in ALT compared to 24% of patients treated with placebo + MTX. ALT elevations >/=3 times the upper limit of normal were observed in 3.9% of patients who received REMICADE + MTX compared with 3.2% of patients who received MTX alone (median follow up approximately 1 year).
In Crohn's disease clinical trials (median follow up 54 weeks), 39% of patients receiving REMICADE-maintenance experienced mild to moderate elevations in ALT, compared to 34% of patients treated with placebo-maintenance. ALT elevations >/=3 times the upper limit of normal were observed in 5.0% of patients who received REMICADE-maintenance compared with 4.0% of patients who received placebo-maintenance.
In an ankylosing spondylitis clinical trial in which patients were not receiving MTX, 40% of patients who received REMICADE experienced mild to moderate elevations in ALT compared to 13% of patients treated with placebo. ALT elevations >/=3 times the upper limit of normal were observed in 12 (6%) REMICADE-treated patients compared to none in placebo-treated patients. Similar rates of mild to moderate ALT elevations and elevations >/=3 times the upper limit of normal were observed in a psoriatic arthritis clinical trial.
Other Adverse Reactions
Safety data are available from 2779 REMICADE-treated patients, including 1304 with rheumatoid arthritis, 1106 with Crohn's disease, 202 with ankylosing spondylitis, 150 with psoriatic arthritis, and 17 with other conditions. Adverse events reported in >/=5% of all patients with rheumatoid arthritis receiving 4 or more infusions are in Table 7. The types and frequencies of adverse reactions observed were similar in REMICADE-treated rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and Crohn's disease patients except for abdominal pain, which occurred in 26% of REMICADE-treated patients with Crohn's disease. In the Crohn's disease studies, there were insufficient numbers and duration of follow-up for patients who never received REMICADE to provide meaningful comparisons.
Table 7
ADVERSE EVENTS OCCURRING IN 5% OR MORE
OF PATIENTS RECEIVING 4 OR MORE INFUSIONS
FOR RHEUMATOID ARTHRITISPlacebo
(n=350)REMICADE
(n=1129)Average weeks of follow-up59 66 GastrointestinalNausea20% 21% Abdominal Pain8% 12% Diarrhea12% 12% Dyspepsia7% 10% RespiratoryUpper respiratory tract
infection25% 32% Sinusitis8% 14% Pharyngitis8% 12% Coughing8% 12% Bronchitis9% 10% Rhinitis5% 8% Skin and appendages disordersRash5% 10% Pruritis2% 7% Body as a whole-general disordersFatigue7% 9% Pain7% 8% Resistance mechanism disordersFever4% 7% Moniliasis3% 5% Central and peripheral nervous
system disordersHeadache14% 18% Musculoskeletal system disordersBack pain5% 8% Arthralgia7% 8% Urinary system disordersUrinary tract infection6% 8% Cardiovascular disorders, generalHypertension5% 7% Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not predict the rates observed in broader patient populations in clinical practice.
The most common serious adverse events observed in clinical trials were infections (see ADVERSE REACTIONS , Infections ). Other serious, medically relevant adverse events >/=0.2% or clinically significant adverse events by body system were as follows:
Body as a whole: allergic reaction, diaphragmatic hernia, edema, surgical/procedural sequela
Blood: pancytopenia
Cardiovascular: circulatory failure, hypotension, syncope
Gastrointestinal: constipation, gastrointestinal hemorrhage, ileus, intestinal obstruction, intestinal perforation, intestinal stenosis, pancreatitis, peritonitis, proctalgia
Central & Peripheral Nervous: meningitis, neuritis, peripheral neuropathy, dizziness
Heart Rate and Rhythm: arrhythmia, bradycardia, cardiac arrest, tachycardia
Liver and Biliary: biliary pain, cholecystitis, cholelithiasis, hepatitis
Metabolic and Nutritional: dehydration
Musculoskeletal: intervertebral disk herniation, tendon disorder
Myo-, Endo-, Pericardial and Coronary Valve: myocardial infarction
Platelet, Bleeding and Clotting: thrombocytopenia
Neoplasms: basal cell, breast, lymphoma
Psychiatric: confusion, suicide attempt
Red Blood Cell: anemia, hemolytic anemia
Reproductive: menstrual irregularity
Resistance Mechanism: cellulitis, sepsis, serum sickness
Respiratory: adult respiratory distress syndrome, lower respiratory tract infection (including pneumonia), pleu-ral effusion, pleurisy, pulmonary edema, respiratory insufficiency
Skin and Appendages: increased sweating, ulceration
Urinary: renal calculus, renal failure
Vascular (Extracardiac): brain infarction, pulmonary embolism, thrombophlebitis
White Cell and Reticuloendothelial: leukopenia, lymphadenopathy
The following adverse events have been reported during post-approval use of REMICADE: neutropenia (see WARNINGS , Hematologic Events ), interstitial pneumonitis/fibrosis, idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, pericardial effusion, systemic and cutaneous vasculitis, Guillain-Barré syndrome, transverse myelitis, and neuropathies (additional neurologic events have also been observed, see WARNINGS , Neurologic Events ). Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to REMICADE exposure.
OVERDOSAGE
Single doses up to 20 mg/kg have been administered without any direct toxic effect. In case of overdosage, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions or effects and appropriate symptomatic treatment instituted immediately.
DOSAGE AND ADMINISTRATION
Rheumatoid Arthritis
The recommended dose of REMICADE is 3 mg/kg given as an intravenous infusion followed with additional similar doses at 2 and 6 weeks after the first infusion then every 8 weeks thereafter. REMICADE should be given in combination with methotrexate. For patients who have an incomplete response, consideration may be given to adjusting the dose up to 10 mg/kg or treating as often as every 4 weeks bearing in mind that risk of serious infections is increased at higher doses (see ADVERSE REACTIONS, Infections).
Crohn's Disease or Fistulizing Crohn's Disease
The recommended dose of REMICADE is 5 mg/kg given as an induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of moderately to severely active Crohn's disease or fistulizing disease. For patients who respond and then lose their response, consideration may be given to treatment with 10 mg/kg. Patients who do not respond by week 14 are unlikely to respond with continued dosing and consideration should be given to discontinue REMICADE in these patients.
Ankylosing Spondylitis
The recommended dose of REMICADE is 5 mg/kg given as an intravenous infusion followed with additional similar doses at 2 and 6 weeks after the first infusion, then every 6 weeks thereafter.
Psoriatic Arthritis
The recommended dose of REMICADE is 5 mg/kg given as an intravenous infusion followed with additional similar doses at 2 and 6 weeks after the first infusion then every 8 weeks thereafter. REMICADE can be used with or without methotrexate.
Preparation and Administration Instructions
Use aseptic technique.
REMICADE vials do not contain antibacterial preservatives. Therefore, the vials after reconstitution should be used immediately, not re-entered or stored. The diluent to be used for reconstitution is 10 mL of Sterile Water for Injection, USP. The total dose of the reconstituted product must be further diluted to 250 mL with 0.9% Sodium Chloride Injection, USP. The infusion concentration should range between 0.4 mg/mL and 4 mg/mL. The REMICADE infusion should begin within 3 hours of preparation.
- Calculate the dose and the number of REMICADE vials needed. Each REMICADE vial contains 100 mg of infliximab. Calculate the total volume of reconstituted REMICADE solution required.
- Reconstitute each REMICADE vial with 10 mL of Sterile Water for Injection, USP, using a syringe equipped with a 21-gauge or smaller needle. Remove the flip-top from the vial and wipe the top with an alcohol swab. Insert the syringe needle into the vial through the center of the rubber stopper and direct the stream of Sterile Water for Injection, USP, to the glass wall of the vial. Do not use the vial if the vacuum is not present. Gently swirl the solution by rotating the vial to dissolve the lyophilized powder. Avoid prolonged or vigorous agitation. DO NOT SHAKE. Foaming of the solution on reconstitution is not unusual. Allow the reconstituted solution to stand for 5 minutes. The solution should be colorless to light yellow and opalescent, and the solution may develop a few translucent particles as infliximab is a protein. Do not use if opaque particles, discoloration, or other foreign particles are present.
- Dilute the total volume of the reconstituted REMICADE solution dose to 250 mL with 0.9% Sodium Chloride Injection, USP, by withdrawing a volume of 0.9% Sodium Chloride Injection, USP, equal to the volume of reconstituted REMICADE from the 0.9% Sodium Chloride Injection, USP, 250 mL bottle or bag. Slowly add the total volume of reconstituted REMICADE solution to the 250 mL infusion bottle or bag. Gently mix.
- The infusion solution must be administered over a period of not less than 2 hours and must use an infusion set with an in-line, sterile, non-pyrogenic, low-protein-binding filter (pore size of 1.2 µm or less). Any unused portion of the infusion solution should not be stored for reuse.
- No physical biochemical compatibility studies have been conducted to evaluate the coadministration of REMICADE with other agents. REMICADE should not be infused concomitantly in the same intravenous line with other agents.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If visibly opaque particles, discoloration or other foreign particulates are observed, the solution should not be used.
Storage
Store the lyophilized product under refrigeration at 2°C to 8°C (36°F to 46°F). Do not freeze. Do not use beyond the expiration date. This product contains no preservative.
HOW SUPPLIED
REMICADE lyophilized concentrate for IV injection is supplied in individually-boxed single-use vials in the following strength:
NDC 57894-030-01 100 mg infliximab in a 20 mL vial
REFERENCES
- American Thoracic Society, Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-S247.
- Knight DM, Trinh H, Le J, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Molec Immunol 1993;30:1443-1453.
- Scallon BJ, Moore MA, Trinh H, et al. Chimeric anti-TNF(alpha) monoclonal antibody cA2 binds recombinant transmembrane TNF(alpha) and activates immune effector functions. Cytokine 1995;7:251-259.
- ten Hove T, van Montfrans C, Peppelenbosch MP, et al. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease. Gut 2002;50:206-211.
- Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor (alpha) monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41(9):1552-1563.
- Elliott MJ, Maini RN, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) vs. placebo in rheumatoid arthritis. Lancet 1994;344(8930):1105-1110.
- Van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective follow-up of patients with early rheumatoid arthritis. Arthritis Rheum 1992;35(1):26-34.
- Targan SR, Hanauer SR, van Deventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor (alpha) for Crohn's disease. N Engl J Med 1997;337(15):1029-1035.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomized trial. Lancet 2002; 359:1541-1549.
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999;340:1398-1405.
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum . 1984;27(4):361-368.
©Centocor, Inc. 2005
Malvern, PA 19355, USA License #1242
1-800-457-6399Revised May 2005
Rx Only
REMICADE® (infliximab)
Patient Information SheetYou should read this information sheet before you start using REMICADE® (pronounced rem-eh-kaid) and before each time you are scheduled to receive REMICADE. This information sheet does not take the place of talking with your doctor. You and your doctor should talk about your health and how you are feeling before you start taking REMICADE, while you are taking it and at regular checkups. If you do not understand any of the information in this sheet, you should ask your doctor to explain what it means.
What is REMICADE?
REMICADE is a medicine that is used to treat adults with moderately to severely active rheumatoid arthritis and Crohn's disease. In Crohn's disease, REMICADE is for people who have not responded well enough to other medicines. REMICADE is used to treat active ankylosing spondylitis and psoriatic arthritis.
How does REMICADE work?
The medicine REMICADE is a type of protein that recognizes, attaches to and blocks the action of a substance in your body called tumor necrosis factor. Tumor necrosis factor (TNF) is made by certain blood cells in your body. REMICADE will not cure rheumatoid arthritis, Crohn's disease, ankylosing spondylitis or psoriatic arthritis, but blocking TNF with REMICADE may reduce the inflammation caused by TNF in your body. You should also know that REMICADE may help you feel better but can also cause serious side effects and can reduce your body's ability to fight infections (see below).
What should I know about the immune system, and taking REMICADE for Rheumatoid Arthritis, Crohn's Disease, Ankylosing Spondylitis or Psoriatic Arthritis?
The immune system protects the body by responding to "invaders" like bacteria, viruses and other foreign matter that enter your body by producing antibodies and putting them into action to fight off the "invaders." In diseases like rheumatoid arthritis, Crohn's disease, ankylosing spondylitis and psoriatic arthritis, TNF can cause your immune system to attack healthy tissues in your body and cause inflammation and damage. If these diseases are untreated, it can cause permanent damage to the body's bones, cartilage and tissue.
While taking REMICADE can block the TNF that causes inflammation, it can also lower your body's ability to fight infections. So, taking REMICADE can make you more prone to getting infections or it can make an infection that you already have worse. You should call your doctor right away if you think you have an infection.
What important information should I know about treatment with REMICADE?
REMICADE, like other medicines that affect your immune system, is a strong medicine that can cause serious side effects. Possible serious side effects include:
Serious Infections:
- Some patients have had serious infections while receiving REMICADE. Some of the patients have died from these infections. Serious infections include TB (tuberculosis), and infections caused by viruses, fungi or bacteria that have spread throughout the body. If you develop a fever, feel very tired, have a cough, or have flu-like symptoms, these could be signs that you may be getting an infection. If you have any of these symptoms while you are taking or after you have taken REMICADE, you should tell your doctor right away.
Heart Failure:
- If you have been told that you have a heart problem called congestive heart failure and you are currently being treated with REMICADE, you will need to be closely monitored by your doctor. If you develop new or worse symptoms that are related to your heart condition, such as shortness of breath or swelling of your ankles or feet, you must contact your doctor immediately.
Blood Problems:
- In some patients the body may fail to produce enough of the blood cells that help your body fight infections or help you stop bleeding. Some of the patients have died from this failure to produce blood cells. If you develop a fever that doesn't go away, bruise or bleed very easily or look very pale, call your doctor right away. Your doctor may decide to stop your treatment.
Allergic Reactions:
- Some patients have had severe allergic reactions to REMICADE. These reactions can happen while you are getting your REMICADE infusion or shortly afterwards. The symptoms of an allergic reaction may include hives (red, raised, itchy patches of skin), difficulty breathing, chest pain and high or low blood pressure. Your doctor may decide to stop REMICADE treatment and give you medicines to treat the allergic reaction.
- Some patients who have been taking REMICADE for Crohn's disease have had allergic reactions 3 to 12 days after receiving their REMICADE treatment. The symptoms of this type of delayed reaction may include fever, rash, headache and muscle or joint pain. Call your doctor right away if you develop any of these symptoms or any other unusual symptoms such as difficulty swallowing.
Nervous System Disorders:
- There have been rare cases where people taking REMICADE or other TNF blockers have developed disorders that affected their nervous system. Signs that you could be having a problem include: changes in your vision, weakness in your arms and/or legs, and numbness or tingling in any part of your body.
Malignancy
- Reports of a type of blood cancer called lymphoma in patients on REMICADE or other TNF blockers are rare but occur more often than expected for people in general. People who have been treated for rheumatoid arthritis, Crohn's disease, ankylosing spondylitis or psoriatic arthritis for a long time, particularly those with highly active disease may be more prone to develop lymphoma. If you take REMICADE or other TNF blockers, your risk for developing lymphoma may increase. You should also tell your doctor if you have had or develop lymphoma or other cancers while you are taking REMICADE.
Liver Injury
- There have been rare cases where people taking REMICADE have developed serious liver problems, some fatal. Signs that you could be having a problem include: jaundice (skin and eyes turning yellow), dark brown-colored urine, right sided abdominal pain, fever, and severe fatigue (tiredness). You should contact your doctor immediately if you develop any of these symptoms.
Other Important Information
Some patients have developed symptoms that can resemble a disease called lupus. Lupus-like symptoms may include chest discomfort or pain that doesn't go away, shortness of breath, joint pain, or a rash on the cheeks or arms that gets worse in the sun. If you develop any of these symptoms your doctor may decide to stop your treatment with REMICADE.
What are the more common side effects of REMICADE?
The more common side effects with REMICADE are respiratory infections (that may include sinus infections and sore throat), coughing and stomach pain.
Who should not take REMICADE?
YOU SHOULD NOT take REMICADE if you have:
- Heart failure, unless your doctor has talked to you and decided that you are able to take REMICADE.
- Had an allergic reaction to REMICADE or any other product that was made with murine (mouse) proteins.
What health concerns should I talk to my doctor about?
Before receiving your first treatment with REMICADE you should tell your doctor if you:
- Have or think you may have any kind of infection. The infection could be in only one place in your body (such as an open cut or sore), or an infection that affects your whole body (such as the flu). Having an infection could put you at risk for serious side effects from REMICADE.
- Have an infection that won't go away or a history of infection that keeps coming back.
- Have had TB (tuberculosis), or if you have recently been with anyone who might have TB. Your doctor will examine you for TB and perform a skin test. If your doctor feels that you are at risk for TB, he or she may start treating you for TB before you begin REMICADE therapy.
- Have lived in or visited an area of the country where an infection called histoplasmosis or coccidioidomycosis (an infection caused by a fungus that affects the lungs) is common. If you don't know if the area you live in is one where histoplasmosis or coccidioidomycosis is common, ask your doctor.
- Have or have previously had heart failure or other heart conditions.
- Have or have had a condition that affects your nervous system, like multiple sclerosis, or Guillain-Barré syndrome, or if you experience any numbness, or tingling, or have had a seizure.
- Are pregnant or nursing.
- Have recently received or are scheduled to receive a vaccine.
Can I take REMICADE while I am on other medicines?
Tell your doctor if you are taking any other medicines including over the counter medicines, supplements or herbal products before you are treated with REMICADE. If you start taking or plan to start taking any new medicine while you are taking REMICADE, tell your doctor.
REMICADE and KINERET should not be taken together.
How will REMICADE be given to me?
REMICADE will be given to you by a healthcare professional. REMICADE will be given to you by an IV. This means that the medicine will be given to you through a needle placed in a vein in your arm. It will take about 2 hours to give you the full dose of medicine. During that time and for a period after you receive REMICADE, you will be monitored by a healthcare professional. Your doctor may ask you to take other medicines along with REMICADE.
Only a health care professional should prepare the medicine and administer it to you.
How often will I receive REMICADE?
Rheumatoid Arthritis
If you are receiving REMICADE for rheumatoid arthritis you will receive your first dose followed by additional doses at 2 and 6 weeks after the first dose. You will then receive a dose every 8 weeks. Your doctor will monitor your response to REMICADE and may change your dose or treat you more frequently (as often as every 4 weeks).
Crohn's Disease or Fistulizing Crohn's Disease
If you are receiving REMICADE for active Crohn's disease or fistulizing Crohn's disease, you will receive your first dose followed by additional doses at 2 and 6 weeks after the first dose. You will then receive a dose every 8 weeks. Your doctor will monitor your response to REMICADE and may change your dose.
Ankylosing Spondylitis
If you are receiving REMICADE for ankylosing spondylitis you will receive your first dose followed by additional doses at 2 and 6 weeks after the first dose. You will then receive a dose every 6 weeks.
Psoriatic Arthritis
If you are receiving REMICADE for psoriatic arthritis you will receive your first dose followed by additional doses at 2 and 6 weeks after the first dose. You will then receive a dose every 8 weeks.
What if I still have questions?
If you have any questions, or problems, always talk first with your doctor. You can also visit the REMICADE internet site at www.remicade.com
Product developed and manufactured by:
Centocor, Inc.
200 Great Valley Parkway
Malvern, PA 19355
Revised May 2005
Subscribe to the "News" RSS Feed 
TOP ۞
