-
Retisert Implant (Bausch & Lomb)
Description: RETISERT™ (fluocinolone acetonide intravitreal implant) 0.59 mg is a sterile implant designed to release fluocinolone acetonide locally to the posterior segment of the eye at a nominal initial rate of 0.6 µg/day, decreasing over the first month to a steady state between 0.3-0.4 µg/day over approximately 30 months. The drug substance is the synthetic corticosteroid fluocinolone acetonide, represented by the following structural formula:
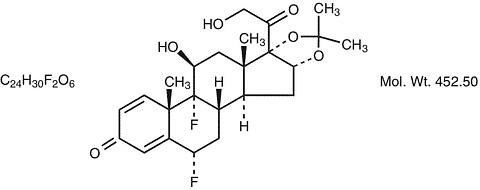
Chemical Name: Pregna-1,4-diene-3,20-dione,6,9-difluoro-11,21-dihydroxy-16,17-[(1-methyl-ethylidene)bis(oxy)]-, (6(alpha) ,11(beta),16(alpha))-.
Fluocinolone acetonide is a white crystalline powder, insoluble in water, and soluble in methanol. It has a melting point of 265-266°C.
Each RETISERT consists of a tablet containing 0.59 mg of the active ingredient, Fluocinolone Acetonide, USP, and the following inactives: microcrystalline cellulose, polyvinyl alcohol, and magnesium stearate.
Clinical Pharmacology: Corticosteroids inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation. There is no generally accepted explanation for the mechanism of action of ocular corticosteroids. However, corticosteroids are thought to act by the induction of phospholipase A 2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2 . Corticosteroids are capable of producing a rise in intraocular pressure.
Pharmacokinetics: In a subset of patients who received the intravitreal implant, and had blood samples taken at various times (weeks 1, 4 and 34) after implantation, plasma levels of fluocinolone acetonide were below the limit of detection (0.2 ng/mL) at all times. Aqueous and vitreous humor samples were assayed for fluocinolone acetonide in a further subset of patients. While detectable concentrations of fluocinolone acetonide were seen throughout the observation interval (up to 34 months), the concentrations were highly variable, ranging from below the limit of detection (0.2 ng/mL) to 589 ng/mL.
Clinical Studies: In two randomized, double-masked, multicenter controlled clinical trials, 227 patients with chronic (a one year or greater history) non-infectious uveitis affecting the posterior segment of one or both eyes who received a 0.59 mg RETISERT were studied. The primary efficacy endpoint in both trials was the rate of recurrence of uveitis affecting the posterior segment of the study eye in the 34 week period post-implantation compared to the rate of recurrence in the 34 week period pre-implantation. The rates of recurrence ranged from approximately 7% (7/108) to 14% (16/116) for the 34 week period post-implantation as compared to approximately 40% (46/116) to 54% (58/108) for the 34 week period pre-implantation.
Indications And Usage: RETISERT is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye.
Contraindications: RETISERT is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infections of the eye and fungal diseases of ocular structures. RETISERT is also contraindicated in individuals with known or suspected hypersensitivity to any of the ingredients of this preparation and to other corticosteroids.
Warnings: As with any surgical procedure there is risk involved. Potential complications accompanying intraocular surgery to place RETISERT into the vitreous cavity may include, but are not limited to, the following: cataract formation, choroidal detachment, temporary decreased visual acuity, endophthalmitis, hypotony, increased intraocular pressure, exacerbation of intraocular inflammation, retinal detachment, vitreous hemorrhage, vitreous loss, and wound dehiscence.
Following implantation of RETISERT, nearly all patients will experience an immediate and temporary decrease in visual acuity in the implanted eye which lasts for approximately one to four weeks post-operatively. This decrease in visual acuity is likely a result of the surgical procedure.
Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision, and in posterior subcapsular cataract formation. Steroids should be used with caution in the presence of glaucoma. Patients must be monitored for elevated IOP.
Based on clinical trials with RETISERT, within 34 weeks post-implantation, approximately 60% of patients will require IOP lowering medications to control intraocular pressure. Within an average post-implantation period of approximately 2 years, approximately 32% of patients are expected to require filtering procedures to control intraocular pressure.
Within an average post-implantation period of approximately 2 years, nearly all phakic eyes are expected to develop cataracts and require cataract surgery.
Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution.
Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions of the eye, steroids may mask infection or enhance existing infection.
The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation.
Precautions:
General
As with all intraocular surgery, sterility of the surgical field and RETISERT should be rigorously maintained.
RETISERT should be handled only by the suture tab in order to avoid damaging the implant since this could affect the release rate of fluocinolone acetonide inside the eye. Care should be taken during implantation and explantation to avoid sheer forces on the implant that could disengage the silicone cup reservoir (which contains a fluocinolone acetonide tablet) from the suture tab. RETISERT should not be resterilized by any method.
RETISERT should be used with caution in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella.
Since resistance to infections is known to be reduced by corticosteroids, simultaneous bilateral implantation should not be carried out, in order to limit the potential for bilateral post-operative infection.
Information for Patients: RETISERT is designed to locally treat inflammation in the eye, but it is not known to treat the underlying disease. Medication to treat the underlying disease may be prescribed concurrently as deemed appropriate by a physician. Patients should be advised to have ophthalmologic follow-up examinations of both eyes at appropriate intervals following implantation of RETISERT.
As with any surgical procedure, there is risk involved. Potential complications accompanying intraocular surgery to place RETISERT into the vitreous cavity may include, but are not limited to, the following: cataract formation, choroidal detachment, temporary decreased visual acuity, endophthalmitis, hypo-tony, increased intraocular pressure, exacerbation of intraocular inflammation, retinal detachment, vitreous hemorrhage, vitreous loss, and wound dehiscence.
Following implantation of RETISERT, nearly all patients will experience an immediate and temporary decrease in visual acuity in the implanted eye which lasts for approximately one to four weeks post-operatively. This decrease in visual acuity is likely a result of the surgical implant procedure.
Based on clinical trials with RETISERT, within 34 weeks post-implantation, approximately 60% of patients will require IOP lowering medications to control intraocular pressure. Within an average post-implantation period of approximately 2 years, approximately 32% of patients are expected to require filtering procedures to control intraocular pressure.
Within an average post-implantation period of approximately 2 years, nearly all phakic eyes are expected to develop cataracts and require cataract surgery.
Carcinogenesis, mutagenesis, impairment of fertility: Long-term animal studies have not been performed on RETISERT to evaluate the carcinogenic potential or the effect on fertility of fluocinolone acetonide.
Fluocinolone acetonide was not genotoxic in vitro in the Ames test, the mouse lymphoma TK assay, or in vivo in the mouse bone marrow micronucleus assay.
Pregnancy: Teratogenic effects: Pregnancy Category C. No adequate animal reproduction studies have been conducted with fluocinolone acetonide.
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Fluocinolone acetonide when administered subcutaneously at a dose of 0.13 mg/kg/day (approximately 10,000 times the daily clinical dose of RETISERT), during Days 6 to 18 of pregnancy in the rabbit, induced abortion at the end of the third and at the beginning of the fourth gestational week. When administered subcutaneously to rats and rabbits during gestation at a maternal toxic dose of 50 µg/kg/day (approximately 4,000 times the clinical dose of RETISERT), fluocinolone acetonide caused abortions and malformations in a few surviving fetuses.
There are no adequate and well-controlled studies in pregnant women. RETISERT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: It is not known whether ocular administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Systemic steroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution should be exercised when RETISERT is implanted in a nursing woman.
Pediatric Use: Safety and effectiveness in pediatric patients below the age of 12 years have not been established.
Geriatric Use: No overall differences in safety and effectiveness have been observed between elderly and younger patients.
Adverse Reactions: Adverse reactions associated with ocular administration of corticosteroids include elevated intraocular pressure with possible development of glaucoma, optic nerve damage, and visual acuity and field defects, posterior subcapsular cataract formation, delayed wound healing, and perforation of the globe where there is thinning of the sclera.
The development of secondary ocular infection (bacterial, fungal, and viral) has occurred after use of ophthalmic steroids. Fungal and viral infections of the cornea are particularly prone to develop coincidentally with long-term applications of steroids. The possibility of fungal invasion should be considered in any persistent corneal ulceration where steroid treatment has been used (see Warnings ).
The most frequently reported ocular adverse events in the overall study population were cataract, increased intraocular pressure, procedural complication, and eye pain. These events occurred in approximately 50-90% of patients. Procedural complication includes cataract fragments in the eye post-op, implant expulsion, injury, mechanical complication of implant, migration of implant, post-op complications, post-op wound complications, and wound dehiscence.
Based on clinical trials with RETISERT, within an average post-implantation period of approximately 2 years, nearly all phakic eyes are expected to develop cataracts and require cataract surgery.
Within 34 weeks post-implantation, approximately 60% of patients will require IOP lowering medications to control intraocular pressure. Within an average post-implantation period of approximately 2 years, approximately 32% of patients are expected to require filtering procedures to control intraocular pressure.
Ocular adverse events occurring in approximately 10-35% of patients include reduced visual acuity, conjunctival hemorrhage, conjunctival hyperemia, glaucoma, blurred vision, abnormal sensation in the eye, eye irritation, hypotony, pruritus, vitreous floaters, macu-lopathy, vitreous hemorrhage, ptosis, eye inflammation, eyelid edema, increased tearing, and dry eye.
Ocular adverse events occurring in approximately 5-9% of patients included macular edema, visual disturbance, eye discharge, conjunctival edema/chemosis, photophobia, blepharitis, corneal edema, photopsia, retinal hemorrhage, choroidal detachment, vitreous opacities, and eye swelling.
The most frequently reported non-ocular adverse event was headache (31%).
Other non-ocular adverse events occurring in approximately 5-15% of patients were nasopharyngitis, arthralgia, sinusitis, dizziness, pyrexia, nausea, cough, influenza, upper respiratory tract infection, vomiting, limb pain, back pain, rash, and pain.
Dosage And Administration: RETISERT is surgically implanted into the posterior segment of the affected eye through a pars plana incision. The implant contains one tablet of 0.59 mg of fluocinolone acetonide. RETISERT is designed to release fluocinolone acetonide at a nominal initial rate of 0.6 µg/day, decreasing over the first month to a steady state between 0.3-0.4 µg/day over approximately 30 months. Following depletion of fluocinolone acetonide from RETISERT as evidenced by recurrence of uveitis, RETISERT may be replaced.
Handling and Disposal: Caution should be exercised in handling RETISERT in order to avoid damage to the implant, which may result in an increased rate of drug release from the implant. Thus, RETISERT should be handled only by the suture tab. Care should be taken during implantation and explantation to avoid sheer forces on the implant that could disengage the silicone cup reservoir (which contains a fluocinolone acetonide tablet) from the suture tab. Aseptic technique should be maintained at all times prior to and during the surgical implantation procedure. RETISERT should not be resterilized by any method.
How Supplied: The implant consists of a tablet encased in a silicone elastomer cup containing a release orifice and a polyvinyl alcohol membrane positioned between the tablet and the orifice. The silicone elastomer cup assembly is attached to a polyvinyl alcohol suture tab with silicone adhesive. Each RETISERT is approximately 3 mm × 2 mm × 5 mm.
Each implant is stored in a clear polycarbonate case within a foil pouch within a Tyvek peelable overwrap. Each packaged implant is provided in a carton which includes the package insert.
0.59 mg RETISERT - NDC 24208-416-01
Storage: Store in the original container at 15° - 25°C (59° - 77°F). Protect from freezing.
Marketed by: Bausch & Lomb Incorporated, Rochester, NY 14609
Manufactured by: Bausch & Lomb Incorporated, Waterford, Ireland
U.S. Patent # 6,217,895, U.S. Patent # 6,548,078
™/® denote trademarks of Bausch & Lomb Incorporated.
© Bausch & Lomb Incorporated
April 2005
Subscribe to the "News" RSS Feed 
TOP ۞
