-
Serevent Diskus (Glaxosmithkline)
WARNING:
Data from a large placebo-controlled US study that compared the safety of salmeterol (SEREVENT® Inhalation Aerosol) or placebo added to usual asthma therapy showed a small but significant increase in asthma-related deaths in patients receiving salmeterol (13 deaths out of 13,176 patients treated for 28 weeks) versus those on placebo (3 of 13,179) (see WARNINGS and CLINICAL TRIALS : Asthma : Salmeterol Multi-center Asthma Research Trial ).
DESCRIPTION
SEREVENT DISKUS (salmeterol xinafoate inhalation powder) contains salmeterol xinafoate as the racemic form of the 1-hydroxy-2-naphthoic acid salt of salmeterol. The active component of the formulation is salmeterol base, a highly selective beta 2 -adrenergic bronchodilator. The chemical name of salmeterol xinafoate is 4-hydroxy-(alpha) 1 -[[[6-(4-phenylbutoxy)hexyl]amino]methyl]-1,3-benzenedimethanol, 1-hydroxy-2-naphthalenecarboxylate.
Salmeterol xinafoate is a white to off-white powder with a molecular weight of 603.8, and the empirical formula is C 25 H 37 NO 4 ·C 11 H 8 O 3 . It is freely soluble in methanol; slightly soluble in ethanol, chloroform, and isopropanol; and sparingly soluble in water.
SEREVENT DISKUS is a specially designed plastic inhalation delivery system containing a double-foil blister strip of a powder formulation of salmeterol xinafoate intended for oral inhalation only. The DISKUS®, which is the delivery component, is an integral part of the drug product. Each blister on the double-foil strip within the unit contains 50 mcg of salmeterol administered as the salmeterol xinafoate salt in 12.5 mg of formulation containing lactose (which contains milk proteins). After a blister containing medication is opened by activating the DISKUS, the medication is dispersed into the airstream created by the patient inhaling through the mouthpiece.
Under standardized in vitro test conditions, SEREVENT DISKUS delivers 47 mcg when tested at a flow rate of 60 L/min for 2 seconds. In adult patients with obstructive lung disease and severely compromised lung function (mean forced expiratory volume in 1 second [FEV 1 ] 20% to 30% of predicted), mean peak inspiratory flow (PIF) through a DISKUS was 82.4 L/min (range, 46.1 to 115.3 L/min).
The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile.
CLINICAL PHARMACOLOGY
Mechanism of Action: Salmeterol is a selective, long-acting beta 2 -adrenergic agonist. In vitro studies and in vivo pharmacologic studies demonstrate that salmeterol is selective for beta 2 -adrenoceptors compared with isoproterenol, which has approximately equal agonist activity on beta 1 - and beta 2 -adrenoceptors. In vitro studies show salmeterol to be at least 50 times more selective for beta 2 -adrenoceptors than albuterol. Although beta 2 -adrenoceptors are the predominant adrenergic receptors in bronchial smooth muscle and beta 1 -adrenoceptors are the predominant receptors in the heart, there are also beta 2 -adrenoceptors in the human heart comprising 10% to 50% of the total beta-adrenoceptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta 2 -agonists may have cardiac effects.
The pharmacologic effects of beta 2 -adrenoceptor agonist drugs, including salmeterol, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3',5'-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
In vitro tests show that salmeterol is a potent and long-lasting inhibitor of the release of mast cell mediators, such as histamine, leukotrienes, and prostaglandin D 2 , from human lung. Salmeterol inhibits histamine-induced plasma protein extravasation and inhibits platelet-activating factor-induced eosinophil accumulation in the lungs of guinea pigs when administered by the inhaled route. In hu-mans, single doses of salmeterol administered via inhalation aerosol attenuate allergen-induced bronchial hyper-responsiveness.
Pharmacokinetics: Salmeterol xinafoate, an ionic salt, dissociates in solution so that the salmeterol and 1-hydroxy-2-naphthoic acid (xinafoate) moieties are absorbed, distributed, metabolized, and excreted independently. Salmeterol acts locally in the lung; therefore, plasma levels do not predict therapeutic effect.
Absorption : Because of the small therapeutic dose, systemic levels of salmeterol are low or undetectable after inhalation of recommended doses (50 mcg of salmeterol inhalation powder twice daily). Following chronic administration of an inhaled dose of 50 mcg of salmeterol inhalation powder twice daily, salmeterol was detected in plasma within 5 to 45 minutes in 7 patients with asthma; plasma concentrations were very low, with mean peak concentrations of 167 pg/mL at 20 minutes and no accumulation with repeated doses.
Distribution : The percentage of salmeterol bound to human plasma proteins averages 96% in vitro over the concentration range of 8 to 7,722 ng of salmeterol base per milliliter, much higher concentrations than those achieved following therapeutic doses of salmeterol.
Metabolism : Salmeterol base is extensively metabolized by hydroxylation, with subsequent elimination predominantly in the feces. No significant amount of unchanged salmeterol base has been detected in either urine or feces.
Elimination : In 2 healthy subjects who received 1 mg of radiolabeled salmeterol (as salmeterol xinafoate) orally, approximately 25% and 60% of the radiolabeled salmeterol was eliminated in urine and feces, respectively, over a period of 7 days. The terminal elimination half-life was about 5.5 hours (1 volunteer only).
The xinafoate moiety has no apparent pharmacologic activity. The xinafoate moiety is highly protein bound (>99%) and has a long elimination half-life of 11 days.
Special Populations : The pharmacokinetics of salmeterol base has not been studied in elderly patients nor in patients with hepatic or renal impairment. Since salmeterol is predominantly cleared by hepatic metabolism, liver function impairment may lead to accumulation of salmeterol in plasma. Therefore, patients with hepatic disease should be closely monitored.
Pharmacodynamics: Inhaled salmeterol, like other beta-adrenergic agonist drugs, can in some patients produce dose-related cardiovascular effects and effects on blood glucose and/or serum potassium (see PRECAUTIONS ). The cardiovascular effects (heart rate, blood pressure) associated with salmeterol inhalation aerosol occur with similar frequency, and are of similar type and severity, as those noted following albuterol administration.
The effects of rising doses of salmeterol and standard inhaled doses of albuterol were studied in volunteers and in patients with asthma. Salmeterol doses up to 84 mcg administered as inhalation aerosol resulted in heart rate increases of 3 to 16 beats/min, about the same as albuterol dosed at 180 mcg by inhalation aerosol (4 to 10 beats/min). Adolescent and adult patients receiving 50-mcg doses of salmeterol inhalation powder (N = 60) underwent continuous electrocardiographic monitoring during two 12-hour periods after the first dose and after 1 month of therapy, and no clinically significant dysrhythmias were noted. Also, pediatric patients receiving 50-mcg doses of salmeterol inhalation powder (N = 67) underwent continuous electrocardiographic monitoring during two 12-hour periods after the first dose and after 3 months of therapy, and no clinically significant dysrhythmias were noted.
In 24-week clinical studies in patients with chronic obstructive pulmonary disease (COPD), the incidence of clinically significant abnormalities on the predose electrocardiograms (ECGs) at Weeks 12 and 24 in patients who received salmeterol 50 mcg was not different compared with placebo.
No effect of treatment with salmeterol 50 mcg was observed on pulse rate and systolic and diastolic blood pressure in a subset of patients with COPD who underwent 12-hour serial vital sign measurements after the first dose (N = 91) and after 12 weeks of therapy (N = 74). Median changes from baseline in pulse rate and systolic and diastolic blood pressure were similar for patients receiving either salmeterol or placebo (see ADVERSE REACTIONS ).
Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of cardiac arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta-agonists and methylxanthines are administered concurrently. The clinical significance of these findings is unknown.
CLINICAL TRIALS
Asthma: During the initial treatment day in several multiple-dose clinical trials with SEREVENT DISKUS in patients with asthma, the median time to onset of clinically significant bronchodilatation (>/=15% improvement in FEV 1 ) ranged from 30 to 48 minutes after a 50-mcg dose.
One hour after a single dose of 50 mcg of SEREVENT DISKUS, the majority of patients had >/=15% improvement in FEV 1 . Maximum improvement in FEV 1 generally occurred within 180 minutes, and clinically significant improvement continued for 12 hours in most patients.
In 2 randomized, double-blind studies, SEREVENT DISKUS was compared with albuterol inhalation aerosol and placebo in adolescent and adult patients with mild-to-moderate asthma (protocol defined as 50% to 80% predicted FEV 1 , actual mean of 67.7% at baseline), including patients who did and who did not receive concurrent inhaled corticosteroids. The efficacy of SEREVENT DISKUS was demonstrated over the 12-week period with no change in effectiveness over this time period (see Figure 1). There were no gender- or age-related differences in safety or efficacy. No development of tachyphylaxis to the bronchodilator effect was noted in these studies. FEV 1 measurements (mean change from baseline) from these two 12-week studies are shown in Figure 1 for both the first and last treatment days.
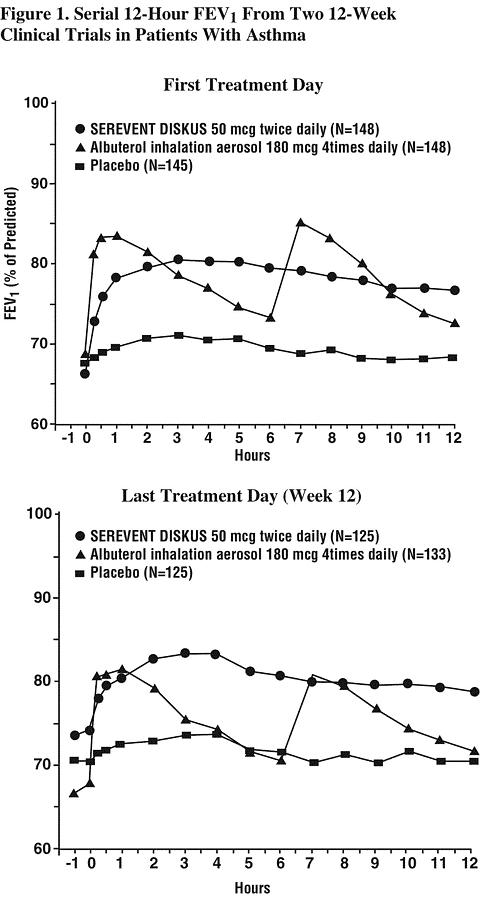
Table 1 shows the treatment effects seen during daily treatment with SEREVENT DISKUS for 12 weeks in adolescent and adult patients with mild-to-moderate asthma.
Table 1. Daily Efficacy Measurements in Two 12-Week Clinical Trials (Combined Data )ParameterTime Placebo SEREVENT
DISKUSAlbuterol
Inhalation
AerosolNo. of randomized subjects152 149 148 Mean AM peak expiratory flow (L/min)baseline
12 weeks394
396395
427 *394
394Mean % days with no asthma symptomsbaseline
12 weeks14
2013
3312
21Mean % nights with no awakeningsbaseline
12 weeks70
7363
85 *68
71Rescue medications (mean no. of inhalations per day)baseline
12 weeks4.2
3.34.3
1.6 **/*4.3
2.2Asthma exacerbations14% 15% 16% * Statistically superior to placebo and albuterol (p<0.001).**/* Statistically superior to placebo (p<0.001).Safe usage with maintenance of efficacy for periods up to 1 year has been documented.
SEREVENT DISKUS and SEREVENT® (salmeterol xinafoate) Inhalation Aerosol were compared to placebo in 2 additional randomized, double-blind clinical trials in adolescent and adult patients with mild-to-moderate asthma. SEREVENT DISKUS 50 mcg and SEREVENT Inhalation Aerosol 42 mcg, both administered twice daily, produced significant improvements in pulmonary function compared with placebo over the 12-week period. While no statistically significant differences were observed between the active treatments for any of the efficacy assessments or safety evaluations performed, there were some efficacy measures on which the metered-dose inhaler appeared to provide better results. Similar findings were noted in 2 randomized, single-dose, crossover comparisons of SEREVENT DISKUS and SEREVENT Inhalation Aerosol for the prevention of exercise-induced bronchospasm (EIB). Therefore, while SEREVENT DISKUS was comparable to SEREVENT Inhalation Aerosol in clinical trials in mild-to-moderate patients with asthma, it should not be assumed that they will produce clinically equivalent outcomes in all patients.
In a randomized, double-blind, controlled study (N = 449), 50 mcg of SEREVENT DISKUS was administered twice daily to pediatric patients with asthma who did and who did not receive concurrent inhaled corticosteroids. The efficacy of salmeterol inhalation powder was demonstrated over the 12-week treatment period with respect to periodic serial peak expiratory flow (PEF) (36% to 39% postdose increase from baseline) and FEV 1 (32% to 33% postdose increase from baseline). Salmeterol was effective in demographic subgroup analyses (gender and age) and was effective when coadministered with other inhaled asthma medications such as short-acting bronchodilators and inhaled corticosteroids. A second randomized, double-blind, placebo-controlled study (N = 207) with 50 mcg of salmeterol inhalation powder via an alternate device supported the findings of the trial with the DISKUS.
Effects in Patients With Asthma on Concomitant Inhaled Corticosteroids: In 4 clinical trials in adult and adolescent patients with asthma (N = 1,922), the effect of adding salmeterol to inhaled corticosteroid therapy was evaluated. The studies utilized the inhalation aerosol formulation of salmeterol xinafoate for a treatment period of 6 months. They compared the addition of salmeterol therapy to an increase (at least doubling) of the inhaled corticosteroid dose.
Two randomized, double-blind, controlled, parallel-group clinical trials (N = 997) enrolled patients (ages 18 to 82 years) with persistent asthma who were previously maintained but not adequately controlled on inhaled corticosteroid therapy. During the 2-week run-in period, all patients were switched to beclomethasone dipropionate 168 mcg twice daily. Patients still not adequately controlled were randomized to either the addition of SEREVENT Inhalation Aerosol 42 mcg twice daily or an increase of beclomethasone dipropionate to 336 mcg twice daily. As compared to the doubled dose of beclomethasone dipropionate, the addition of SEREVENT Inhalation Aerosol resulted in statistically significantly greater improvements in pulmonary function and asthma symptoms, and statistically significantly greater reduction in supplemental albuterol use. The percent of patients who experienced asthma exacerbations overall was not different between groups (i.e., 16.2% in the group receiving SEREVENT Inhalation Aerosol versus 17.9% in the higher dose beclomethasone dipropionate group).
Two randomized, double-blind, parallel-group clinical trials (N = 925) enrolled patients (ages 12 to 78 years) with persistent asthma who were previously maintained but not adequately controlled on prior therapy. During the 2- to 4-week run-in period, all patients were switched to fluticasone propionate 88 mcg twice daily. Patients still not adequately controlled were randomized to either the addition of SEREVENT Inhalation Aerosol 42 mcg twice daily or an increase of fluticasone propionate to 220 mcg twice daily. As compared to the increased (2.5 times) dose of fluticasone propionate, the addition of SEREVENT Inhalation Aerosol resulted in statistically significantly greater improvements in pulmonary function and asthma symptoms, and statistically significantly greater reductions in supplemental albuterol use. Fewer patients receiving SEREVENT Inhalation Aerosol experienced asthma exacerbations than those receiving the higher dose of fluticasone propionate (8.8% versus 13.8%).
Exercise-Induced Bronchospasm: In 2 randomized, single-dose, crossover studies in adolescents and adults with EIB (N = 53), 50 mcg of SEREVENT DISKUS prevented EIB when dosed 30 minutes prior to exercise. For many patients, this protective effect against EIB was still apparent up to 8.5 hours following a single dose.
Table 2. Results of 2 Exercise-Induced Bronchospasm Studies in Adolescents and AdultsPlacebo
(N = 52)SEREVENT DISKUS
(N = 52)n % Total n % Total 0.5-Hour% Fall in FEV 1 postdose<10% 15 29 31 60 exercise>/=10%, <20% 3 6 11 21 challenge>/=20% 34 65 10 19 Mean maximal % fall in FEV 1 (SE)-25% (1.8) -11% (1.9) 8.5-Hour% Fall in FEV 1 postdose<10% 12 23 26 50 exercise>/=10%, <20% 7 13 12 23 challenge>/=20% 33 63 14 27 Mean maximal % fall in FEV 1 (SE)-27% (1.5) -16% (2.0) In 2 randomized studies in children 4 to 11 years old with asthma and EIB (N = 50), a single 50-mcg dose of SEREVENT DISKUS prevented EIB when dosed 30 minutes prior to exercise, with protection lasting up to 11.5 hours in repeat testing following this single dose in many patients.
Salmeterol Multi-center Asthma Research Trial: The Salmeterol Multi-center Asthma Research Trial (SMART) was a randomized, double-blind study that enrolled long-acting beta 2 -agonist-naive patients with asthma (average age of 39 years, 71% Caucasian, 18% African American, 8% Hispanic) to assess the safety of salmeterol (SEREVENT Inhalation Aerosol, 42 mcg twice daily over 28 weeks) compared to placebo when added to usual asthma therapy. The primary endpoint was the combined number of respiratory-related deaths or respiratory-related life-threatening experiences (intubation and mechanical ventilation). Secondary endpoints included combined asthma-related deaths or life-threatening experiences and asthma-related deaths. A planned interim analysis was conducted when approximately half of the intended number of patients had been enrolled (N = 26,355).
Due to the low rate of primary events in the study, the findings of the planned interim analysis were not conclusive. However, analyses of secondary endpoints suggested that patients receiving salmeterol may be at increased risk for some of these events compared to patients receiving placebo. The analysis for the total population showed a relative risk of 1.40 (95% CI 0.91, 2.14) for the primary endpoint in the salmeterol group relative to the placebo group (50 out of 13,176 vs. 36 out of 13,179, respectively). In the total population, a higher number of asthma-related deaths (13 vs. 3, RR 4.37, 95% CI 1.25, 15.34) and combined asthma-related deaths or life-threatening experiences (37 vs. 22, RR 1.71, 95% CI 1.01, 2.89) occurred in patients treated with salmeterol than those treated with placebo. The analysis of the African American subgroup showed a relative risk of 4.10 (95% CI 1.54, 10.90) for the primary endpoint in patients treated with salmeterol relative to those treated with placebo (20 out of 2,366 vs. 5 out of 2,319, respectively). In African Americans, a higher number of asthma-related deaths (7 vs. 1, RR 7.26, 95% CI 0.89, 58.94) and combined asthma-related deaths or life-threatening experiences (19 vs. 4, RR 4.92, 95% CI 1.68, 14.45) occurred in patients treated with salmeterol than those treated with placebo. Analysis of the Caucasian population showed a relative risk of 1.05 (95% CI 0.62, 1.76) for the primary endpoint for those treated with salmeterol relative to those treated with placebo (29 out of 9,281 vs. 28 out of 9,361, respectively). In Caucasians, a higher number of asthma-related deaths (6 vs. 1, RR 5.82, 95% CI 0.70, 48.37) occurred in patients treated with salmeterol than in patients treated with placebo. In Caucasians, the relative risk was 1.08 (17 vs. 16, 95% CI 0.55, 2.14) for combined asthma-related deaths or life-threatening experiences in patients treated with salmeterol relative to placebo. The numbers of patients from other ethnic groups were too small to draw any conclusions in these populations. Even though SMART did not reach predetermined stopping criteria for the total population, the study was stopped due to the findings in African American patients and difficulties in enrollment.
Chronic Obstructive Pulmonary Disease: In 2 clinical trials evaluating twice-daily treatment with SEREVENT DISKUS 50 mcg (N = 336) compared to placebo (N = 366) in patients with chronic bronchitis with airflow limitation, with or without emphysema, improvements in pulmonary function endpoints were greater with salmeterol 50 mcg than with placebo. Treatment with SEREVENT DISKUS did not result in significant improvements in secondary endpoints assessing COPD symptoms in either clinical trial. Both trials were randomized, double-blind, parallel-group studies of 24 weeks' duration and were identical in design, patient entrance criteria, and overall conduct.
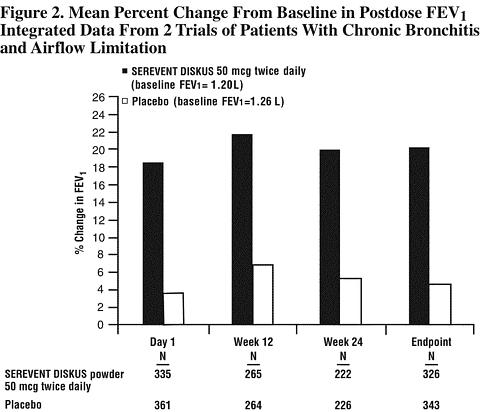
Figure 2 displays the integrated 2-hour postdose FEV 1 results from the 2 clinical trials. The percent change in FEV 1 refers to the change from baseline, defined as the predose value on Treatment Day 1. To account for patient withdrawals during the study, Endpoint (last evaluable FEV 1 ) data are provided. Patients receiving SEREVENT DISKUS 50 mcg had significantly greater improvements in 2-hour postdose FEV 1 at Endpoint (216 mL, 20%) compared to placebo (43 mL, 5%). Improvement was apparent on the first day of treatment and maintained throughout the 24 weeks of treatment.
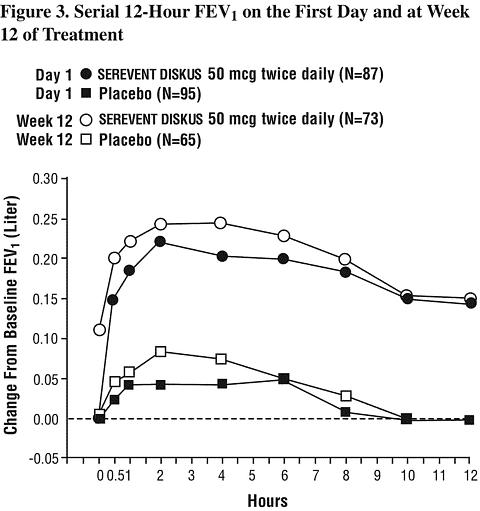
Onset of Action and Duration of Effect : The onset of action and duration of effect of SEREVENT DISKUS were evaluated in a subset of patients (n = 87) from 1 of the 2 clinical trials discussed above. Following the first 50-mcg dose, significant improvement in pulmonary function (mean FEV 1 increase of 12% or more and at least 200 mL) occurred at 2 hours. The mean time to peak bronchodilator effect was 4.75 hours. As seen in Figure 3, evidence of bronchodilatation was seen throughout the 12-hour period. Figure 3 also demonstrates that the bronchodilating effect after 12 weeks of treatment was similar to that observed after the first dose. The mean time to peak bronchodilator effect after 12 weeks of treatment was 3.27 hours.
INDICATIONS AND USAGE
Asthma: SEREVENT DISKUS is indicated for long-term, twice-daily (morning and evening) administration in the maintenance treatment of asthma and in the prevention of bronchospasm in patients 4 years of age and older with reversible obstructive airway disease, including patients with symptoms of nocturnal asthma, who require regular treatment with inhaled, short-acting beta 2 -agonists. It is not indicated for patients whose asthma can be managed by occasional use of inhaled, short-acting beta 2 -agonists.
SEREVENT DISKUS is also indicated for prevention of exercise-induced bronchospasm in patients 4 years of age and older.
SEREVENT DISKUS may be used alone or in combination with inhaled or systemic corticosteroid therapy.
Chronic Obstructive Pulmonary Disease: SEREVENT DISKUS is indicated for the long-term, twice-daily (morning and evening) administration in the maintenance treatment of bronchospasm associated with COPD (including emphysema and chronic bronchitis).
CONTRAINDICATIONS
SEREVENT DISKUS is contraindicated in patients with a history of hypersensitivity to salmeterol or any other component of the drug product (see DESCRIPTION and ADVERSE REACTIONS : Observed During Clinical Practice : Non-Site Specific ).
WARNINGS
DATA FROM A LARGE PLACEBO-CONTROLLED SAFETY STUDY THAT WAS STOPPED EARLY SUGGEST THAT SALMETEROL MAY BE ASSOCIATED WITH RARE SERIOUS ASTHMA EPISODES OR ASTHMA-RELATED DEATHS. Data from this study, called the Salmeterol Multi-center Asthma Research Trial (SMART), further suggest that the risk might be greater in African American patients. These results led to stopping the study prematurely (see CLINICAL TRIALS : Asthma : Salmeterol Multi-center Asthma Research Trial ). The data from the SMART study are not adequate to determine whether concurrent use of inhaled corticosteroids provides protection from this risk. Given the similar basic mechanisms of action of beta 2 -agonists, it is possible that the findings seen in the SMART study may be consistent with a class effect.
Findings similar to the SMART study findings were reported in a prior 16-week clinical study performed in the United Kingdom, the Salmeterol Nationwide Surveillance (SNS) study. In the SNS study, the incidence of asthma-related death was numerically, though not statistically, greater in patients with asthma treated with salmeterol (42 mcg twice daily) versus albuterol (180 mcg 4 times daily) added to usual asthma therapy.
SEREVENT DISKUS SHOULD NOT BE INITIATED IN PATIENTS WITH SIGNIFICANTLY WORSENING OR ACUTELY DETERIORATING ASTHMA, WHICH MAY BE A LIFE-THREATENING CONDITION. Serious acute respiratory events, including fatalities, have been reported both in the United States and worldwide when SEREVENT has been initiated in this situation.
Although it is not possible from these reports to determine whether SEREVENT contributed to these adverse events or simply failed to relieve the deteriorating asthma, the use of SEREVENT DISKUS in this setting is inappropriate.
SEREVENT DISKUS SHOULD NOT BE USED TO TREAT ACUTE SYMPTOMS. It is crucial to inform patients of this and prescribe an inhaled, short-acting beta 2 -agonist for this purpose as well as warn them that increasing inhaled beta 2 -agonist use is a signal of deteriorating asthma.
SEREVENT DISKUS IS NOT A SUBSTITUTE FOR INHALED OR ORAL CORTICOSTEROIDS. Corticosteroids should not be stopped or reduced when SEREVENT DISKUS is initiated.
(See PRECAUTIONS : Information for Patients and the Patient's Instructions for Use accompanying the product.)
- Do Not Introduce SEREVENT DISKUS as a Treatment for Acutely Deteriorating Asthma: SEREVENT DISKUS is intended for the maintenance treatment of asthma (see INDICATIONS AND USAGE ) and should not be introduced in acutely deteriorating asthma, which is a potentially life-threatening condition. There are no data demonstrating that SEREVENT DISKUS provides greater efficacy than or additional efficacy to inhaled, short-acting beta 2 -agonists in patients with worsening asthma. Serious acute respiratory events, including fatalities, have been reported both in the United States and worldwide in patients receiving SEREVENT. In most cases, these have occurred in patients with severe asthma (e.g., patients with a history of corticosteroid dependence, low pulmonary function, intubation, mechanical ventilation, frequent hospitalizations, or previous life-threatening acute asthma exacerbations) and/or in some patients in whom asthma has been acutely deteriorating (e.g., unresponsive to usual medications; increasing need for inhaled, short-acting beta 2 -agonists; increasing need for systemic corticosteroids; significant increase in symptoms; recent emergency room visits; sudden or progressive deterioration in pulmonary function). However, they have occurred in a few patients with less severe asthma as well. It was not possible from these reports to determine whether SEREVENT contributed to these events or simply failed to relieve the deteriorating asthma.
-
Do Not Use SEREVENT DISKUS to Treat Acute Symptoms:
An inhaled, short-acting beta
2
-agonist, not SEREVENT DISKUS, should be used to relieve acute asthma or COPD symptoms. When prescribing SEREVENT DISKUS, the physician must also provide the patient with an inhaled, short-acting beta
2
-agonist (e.g., albuterol) for treatment of symptoms that occur acutely, despite regular twice-daily (morning and evening) use of SEREVENT DISKUS.
When beginning treatment with SEREVENT DISKUS, patients who have been taking inhaled, short-acting beta 2 -agonists on a regular basis (e.g., 4 times a day) should be instructed to discontinue the regular use of these drugs and use them only for symptomatic relief of acute asthma or COPD symptoms (see PRECAUTIONS : Information for Patients ). - Watch for Increasing Use of Inhaled, Short-Acting Beta 2 -Agonists, Which Is a Marker of Deteriorating Asthma or COPD: The patient's condition may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient's inhaled, short-acting beta 2 -agonist becomes less effective, the patient needs more inhalations than usual, or the patient develops a significant decrease in PEF or lung function, these may be markers of destabilization of their disease. In this setting, the patient requires immediate reevaluation with reassessment of the treatment regimen, giving special consideration to the possible need for corticosteroids. If the patient uses 4 or more inhalations per day of an inhaled, short-acting beta 2 -agonist for 2 or more consecutive days, or if more than 1 canister (200 inhalations per canister) of inhaled, short-acting beta 2 -agonist is used in an 8-week period in conjunction with SEREVENT DISKUS, then the patient should consult the physician for reevaluation. Increasing the daily dosage of SEREVENT DISKUS in this situation is not appropriate. SEREVENT DISKUS should not be used more frequently than twice daily (morning and evening) at the recommended dose of 1 inhalation.
- Do Not Use SEREVENT DISKUS as a Substitute for Oral or Inhaled Corticosteroids: The use of beta-adrenergic agonist bronchodilators alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents, e.g., corticosteroids. There are no data demonstrating that SEREVENT DISKUS has a clinical anti-inflammatory effect and could be expected to take the place of corticosteroids. Patients who already require oral or inhaled corticosteroids for treatment of asthma should be continued on a suitable dose to maintain clinical stability even if they feel better as a result of initiating SEREVENT DISKUS. Any change in corticosteroid dosage should be made ONLY after clinical evaluation (see PRECAUTIONS : Information for Patients ).
- Do Not Exceed Recommended Dosage: As with other inhaled beta 2 -adrenergic drugs, SEREVENT DISKUS should not be used more often or at higher doses than recommended. Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Large doses of inhaled or oral salmeterol (12 to 20 times the recommended dose) have been associated with clinically significant prolongation of the QTc interval, which has the potential for producing ventricular arrhythmias.
- Paradoxical Bronchospasm: As with other inhaled asthma and COPD medications, SEREVENT DISKUS can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with SEREVENT DISKUS, it should be treated with a short-acting, inhaled bronchodilator; SEREVENT DISKUS should be discontinued immediately; and alternative therapy should be instituted.
- Immediate Hypersensitivity Reactions: Immediate hypersensitivity reactions may occur after administration of SEREVENT DISKUS, as demonstrated by cases of urticaria, angioedema, rash, and bronchospasm.
- Upper Airway Symptoms: Symptoms of laryngeal spasm, irritation, or swelling, such as stridor and choking, have been reported in patients receiving SEREVENT DISKUS.
- Cardiovascular Disorders: SEREVENT DISKUS, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension. SEREVENT DISKUS, like all other beta-adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of SEREVENT DISKUS at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce ECG changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown.
PRECAUTIONS
General: 1. Cardiovascular and Other Effects: No effect on the cardiovascular system is usually seen after the administration of inhaled salmeterol at recommended doses, but the cardiovascular and central nervous system effects seen with all sympathomimetic drugs (e.g., increased blood pressure, heart rate, excitement) can occur after use of salmeterol and may require discontinuation of SEREVENT DISKUS. SEREVENT DISKUS, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension; in patients with convulsive disorders or thyrotoxicosis; and in patients who are unusually responsive to sympathomimetic amines.
As has been described with other beta-adrenergic agonist bronchodilators, clinically significant changes in systolic and/or diastolic blood pressure, pulse rate, and ECGs have been seen infrequently in individual patients in controlled clinical studies with salmeterol.
2. Metabolic Effects: Doses of the related beta 2 -adrenoceptor agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis. Beta-adrenergic agonist medications may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually transient, not requiring supplementation.
Clinically significant changes in blood glucose and/or serum potassium were seen rarely during clinical studies with long-term administration of SEREVENT DISKUS at recommended doses.
Information for Patients: Patients being treated with SEREVENT DISKUS should receive the following information and instructions. This information is intended to aid them in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
It is important that patients understand how to use the DISKUS appropriately and how to use SEREVENT DISKUS in relation to other asthma or COPD medications they are taking. Patients should be given the following information:
- The action of SEREVENT DISKUS may last up to 12 hours or longer. The recommended dosage (1 inhalation twice daily, morning and evening) should not be exceeded.
- Most patients are able to taste or feel a dose delivered from SEREVENT DISKUS. However, whether or not patients are able to sense delivery of a dose, you should instruct them not to exceed the recommended dose of 1 inhalation twice daily, morning and evening. You should instruct them to contact you or the pharmacist if they have questions.
- SEREVENT DISKUS is not meant to relieve acute asthma or COPD symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting bronchodilator (the physician should provide the patient with such medication and instruct the patient in how it should be used).
- Patients should not stop therapy with SEREVENT DISKUS for asthma or COPD without physician/provider guidance since symptoms may worsen after discontinuation.
-
·When used for the treatment of EIB, 1 inhalation of SEREVENT DISKUS should be taken 30 minutes before exercise.
- Additional doses of SEREVENT should not be used for 12 hours.
- Patients who are receiving SEREVENT DISKUS twice daily should not use additional SEREVENT for prevention of EIB.
-
The physician should be notified immediately if any of the following situations occur, which may be a sign of seriously worsening asthma or COPD:
- Decreasing effectiveness of inhaled, short-acting beta 2 -agonists
- Need for more inhalations than usual of inhaled, short-acting beta 2 -agonists
- Significant decrease in PEF or lung function as outlined by the physician
- Use of 4 or more inhalations per day of a short-acting beta 2 -agonist for 2 or more days consecutively
- Use of more than 1 canister (200 inhalations per canister) of an inhaled, short-acting beta 2 -agonist in an 8-week period.
- SEREVENT DISKUS should not be used as a substitute for oral or inhaled corticosteroids. The dosage of these medications should not be changed and they should not be stopped without consulting the physician, even if the patient feels better after initiating treatment with SEREVENT DISKUS.
- Patients should be cautioned regarding adverse effects associated with beta 2 -agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness.
- When patients are prescribed SEREVENT DISKUS, other medications for asthma and COPD should be used only as directed by the physician.
- SEREVENT DISKUS should not be used with a spacer device.
- Patients who are pregnant or nursing should contact the physician about the use of SEREVENT DISKUS.
-
Effective and safe use of SEREVENT DISKUS includes an understanding of the way that it should be used:
- Never exhale into the DISKUS.
- Never attempt to take the DISKUS apart.
- Always activate and use the DISKUS in a level, horizontal position.
- Never wash the mouthpiece or any part of the DISKUS. KEEP IT DRY.
- Always keep the DISKUS in a dry place.
- Discard 6 weeks after removal from the moisture-protective foil overwrap pouch or after all blisters have been used (when the dose indicator reads "0"), whichever comes first.
- For the proper use of SEREVENT DISKUS and to attain maximum benefit, the patient should read and follow carefully the Patient's Instructions for Use accompanying the product.
Drug Interactions: Short-Acting Beta 2 -Agonists: In two 12-week, repetitive-dose adolescent and adult clinical trials in patients with asthma (N = 149), the mean daily need for additional beta 2 -agonist in patients using SEREVENT DISKUS was approximately 1 ½ inhalations/day. Twenty-six percent (26%) of the patients in these trials used between 8 and 24 inhalations of short-acting beta-agonist per day on 1 or more occasions. Nine percent (9%) of the patients in these trials averaged over 4 inhalations/day over the course of the 12-week trials. No increase in frequency of cardiovascular events was observed among the 3 patients who averaged 8 to 11 inhalations/day; however, the safety of concomitant use of more than 8 inhalations/day of short-acting beta 2 -agonist with SEREVENT DISKUS has not been established. In 29 patients who experienced worsening of asthma while receiving SEREVENT DISKUS during these trials, albuterol therapy administered via either nebulizer or inhalation aerosol (1 dose in most cases) led to improvement in FEV 1 and no increase in occurrence of cardiovascular adverse events.
In 2 clinical trials in patients with COPD, the mean daily need for additional beta 2 -agonist for patients using SEREVENT DISKUS was approximately 4 inhalations/day. Twenty-four percent (24%) of the patients using SEREVENT DISKUS in these trials averaged 6 or more inhalations of albuterol per day over the course of the 24-week trials. No increase in frequency of cardiovascular events was observed among patients who averaged 6 or more inhalations per day.
Monoamine Oxidase Inhibitors and Tricyclic Antidepressants: Salmeterol should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, because the action of salmeterol on the vascular system may be potentiated by these agents.
Corticosteroids and Cromoglycate: In clinical trials, inhaled corticosteroids and/or inhaled cromolyn sodium did not alter the safety profile of salmeterol when administered concurrently.
Methylxanthines: The concurrent use of intravenously or orally administered methylxanthines (e.g., aminophylline, theophylline) by patients receiving salmeterol has not been completely evaluated. In 1 clinical asthma trial, 87 patients receiving SEREVENT Inhalation Aerosol 42 mcg twice daily concurrently with a theophylline product had adverse event rates similar to those in 71 patients receiving SEREVENT Inhalation Aerosol without theophylline. Resting heart rates were slightly higher in the patients on theophylline but were little affected by therapy with SEREVENT Inhalation Aerosol.
In 2 clinical trials in patients with COPD, 39 subjects receiving SEREVENT DISKUS concurrently with a theophylline product had adverse event rates similar to those in 302 patients receiving SEREVENT DISKUS without theophylline. Based on the available data, the concomitant administration of methylxanthines with SEREVENT DISKUS did not alter the observed adverse event profile.
Beta-Adrenergic Receptor Blocking Agents: Beta-blockers not only block the pulmonary effect of beta-agonists, such as SEREVENT DISKUS, but may also produce severe bronchospasm in patients with asthma or COPD. Therefore, patients with asthma or COPD should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents in patients with asthma or COPD. In this setting, cardioselective beta-blockers could be considered, although they should be administered with caution.
Diuretics: The ECG changes and/or hypokalemia that may result from the administration of nonpotassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of beta-agonists with nonpotassium-sparing diuretics.
Carcinogenesis, Mutagenesis, Impairment of Fertility: In an 18-month oral carcinogenicity study in CD-mice, salmeterol xinafoate caused a dose-related increase in the incidence of smooth muscle hyperplasia, cystic glandular hyperplasia, leiomyomas of the uterus, and ovarian cysts at doses of 1.4 mg/kg and above (approximately 20 times the maximum recommended daily inhalation dose in adults and children based on comparison of the area under the plasma concentration versus time curves [AUCs]). The incidence of leiomyosarcomas was not statistically significant. No tumors were seen at 0.2 mg/kg (approximately 3 times the maximum recommended daily inhalation doses in adults and children based on comparison of the AUCs).
In a 24-month oral and inhalation carcinogenicity study in Sprague Dawley rats, salmeterol caused a dose-related increase in the incidence of mesovarian leiomyomas and ovarian cysts at doses of 0.68 mg/kg and above (approximately 55 times the maximum recommended daily inhalation dose in adults and approximately 25 times the maximum recommended daily inhalation dose in children on a mg/m 2 basis). No tumors were seen at 0.21 mg/kg (approximately 15 times the maximum recommended daily inhalation dose in adults and approximately 8 times the maximum recommended daily inhalation dose in children on a mg/m 2 basis). These findings in rodents are similar to those reported previously for other beta-adrenergic agonist drugs. The relevance of these findings to human use is unknown.
Salmeterol produced no detectable or reproducible increases in microbial and mammalian gene mutation in vitro. No clastogenic activity occurred in vitro in human lymphocytes or in vivo in a rat micronucleus test. No effects on fertility were identified in male and female rats treated with salmeterol at oral doses up to 2 mg/kg (approximately 160 times the maximum recommended daily inhalation dose in adults on a mg/m 2 basis).
Pregnancy: Teratogenic Effects: Pregnancy Category C. No teratogenic effects occurred in rats at oral doses up to 2 mg/kg (approximately 160 times the maximum recommended daily inhalation dose in adults on a mg/m 2 basis). In pregnant Dutch rabbits administered oral doses of 1 mg/kg and above (approximately 50 times the maximum recommended daily inhalation dose in adults based on comparison of the AUCs), salmeterol exhibited fetal toxic effects characteristically resulting from beta-adrenoceptor stimulation. These included precocious eyelid openings, cleft palate, sternebral fusion, limb and paw flexures, and delayed ossification of the frontal cranial bones. No significant effects occurred at an oral dose of 0.6 mg/kg (approximately 20 times the maximum recommended daily inhalation dose in adults based on comparison of the AUCs).
New Zealand White rabbits were less sensitive since only delayed ossification of the frontal bones was seen at an oral dose of 10 mg/kg (approximately 1,600 times the maximum recommended daily inhalation dose in adults on a mg/m 2 basis). Extensive use of other beta-agonists has provided no evidence that these class effects in animals are relevant to their use in humans. There are no adequate and well-controlled studies with SEREVENT DISKUS in pregnant women. SEREVENT DISKUS should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Salmeterol xinafoate crossed the placenta following oral administration of 10 mg/kg to mice and rats (approximately 410 and 810 times, respectively, the maximum recommended daily inhalation dose in adults on a mg/m 2 basis).
Use in Labor and Delivery: There are no well-controlled human studies that have investigated effects of salmeterol on preterm labor or labor at term. Because of the potential for beta-agonist interference with uterine contractility, use of SEREVENT DISKUS during labor should be restricted to those patients in whom the benefits clearly outweigh the risks.
Nursing Mothers: Plasma levels of salmeterol after inhaled therapeutic doses are very low. In rats, salmeterol xinafoate is excreted in the milk. However, since there are no data from controlled trials on the use of salmeterol by nursing mothers, a decision should be made whether to discontinue nursing or to discontinue SEREVENT DISKUS, taking into account the importance of SEREVENT DISKUS to the mother. Caution should be exercised when SEREVENT DISKUS is administered to a nursing woman.
Pediatric Use: The safety and efficacy of SEREVENT DISKUS has been evaluated in over 2,500 patients aged 4 to 11 years with asthma, 346 of whom were administered SEREVENT DISKUS for 1 year. Based on available data, no adjustment of dosage of SEREVENT DISKUS in pediatric patients is warranted for either asthma or EIB (see DOSAGE AND ADMINISTRATION ).
In 2 randomized, double-blind, controlled clinical trials of 12 weeks' duration, SEREVENT DISKUS 50-mcg was administered to 211 pediatric patients with asthma who did and who did not receive concurrent inhaled corticosteroids. The efficacy of SEREVENT DISKUS was demonstrated over the 12-week treatment period with respect to PEF and FEV 1 . SEREVENT DISKUS was effective in demographic subgroups (gender and age) of the population. SEREVENT DISKUS was effective when coadministered with other inhaled asthma medications, such as short-acting bronchodilators and inhaled corticosteroids. SEREVENT DISKUS was well tolerated in the pediatric population, and there were no safety issues identified specific to the administration of SEREVENT DISKUS to pediatric patients.
In 2 randomized studies in children 4 to 11 years old with asthma and EIB, a single 50-mcg dose of SEREVENT DISKUS prevented EIB when dosed 30 minutes prior to exercise, with protection lasting up to 11.5 hours in repeat testing following this single dose in many patients.
Geriatric Use: Of the total number of adolescent and adult patients with asthma who received SEREVENT DISKUS in chronic dosing clinical trials, 209 were 65 years of age and older. Of the total number of patients with COPD who received SEREVENT DISKUS in chronic dosing clinical trials, 167 were 65 years of age or older and 45 were 75 years of age or older. No apparent differences in the safety of SEREVENT DISKUS were observed when geriatric patients were compared with younger patients in clinical trials. As with other beta 2 -agonists, however, special caution should be observed when using SEREVENT DISKUS in geriatric patients who have concomitant cardiovascular disease that could be adversely affected by this class of drug. Data from the trials in patients with COPD suggested a greater effect on FEV 1 of SEREVENT DISKUS in the <65 years age-group, as compared with the >/=65 years age-group. However, based on available data, no adjustment of dosage of SEREVENT DISKUS in geriatric patients is warranted.
ADVERSE REACTIONS
Adverse reactions to salmeterol are similar in nature to reactions to other selective beta 2 -adrenoceptor agonists, i.e., tachycardia; palpitations; immediate hypersensitivity reactions, including urticaria, angioedema, rash, bronchospasm (see WARNINGS ); headache; tremor; nervousness; and paradoxical bronchospasm (see WARNINGS ).
Asthma: Two multicenter, 12-week, controlled studies have evaluated twice-daily doses of SEREVENT DISKUS in patients 12 years of age and older with asthma. Table 3 reports the incidence of adverse events in these 2 studies.
Table 3. Adverse Event Incidence in Two 12-Week Adolescent and Adult Clinical Trials in Patients With AsthmaPercent of Patients Adverse EventPlacebo
(N = 152)SEREVENT
DISKUS
50 mcg Twice Daily
(N = 149)Albuterol
Inhalation Aerosol
180 mcg 4 Times Daily
(N = 150)Ear, nose, and throatNasal/sinus congestion, pallor6 9 8 Rhinitis4 5 4 NeurologicalHeadache9 13 12 RespiratoryAsthma1 3 <1 Tracheitis/bronchitis4 7 3 Influenza2 5 5 Table 3 includes all events (whether considered drug-related or nondrug-related by the investigator) that occurred at a rate of 3% or greater in the group receiving SEREVENT DISKUS and were more common than in the placebo group.
Pharyngitis, sinusitis, upper respiratory tract infection, and cough occurred at >/=3% but were more common in the placebo group. However, throat irritation has been described at rates exceeding that of placebo in other controlled clinical trials.
Other adverse events that occurred in the group receiving SEREVENT DISKUS in these studies with an incidence of 1% to 3% and that occurred at a greater incidence than with placebo were:
Ear, Nose, and Throat: Sinus headache.
Gastrointestinal: Nausea.
Mouth and Teeth: Oral mucosal abnormality.
Musculoskeletal: Pain in joint.
Neurological: Sleep disturbance, paresthesia.
Skin: Contact dermatitis, eczema.
Miscellaneous: Localized aches and pains, pyrexia of unknown origin.
Two multicenter, 12-week, controlled studies have evaluated twice-daily doses of SEREVENT DISKUS in patients aged 4 to 11 years with asthma. Table 4 includes all events (whether considered drug-related or nondrug-related by the investigator) that occurred at a rate of 3% or greater in the group receiving SEREVENT DISKUS and were more common than in the placebo group.
Table 4. Adverse Event Incidence in Two 12-Week Pediatric Clinical Trials in Patients With AsthmaPercent of Patients Adverse EventPlacebo
(N = 215)SEREVENT
DISKUS
50 mcg Twice Daily
(N = 211)Albuterol Inhalation
Powder
200 mcg 4 Times Daily
(N = 115)Ear, nose, and throatEar signs and symptoms3 4 9 Pharyngitis3 6 3 NeurologicalHeadache14 17 20 RespiratoryAsthma2 4 <1 SkinSkin rashes3 4 2 Urticaria0 3 2 The following events were reported at an incidence of 1% to 2% (3 to 4 patients) in the salmeterol group and with a higher incidence than in the albuterol and placebo groups: gastrointestinal signs and symptoms, lower respiratory signs and symptoms, photodermatitis, and arthralgia and articular rheumatism.
In clinical trials evaluating concurrent therapy of salmeterol with inhaled corticosteroids, adverse events were consistent with those previously reported for salmeterol, or might otherwise be expected with the use of inhaled corticosteroids.
Chronic Obstructive Pulmonary Disease: Two multicenter, 24-week, controlled studies have evaluated twice-daily doses of SEREVENT DISKUS in patients with COPD. For presentation (Table 5), the placebo data from a third trial, identical in design, patient entrance criteria, and overall conduct but comparing fluticasone propionate with placebo, were integrated with the placebo data from these 2 studies (total N = 341 for salmeterol and 576 for placebo).
Table 5. Adverse Events With >/=3% Incidence in US Controlled Clinical Trials With SEREVENT DISKUS in Patients With Chronic Obstructive Pulmonary Disease *Percent of Patients Adverse EventPlacebo
(N = 576)SEREVENT DISKUS
50 mcg Twice Daily
(N = 341)CardiovascularHypertension2 4 Ear, nose, and throatThroat irritation6 7 Nasal congestion/blockage3 4 Sinusitis2 4 Ear signs and symptoms1 3 GastrointestinalNausea and vomiting3 3 Lower respiratoryCough4 5 Rhinitis2 4 Viral respiratory infection4 5 MusculoskeletalMusculoskeletal pain10 12 Muscle cramps and spasms1 3 NeurologicalHeadache11 14 Dizziness2 4 Average duration of exposure (days)128.9 138.5 *Table 5 includes all events (whether considered drug-related or nondrug-related by the investigator) that occurred at a rate of 3% or greater in the group receiving SEREVENT DISKUS and were more common in the group receiving SEREVENT DISKUS than in the placebo group.Other events occurring in the group receiving SEREVENT DISKUS that occurred at a frequency of 1% to <3% and were more common than in the placebo group were as follows:
Endocrine and Metabolic: Hyperglycemia.
Eye: Keratitis and conjunctivitis.
Gastrointestinal: Candidiasis mouth/throat, dyspeptic symptoms, hyposalivation, dental discomfort and pain, gastrointestinal infections.
Lower Respiratory: Lower respiratory signs and symptoms.
Musculoskeletal: Arthralgia and articular rheumatism; muscle pain; bone and skeletal pain; musculoskeletal inflammation; muscle stiffness, tightness, and rigidity.
Neurology: Migraines.
Non-Site Specific: Pain, edema and swelling.
Psychiatry: Anxiety.
Skin: Skin rashes.
Observed During Clinical Practice: In addition to adverse events reported from clinical trials, the following events have been identified during postapproval use of salmeterol. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to salmeterol or a combination of these factors.
In extensive US and worldwide postmarketing experience with salmeterol, serious exacerbations of asthma, including some that have been fatal, have been reported. In most cases, these have occurred in patients with severe asthma and/or in some patients in whom asthma has been acutely deteriorating (see WARNINGS no. 1), but they have also occurred in a few patients with less severe asthma. It was not possible from these reports to determine whether salmeterol contributed to these events or simply failed to relieve the deteriorating asthma.
Respiratory: Reports of upper airway symptoms of laryngeal spasm, irritation, or swelling such as stridor or choking; oropharyngeal irritation.
Cardiovascular: Arrhythmias (including atrial fibrillation, supraventricular tachycardia, extrasystoles), and anaphylaxis.
Non-Site Specific: Very rare anaphylactic reaction in patients with severe milk protein allergy.
OVERDOSAGE
The expected signs and symptoms with overdosage of SEREVENT DISKUS are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the signs and symptoms listed under ADVERSE REACTIONS, e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, muscle cramps, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, and insomnia. Overdosage with SEREVENT DISKUS may be expected to result in exaggeration of the pharmacologic adverse effects associated with beta-adrenoceptor agonists, including tachycardia and/or arrhythmia, tremor, headache, and muscle cramps. Overdosage with SEREVENT DISKUS can lead to clinically significant prolongation of the QTc interval, which can produce ventricular arrhythmias. Other signs of overdosage may include hypokalemia and hyperglycemia.
As with all sympathomimetic medications, cardiac arrest and even death may be associated with abuse of SEREVENT DISKUS.
Treatment consists of discontinuation of SEREVENT DISKUS together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of SEREVENT DISKUS. Cardiac monitoring is recommended in cases of overdosage.
No deaths were seen in rats at an inhalation dose of 2.9 mg/kg (approximately 240 times the maximum recommended daily inhalation dose in adults and approximately 110 times the maximum recommended daily inhalation dose in children on a mg/m 2 basis) and in dogs at an inhalation dose of 0.7 mg/kg (approximately 190 times the maximum recommended daily inhalation dose in adults and approximately 90 times the maximum recommended daily inhalation dose in children on a mg/m 2 basis). By the oral route, no deaths occurred in mice at 150 mg/kg (approximately 6,100 times the maximum recommended daily inhalation dose in adults and approximately 2,900 times the maximum recommended daily inhalation dose in children on a mg/m 2 basis) and in rats at 1,000 mg/kg (approximately 81,000 times the maximum recommended daily inhalation dose in adults and approximately 38,000 times the maximum recommended daily inhalation dose in children on a mg/m 2 basis).
DOSAGE AND ADMINISTRATION
SEREVENT DISKUS should be administered by the orally inhaled route only (see Patient's Instructions for Use). The patient must not exhale into the DISKUS and the DISKUS should only be activated and used in a level, horizontal position.
Asthma: For maintenance of bronchodilatation and prevention of symptoms of asthma, including the symptoms of nocturnal asthma, the usual dosage for adults and children 4 years of age and older is 1 inhalation (50 mcg) twice daily (morning and evening, approximately 12 hours apart). If a previously effective dosage regimen fails to provide the usual response, medical advice should be sought immediately as this is often a sign of destabilization of asthma. Under these circumstances, the therapeutic regimen should be reevaluated and additional therapeutic options, such as inhaled or systemic corticosteroids, should be considered. If symptoms arise in the period between doses, an inhaled, short-acting beta 2 -agonist should be taken for immediate relief.
Chronic Obstructive Pulmonary Disease: For maintenance treatment of bronchospasm associated with COPD (including chronic bronchitis and emphysema), the usual dosage for adults is 1 inhalation (50 mcg) twice daily (morning and evening, approximately 12 hours apart).
For both asthma and COPD, adverse effects are more likely to occur with higher doses of salmeterol, and more frequent administration or administration of a larger number of inhalations is not recommended.
To gain full therapeutic benefit, SEREVENT DISKUS should be administered twice daily (morning and evening) in the treatment of reversible airway obstruction.
Geriatric Use: Based on available data for SEREVENT DISKUS, no dosage adjustment is recommended.
Prevention of Exercise-Induced Bronchospasm: One inhalation of SEREVENT DISKUS at least 30 minutes before exercise has been shown to protect patients against EIB. When used intermittently as needed for prevention of EIB, this protection may last up to 9 hours in adolescents and adults and up to 12 hours in patients 4 to 11 years of age. Additional doses of SEREVENT should not be used for 12 hours after the administration of this drug. Patients who are receiving SEREVENT DISKUS twice daily should not use additional SEREVENT for prevention of EIB. If regular, twice-daily dosing is not effective in preventing EIB, other appropriate therapy for EIB should be considered.
HOW SUPPLIED
SEREVENT DISKUS is supplied as a disposable, teal green unit containing 60 blisters. The drug product is packaged within a teal green, plastic-coated, moisture-protective foil pouch (NDC 0173-0521-00).
SEREVENT DISKUS is also supplied in an institutional pack of 1 teal green, disposable unit containing 28 blisters. The drug product is packaged within a teal green, plastic-coated, moisture-protective foil pouch (NDC 0173-0520-00).
Store at controlled room temperature (see USP), 20° to 25°C (68° to 77°F) in a dry place away from direct heat or sunlight. Keep out of reach of children. SEREVENT DISKUS should be discarded 6 weeks after removal from the moisture-protective foil overwrap pouch or after all blisters have been used (when the dose indicator reads "0"), whichever comes first. The DISKUS is not reusable. Do not attempt to take the DISKUS apart.
Glaxosmithkline, Research Triangle Park, NC 27709
©2004, Glaxosmithkline. All rights reserved.
September 2004/RL-2129
Subscribe to the "News" RSS Feed 
TOP ۞
