-
Solage Topical Solution (Barrier)
DESCRIPTION
Solagé ® Topical Solution contains mequinol 2% and tretinoin 0.01%, by weight, in a solution base of ethyl alcohol (77.8% v/v), polyethylene glycol 400, butylated hydroxytoluene, ascorbic acid, citric acid, ascorbyl palmitate, edetate disodium and purified water.
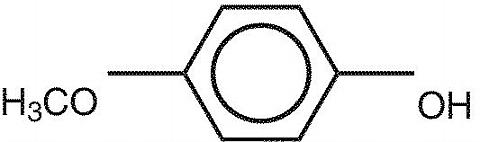
Mequinol is 4-hydroxyanisole, the monomethyl ether of hydroquinone or 1-hydroxy-4-methoxybenzene. It has the chemical formula, C 7 H 8 O 2 , a molecular weight of 124.14, and the structural formula:
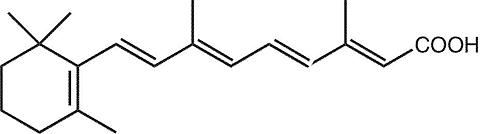
The chemical name for tretinoin, a retinoid, is (all- E )-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid, also referred to as all- trans -retinoic acid. It has the chemical formula, C 20 H 28 O 2 , a molecular weight of 300.44, and the structural formula:
CLINICAL PHARMACOLOGY
Solar lentigines are localized, pigmented, macular lesions of the skin on the areas of the body which have been chronically exposed to sunlight.
Biopsy specimens of solar lentigines were collected in a clinical study with Solagé Solution at baseline, at the end of a 24 week treatment period and at the end of a subsequent 24 week, no treatment, follow-up period. The end of treatment specimens showed a decrease in melanin pigmentation in both melanocytes and keratinocytes, and an increased lymphocytic infiltration, which may have been the result of irritation or an immunologic reaction. The end of follow-up period specimens showed repigmentation of the melanocytes and keratinocytes to a state similar to the baseline specimens. These results indicate that there is no assurance that any improvement obtained would persist upon discontinuation of drug therapy.
The mechanism of action of mequinol is unknown. Although mequinol is a substrate for the enzyme tyrosinase and acts as a competitive inhibitor of the formation of melanin precursors, the clinical significance of these findings is unknown. The mechanism of action of tretinoin as a depigmenting agent also is unknown.
PHARMACOKINETICS
The percutaneous absorption of tretinoin and the systemic exposure to tretinoin and mequinol were assessed in healthy subjects (n=8) following two weeks of twice daily topical treatment of Solagé Solution. Approximately 0.8 mL of Solagé Solution was applied to a 400 cm 2 area of the back, corresponding to a dose of 37.3 mg/cm 2 for mequinol and 0.23 mg/cm 2 for tretinoin. The percutaneous absorption of tretinoin was approximately 4.4%, and systemic concentrations did not increase over endogenous levels. The mean C max for mequinol was 9.92 ng/mL (range 4.22 to 23.62 ng/mL) and the T max was 2 hours (range 1 to 2 hours).
INDICATIONS AND USAGE
(To understand fully the indication for this product, please read the entire INDICATIONS AND USAGE section of the labeling).
Solagé (mequinol 2%, tretinoin 0.01%) Topical Solution is indicated for the treatment of solar lentigines.
Solagé Solution should only be used under medical supervision as an adjunct to a comprehensive skin care and sun avoidance program where the patient should primarily either avoid the sun or use protective clothing.
Neither the safety nor effectiveness of Solagé Solution for the prevention or treatment of melasma or postinflammatory hyperpigmentation has been established.
The efficacy of using Solagé Solution daily for greater than 24 weeks has not been established.
The local cutaneous safety of using Solagé Solution in non-Caucasians has not been adequately established (see Clinical Studies section).
CONTRAINDICATIONS
The combination of mequinol and tretinoin may cause fetal harm when administered to a pregnant woman. Due to the known effects of these active ingredients, Solagé Topical Solution should not be used in women of childbearing potential.
In a dermal teratology study in New Zealand White rabbits, there were no statistically significant differences among treatment groups in fetal malformation data; however, marked hydrocephaly with visible doming of the head was observed in one mid-dose litter (12 and 0.06 mg/kg or 132 and 0.66 mg/m 2 of mequinol and tretinoin, respectively) and two fetuses in one high dose litter (40 and 0.2 mg/kg or 440 and 2.2 mg/m 2 of mequinol and tretinoin, respectively) of Solagé Solution, and two high-dose tretinoin (0.2 mg/kg, 2.2 mg/m 2 ) treated litters. These malformations were considered to be treatment related and due to the known effects of tretinoin. This was further supported by coincident appearance of other malformations associated with tretinoin, such as cleft palate and appendicular skeletal defects. No effects attributed to treatment were observed in rabbits in that study treated topically with mequinol alone (dose 40 mg/kg, 440 mg/m 2 ). A no-observed-effect level (NOEL) for teratogenicity in rabbits was established at 4 and 0.02 mg/kg (44 and 0.22 mg/m 2 mequinol and tretinoin, respectively) for Solagé Solution which is approximately the maximum possible human daily dose, based on clinical application to 5% of total body surface area. Plasma tretinoin concentrations were not raised above endogenous levels, even at teratogenic doses. Plasma mequinol concentrations in rabbits at the NOEL at one hour after application were 124 ng/mL or approximately twelve times the mean peak plasma concentrations of that substance seen in human subjects in a clinical pharmacokinetic study. In a repeated study in pregnant rabbits administered the same dose levels as the study described above, additional precautionary measures were taken to prevent ingestion, although there is no evidence to confirm that ingestion occurred in the initial study. Precautionary measures additionally limited transdermal absorption to a six hour exposure period, or approximately one-fourth of the human clinical daily continuous exposure time. This study did not show any significant teratogenic effects at doses up to approximately 13 times the human dose on a mg/m 2 basis. However, a concurrent tretinoin dose group (0.2 mg/kg/day) did include two litters with limb malformations.
In a published study in albino rats (J. Am. Coll. Toxicology 4(5):31-63, 1985), topical application of 5% of mequinol in a cream vehicle during gestation was embryotoxic and embryolethal. Embryonic loss prior to implantation was noted in that study where animals were treated throughout gestation. Coincidentally, mean preimplantation embryonic loss was increased in the first rabbit study in all mequinol treated groups, relative to control, and in the high dose mequinol/tretinoin and tretinoin only treated groups in the second study. In those studies, dosing began at gestation day 6, when implantation is purported to occur. Increased preimplantation loss was also noted at the high combination dose in a study of early embryonic effects in rats, as was decreased body weight in male pups; these findings are consistent with the published study.
Solagé Solution was not teratogenic in Sprague-Dawley rats when given in topical doses of 80 and 0.4 mg/kg mequinol and tretinoin, respectively (480 and 2.4 mg/m 2 or 11 times the maximum human daily dose). The maximum human dose is defined as the amount of solution applied daily to 5% of the total body surface area.
With widespread use of any drug, a small number of birth defect reports associated temporally with the administration of the drug would be expected by chance alone. Thirty cases of temporally-associated congenital malformations have been reported during two decades of clinical use of another formulation of topical tretinoin. Although no definite pattern of teratogenicity and no casual association has been established from these cases, 6 of the reports describe the rare birth defect category holoprosencephaly (defects associated with incomplete midline development of the forebrain). The significance of these spontaneous reports in terms of risk to the fetus is not known. No adequate or well-controlled trials have been conducted with Solagé Solution in pregnant women.
Solagé Topical Solution is contraindicated in individuals with a history of sensitivity reactions to any of its ingredients. It should be discontinued if hypersensitivity to any of its ingredients is noted.
WARNINGS
Solagé Solution is a dermal irritant and the results of continued irritation of the skin for greater than 52 weeks in chronic, long-term use are not known. Tretinoin has been reported to cause severe irritation on eczematous skin and should be used only with utmost caution in patients with this condition.
Safety and effectiveness of Solagé Solution in individuals with moderately or heavily pigmented skin have not been established.
Solagé Solution should not be administered if the patient is also taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the possibility of augmented phototoxicity.
Because of heightened burning susceptibility, exposure to sunlight (including sunlamps) to treated areas should be avoided or minimized during the use of Solagé Solution. Patients must be advised to use protective clothing and comply with a comprehensive sun avoidance program when using Solagé Solution. Data are not available to establish how or whether Solagé Solution is degraded (either by sunlight or by normal interior lighting) following application to the skin. Patients with sunburn should be advised not to use Solagé Solution until fully recovered. Patients who may have considerable sun exposure due to their occupation and those patients with inherent sensitivity to sunlight should exercise particular caution when using Solagé Solution and ensure that the precautions outlined in the Patient Medication Guide are observed.
Solagé Solution should be kept out of the eyes, mouth, paranasal creases, and mucous membranes. Solagé Solution may cause skin irritation, erythema, burning, stinging or tingling, peeling, and pruritis. If the degree of such local irritation warrants, patients should be directed to use less medication, decrease the frequency of application, discontinue use temporarily, or discontinue use altogether. The efficacy at reduced frequencies of application has not been established.
Solagé Solution should be used with caution by patients with a history, or family history, of vitiligo. One patient in the trials, whose brother had vitiligo, experienced hypopigmentation in areas that had not been treated with study medication. Some of these areas continued to worsen for at least one month post treatment with Solagé Solution. Six weeks later the severity of the hypopigmentation had decreased from moderate to mild and 106 days post treatment, the patient had resolution of some but not all lesions.
Application of larger amounts of medication than recommended will not lead to more rapid or better results, and marked redness, peeling, discomfort, or hypopigmentation of the skin may occur.
PRECAUTIONS
General
For external use only.
Solagé Solution should only be used as an adjunct to a comprehensive skin care and sun avoidance program. (See INDICATIONS AND USAGE section).
If a drug sensitivity, chemical irritation, or a systemic adverse reaction develops, use of Solagé Solution should be discontinued. Weather extremes, such as wind or cold, may be more irritating to patients using Solagé Solution.
Information for patients
Patients require detailed instruction to obtain maximal benefits and to understand all the precautions necessary to use this product with greatest safety.
Drug Interactions
Concomitant topical products with a strong skin drying effect, products with high concentrations of alcohol, astringents, spices or lime, medicated soaps or shampoos, permanent wave solutions, electrolysis, hair depilatories or waxes, or other preparations that might dry or irritate the skin should be used with caution in patients being treated with Solagé Solution because they may increase irritation when used with Solagé Solution.
Solagé Solution should not be administered if the patient is also taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the possibility of augmented phototoxicity.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Although a dermal carcinogenicity study in CD-1 mice indicated that Solagé Solution applied topically at daily doses up to 80 and 0.4 mg/kg (240 and 1.2 mg/m 2 ) of mequinol and tretinoin, respectively, representing approximately 5 times the maximum possible systemic human exposure was not carcinogenic, in a photocarcinogenicity study utilizing Crl:Skh-1(hr/hr BR) hairless albino mice, median time to onset of tumors decreased. Also, the number of tumors increased in all dose groups administered 1.4, 4.3 or 14 µl of Solagé Solution/cm 2 of skin (24 and 0.12, 72 and 0.36, or 240 and 1.2 mg/m 2 of mequinol and tretinoin, respectively; 0.6, 1.9, or 6.5 times the daily human dose on a mg/m 2 basis) following chronic topical dosing with intercurrent exposure to ultraviolet radiation for up to 40 weeks. Similar animal studies have shown an increased tumorigenic risk with the use of retinoids when followed by ultraviolet radiation. Although the significance of these studies to human use is not clear, patients using this product should be advised to avoid or minimize exposure to either sunlight or artificial ultraviolet irradiation sources.
Mequinol was non-mutagenic in the Ames/Salmonella assay using strains TA98, TA100, TA1535, and TA1537, all of which are insensitive to mutagenic effects of structurally-related quinones. Solagé Solution was non-genotoxic in an in vivo dermal micronucleus assay in rats, but exposure of bone marrow to drug was not demonstrated.
A dermal reproduction study with Solagé Solution in Sprague-Dawley rats at a daily dose of 80 and 0.4 mg/kg (480 and 2.4 mg/m 2 ) of mequinol and tretinoin, respectively, approximately 11 times the corresponding maximum possible human exposure, assuming 100% bioavailability following topical application to 5% of the total body surface area, showed no impairment of fertility.
Pregnancy: Teratogenic effects: Pregnancy Category X.
Although the magnitude of the potential for teratogenicity may not be well-defined, Solagé Solution is labeled as an "X" because the potential risk of the use of this drug to treat this particular indication (solar lentigines) in a pregnant woman clearly outweighs any possible benefit (see CONTRAINDICATIONS section).
Nursing Mothers: It is not known to what extent mequinol and/or tretinoin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Solagé Solution is administered to a nursing woman.
Pediatric Use: The safety and effectiveness of this product have not been established in pediatric patients. Solagé Solution should not be used on children.
Geriatric Use: Of the total number of patients in clinical studies of Solagé Solution, approximately 43% were 65 and older, while approximately 8% were 75 and over. No overall differences in effectiveness or safety were observed between these patients and younger patients.
ADVERSE REACTIONS
In clinical trials, adverse reactions were primarily mild to moderate in intensity, occurring in 66% and 30% of patients, respectively. The majority of these events were limited to the skin and 64% had an onset of a skin related adverse reaction early in treatment (by week 8). The most frequent adverse reactions in patients treated with Solagé Solution were erythema (49% of patients), burning, stinging, or tingling (26%), desquamation (14%), pruritus (12%), and skin irritation (5%).
Some patients experienced temporary hypopigmentation of treated lesions (5%) or of the skin surrounding treated lesions (7%). Ninety-four of 106 patients (89%) had resolution of hypopigmentation upon discontinuation of treatment to the lesion, and/or re-instruction on proper application to the lesion only. Another 8% (9/106) of patients with hypopigmentation events had resolution within 120 days after the end of treatment. Three of the 106 patients (2.8%) had persistence of hypopigmentation beyond 120 days. Approximately 6% of patients discontinued study participation with Solagé Solution due to adverse reactions. These discontinuations were due primarily to skin redness (erythema) or related cutaneous adverse reactions. Solagé Solution was generally well tolerated.
Adverse Events Occurring in >1% of the Population --All Studies Body SystemSolagé Solution
(mequinol 2%,
tretinoin 0.01%)Tretinoin,
0.01%Mequinol,
2%Vehicle Skin and AppendagesN % N % N % N % Erythema549 44.6 261 55.3 13 5.1 8 4.6 Burning/Stinging/ Tingling270 21.9 173 36.7 26 10.2 20 11.4 Desquamation155 12.6 93 19.7 7 2.8 2 1.1 Pruritus135 11.0 66 14.0 12 4.7 3 1.7 Irritation Skin90 7.3 25 5.3 1 0.4 1 0.6 Halo Hypopigmentation76 6.2 16 3.4 2 0.8 2 1.1 Hypopigmentation50 4.1 8 1.7 2 0.8 0 0.0 Skin Dry38 3.1 18 3.8 3 1.2 1 0.6 Rash31 2.5 21 4.4 0 0.0 1 0.6 Crusting30 2.4 18 3.8 0 0.0 1 0.6 Rash Vesicular Bullae18 2.1 8 1.7 0 0.0 0 0.0 Dermatitis25 2.0 0 0.0 0 0.0 0 0.0 OVERDOSAGE
If Solagé Solution is applied excessively, no more rapid or better results will be obtained and marked redness, peeling, discomfort, or hypopigmentation may occur. Oral ingestion of the drug may lead to the same adverse effects as those associated with excessive oral intake of vitamin A (hypervitaminosis A). If oral ingestion occurs, the patient should be monitored, and appropriate supportive measures should be administered as necessary. The maximal no-effect level for oral administration of Solagé Solution in rats was 5.0 mL/kg (30 mg/m 2 ). Clinical signs observed were attributed to the high alcohol content (77%) of the drug formulation.
DOSAGE AND ADMINISTRATION
Patients require detailed instruction to obtain maximal benefits and to understand all the precautions necessary to use this product with greatest safety. The physician should review the Patient Medication Guide.
Apply Solagé Solution to the solar lentigines using the applicator tip while avoiding application to the surrounding skin. Use twice daily, morning and evening at least 8 hours apart, or as directed by a physician. Patients should not shower or bathe the treatment areas for at least 6 hours after application of Solagé Solution. Special caution should be taken when applying Solagé Solution to avoid the eyes, mouth, paranasal creases, and mucous membranes.
Application of Solagé Solution may cause transitory stinging, burning or irritation.
Improvement continues gradually through the course of therapy and should be apparent by 24 weeks. Patients should avoid exposure to sunlight (including sunlamps) or wear protective clothing while using Solagé Solution. Data are not available to establish how or whether Solagé Solution is degraded (either by sunlight or by normal interior lighting) following application to the skin.
With discontinuation of Solagé Solution therapy, a majority of patients will experience some repigmentation over time of their lesions.
Applications of larger amounts of medication or more frequently than recommended will not lead to more rapid or better results, and marked redness, peeling, irritation, or hypopigmentation (abnormal lightening) of the skin may occur.
Patients treated with Solagé Solution may use cosmetics but should wait 30 minutes before applying.
CLINICAL STUDIES
Two adequate and well-controlled trials evaluated changes in treated hyperpigmented lesions on the face, forearms/back of hands in 421 patients treated with Solagé Topical Solution, 422 patients treated with tretinoin topical solution, 209 patients treated with mequinol topical solution and 107 patients treated with vehicle for up to 24 weeks. In these studies, patients were to avoid sun exposure and use protective clothing, and use of suncreens was prohibited. Patients were allowed to apply Moisturel® Lotion 30 minutes after application of Solagé Solution. Physicians assessed the extent of improvement or worsening of all the treated lesions from the baseline condition on a 7 point scale. The results of these evaluations are shown below.
Face Forearms/Back of Hands Solagé Solution Vehicle Solagé Solution Vehicle Moderate Improvement
or greater 157% 15% 54% 14% Slight Improvement28% 36% 26% 33% No Change 215% 49% 20% 53% 1 Includes the following grades: Moderate Improvement, Marked Improvement, Almost Clear, Completely Clear. Moderate Improvement or greater was considered clinically meaningful. 2 Includes the following grades: No Change, Worse (less than 1% of patients treated with Solagé Solution were rated as worse).
Improvement (lightening) of the solar lentigines occurred gradually over time during the 24 week treatment period. At 24 weeks of treatment, 57% and 54% of patients experienced moderate improvement or greater, and 3% and 1% of patients were completely clear of all treated lesions for the face and forearms/back of hands, respectively. It should be noted that approximately 9% of patients, from both treatment areas in these studies, with moderate improvement or greater also experienced hypopigmentation of the skin surrounding at least one treated lesion. There are no vehicle-controlled effectiveness data on the course of lesions treated beyond 24 weeks.
After 24 weeks of treatment, for the forearm/back of hands treatment site, the percentage of patients treated with tretinoin topical solution with moderate improvement or greater, slight improvement, or no change, were 38%, 37%, and 26%, respectively, and for mequinol topical solution were 24%, 40%, and 36%, respectively. For the face treatment site, the percentage of patients treated with tretinoin topical solution with moderate improvement or greater, slight improvement, or no change, were 46%, 33%, and 21%, respectively, and for mequinol topical solution were 33%, 30%, and 37% respectively.
The duration of effect was investigated during a period of up to 24 weeks following the discontinuation of treatment. Results from these studies showed that patients may maintain the level of clinical improvement of their treated lesions from the end of treatment through the 24 week follow-up period. However, some degree of repigmentation of treated lesions was observed over time, demonstrating reversibility of the depigmenting action of Solagé Solution.
In the clinical studies, some patients experienced temporary hypopigmentation of treated lesions (5%) or of the skin surrounding treated lesions (7%). Hypopigmentation of the skin surrounding treated lesions occurs even in the setting of proper application of the drug within the lesion border. The majority (94/106 - 89%) resolved upon discontinuation of treatment to the lesion, and/or re-instruction on proper application to the lesion only. Another 8% (9/106) of patients with hypopigmentation events had resolution within 120 days after the end of treatment.
Three of the 106 patients (2.8%) had persistence of hypopigmentation beyond 120 days. This further demonstrates the reversibility of the depigmenting action of Solagé Solution.
Over 150 patients used Solagé Solution twice daily for 52 weeks in an open label clinical study. The safety profile for Solagé Solution in this long-term study was similar to that seen in the 24 week studies although burning/stinging/ tingling, desquamation, pruritis, and irritation of the skin occurred at lower rates and halo hypopigmentation and hypopigmentation occurred at a slightly greater rate.
Over 90 patients used Solagé Solution twice daily and a concomitant sunscreen (PreSun® 29) daily for up to 24 weeks in an open label clinical study. The safety profile for Solagé Solution in this study was similar to that seen in studies which prohibited sunscreen use although desquamation, pruritis, and halo hypopigmentation occurred at slightly lower rates.
The clinical studies of Solagé Solution included 1794 individuals of Skin Type I-V, 94.5% of whom were Caucasian. The trials also included 5% of individuals who were Asian/Pacific Islander-1.2%, African-American-0.8%, and Hispanic/Latino-3.5%. Safety in Asian/Pacific Islander, African-American, and Hispanic/Latino individuals has not been adequately established. Safety and effectiveness of Solagé Solution in individuals with Skin Type VI (never burns from the sun, deeply pigmented skin) or women of childbearing potential have not been established (see CONTRAINDICATIONS ).
HOW SUPPLIED
Solagé (mequinol 2%, tretinoin 0.01%) Topical Solution is available in 30 mL plastic bottles with an applicator. NDC 13478-001-01
STORAGE: The bottle should be protected from light by continuing to store in the carton after opening. Store at 25° C (77° F): excursions permitted to 15-30° C (59-86° F).
Note: FLAMMABLE. Keep away from heat and open flame.
Marketed by:
Barrier Therapeutics, Inc.
Barrier Princeton, New Jersey 08540-6697 USA
Therapeutics Manufactured by:
Bristol-Myers Squibb, Buffalo, NY 14213 USA
SO-001 Revised February 2005
Subscribe to the "News" RSS Feed 
TOP ۞
