-
Tamiflu Capsules, Tamiflu Oral Suspension (Roche Laboratories)
DESCRIPTION
TAMIFLU (oseltamivir phosphate) is available as a capsule containing 75 mg oseltamivir for oral use, in the form of oseltamivir phosphate, and as a powder for oral suspension, which when constituted with water as directed contains 12 mg/mL oseltamivir base. In addition to the active ingredient, each capsule contains pregelatinized starch, talc, povidone K 30, croscarmellose sodium, and sodium stearyl fumarate. The capsule shell contains gelatin, titanium dioxide, yellow iron oxide, black iron oxide, and red iron oxide. Each capsule is printed with blue ink, which includes FD&C Blue No. 2 as the colorant. In addition to the active ingredient, the powder for oral suspension contains xanthan gum, monosodium citrate, sodium benzoate, sorbitol, saccharin sodium, titanium dioxide, and tutti-frutti flavoring.
Oseltamivir phosphate is a white crystalline solid with the chemical name (3R,4R,5S)-4-acetylamino-5-amino-3(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid, ethyl ester, phosphate (1:1). The chemical formula is C 16 H 28 N 2 O 4 (free base). The molecular weight is 312.4 for oseltamivir free base and 410.4 for oseltamivir phosphate salt.
MICROBIOLOGY
Mechanism of Action
Oseltamivir is an ethyl ester prodrug requiring ester hydrolysis for conversion to the active form, oseltamivir carboxylate. The proposed mechanism of action of oseltamivir is inhibition of influenza virus neuraminidase with the possibility of alteration of virus particle aggregation and release.
Antiviral Activity In Vitro
The antiviral activity of oseltamivir carboxylate against laboratory strains and clinical isolates of influenza virus was determined in cell culture assays. The concentrations of oseltamivir carboxylate required for inhibition of influenza virus were highly variable depending on the assay method used and the virus tested. The 50% and 90% inhibitory concentrations (IC 50 and IC 90 ) were in the range of 0.0008 µM to >35 µM and 0.004 µM to >100 µM, respectively (1 µM=0.284 µg/mL). The relationship between the in vitro antiviral activity in cell culture and the inhibition of influenza virus replication in humans has not been established.
Resistance
Influenza A virus isolates with reduced susceptibility to oseltamivir carboxylate have been recovered in vitro by passage of virus in the presence of increasing concentrations of oseltamivir carboxylate. Genetic analysis of these isolates showed that reduced susceptibility to oseltamivir carboxy-late is associated with mutations that result in amino acid changes in the viral neuraminidase or viral hemagglutinin or both. Resistance mutations selected in vitro in neuraminidase are I222T and H274Y in influenza A N1 and I222T and R292K in influenza A N2. Mutations E119V, R292K and R305Q have been selected in avian influenza A neuraminidase N9. Mutations A28T and R124M have been selected in the hemagglutinin of influenza A H3N2 and mutation H154Q in the hemagglutinin of a reassortant human/avian virus H1N9.
In clinical studies in the treatment of naturally acquired infection with influenza virus, 1.3% (4/301) of posttreatment isolates in adult patients and adolescents, and 8.6% (9/105) in pediatric patients aged 1 to 12 years showed emergence of influenza variants with decreased neuraminidase susceptibility in vitro to oseltamivir carboxylate. Mutations in influenza A resulting in decreased susceptibility were H274Y in neuraminidase N1 and E119V and R292K in neuraminidase N2. Insufficient information is available to fully characterize the risk of emergence of TAMIFLU resistance in clinical use.
In clinical studies of postexposure and seasonal prophylaxis, determination of resistance was limited by the low overall incidence rate of influenza infection and prophylactic effect of TAMIFLU.
Cross-resistance
Cross-resistance between zanamivir-resistant influenza mutants and oseltamivir-resistant influenza mutants has been observed in vitro. Due to limitations in the assays available to detect drug-induced shifts in virus susceptibility, an estimate of the incidence of oseltamivir resistance and possible cross-resistance to zanamivir in clinical isolates cannot be made. However, two of the three oseltamivir-induced mutations (E119V, H274Y and R292K) in the viral neuraminidase from clinical isolates occur at the same amino acid residues as two of the three mutations (E119G/A/D, R152K and R292K) observed in zanamivir-resistant virus.
Immune Response
No influenza vaccine interaction study has been conducted. In studies of naturally acquired and experimental influenza, treatment with TAMIFLU did not impair normal humoral antibody response to infection.
CLINICAL PHARMACOLOGY
PharmacokineticsAbsorption and Bioavailability
Oseltamivir is readily absorbed from the gastrointestinal tract after oral administration of oseltamivir phosphate and is extensively converted predominantly by hepatic esterases to oseltamivir carboxylate. At least 75% of an oral dose reaches the systemic circulation as oseltamivir carboxylate. Exposure to oseltamivir is less than 5% of the total exposure after oral dosing (Table 1).
Table 1. Mean (% CV) Pharmacokinetic Parameters of Oseltamivir and Oseltamivir Carboxylate After a
Multiple 75 mg Capsule Twice Daily Oral Dose (n=20)ParameterOseltamivir Oseltamivir Carboxylate C max (ng/mL)65.2 (26) 348 (18) AUC 0-12h (ng·h/mL)112 (25) 2719 (20) Plasma concentrations of oseltamivir carboxylate are proportional to doses up to 500 mg given twice daily (see DOSAGE AND ADMINISTRATION ).
Coadministration with food has no significant effect on the peak plasma concentration (551 ng/mL under fasted conditions and 441 ng/mL under fed conditions) and the area under the plasma concentration time curve (6218 ng·h/mL under fasted conditions and 6069 ng·h/mL under fed conditions) of oseltamivir carboxylate.
Distribution
The volume of distribution (V ss ) of oseltamivir carboxylate, following intravenous administration in 24 subjects, ranged between 23 and 26 liters.
The binding of oseltamivir carboxylate to human plasma protein is low (3%). The binding of oseltamivir to human plasma protein is 42%, which is insufficient to cause significant displacement-based drug interactions.
Metabolism
Oseltamivir is extensively converted to oseltamivir carboxylate by esterases located predominantly in the liver. Neither oseltamivir nor oseltamivir carboxylate is a substrate for, or inhibitor of, cytochrome P450 isoforms.
Elimination
Absorbed oseltamivir is primarily (>90%) eliminated by conversion to oseltamivir carboxylate. Plasma concentrations of oseltamivir declined with a half-life of 1 to 3 hours in most subjects after oral administration. Oseltamivir carboxylate is not further metabolized and is eliminated in the urine. Plasma concentrations of oseltamivir carboxylate declined with a half-life of 6 to 10 hours in most subjects after oral administration. Oseltamivir carboxylate is eliminated entirely (>99%) by renal excretion. Renal clearance (18.8 L/h) exceeds glomerular filtration rate (7.5 L/h) indicating that tubular secretion occurs, in addition to glomerular filtration. Less than 20% of an oral radiolabeled dose is eliminated in feces.
Special Populations
Renal Impairment
Administration of 100 mg of oseltamivir phosphate twice daily for 5 days to patients with various degrees of renal impairment showed that exposure to oseltamivir carboxy-late is inversely proportional to declining renal function. Oseltamivir carboxylate exposures in patients with normal and abnormal renal function administered various dose regimens of oseltamivir are described in Table 2.
Table 2. Oseltamivir Carboxylate Exposures in Patients With Normal and Reduced Serum Creatinine ClearanceParameterNormal Renal Function Impaired Renal Function 75 mg
qd75 mg
bid150 mg
bidCreatinine Clearance
<10 mL/minCreatinine Clearance
>10 and <30 mL/minCAPD Hemodialysis 75 mg
daily75 mg
alternate
days30 mg
daily30 mg
weekly30 mg alternate
HD cycleC max259 * 348 * 705 * 766 850 1638 1175 655 C min39 * 138 * 288 * 62 48 864 209 346 AUC 487476 * 10876 * 21864 * 17381 12429 62636 21999 25054 *Observed values. All other values are predicted.
AUC normalized to 48 hours.Pediatric Patients
The pharmacokinetics of oseltamivir and oseltamivir carboxylate have been evaluated in a single dose pharmacokinetic study in pediatric patients aged 5 to 16 years (n=18) and in a small number of pediatric patients aged 3 to 12 years (n=5) enrolled in a clinical trial. Younger pediatric patients cleared both the prodrug and the active metabolite faster than adult patients resulting in a lower exposure for a given mg/kg dose. For oseltamivir carboxylate, apparent total clearance decreases linearly with increasing age (up to 12 years). The pharmacokinetics of oseltamivir in pediatric patients over 12 years of age are similar to those in adult patients.
Geriatric Patients
Exposure to oseltamivir carboxylate at steady-state was 25% to 35% higher in geriatric patients (age range 65 to 78 years) compared to young adults given comparable doses of oseltamivir. Half-lives observed in the geriatric patients were similar to those seen in young adults. Based on drug exposure and tolerability, dose adjustments are not required for geriatric patients for either treatment or prophylaxis (see DOSAGE AND ADMINISTRATION: Special Dosage Instructions ).
INDICATIONS AND USAGE
Treatment of Influenza
TAMIFLU is indicated for the treatment of uncomplicated acute illness due to influenza infection in patients 1 year and older who have been symptomatic for no more than 2 days.
Prophylaxis of Influenza
TAMIFLU is indicated for the prophylaxis of influenza in adult patients and adolescents 13 years and older.
TAMIFLU is not a substitute for early vaccination on an annual basis as recommended by the Centers for Disease Control's Immunization Practices Advisory Committee.
Description of Clinical Studies: Studies in Naturally Occurring Influenza
Treatment of Influenza
Adult Patients
Two phase III placebo-controlled and double-blind clinical trials were conducted: one in the USA and one outside the USA. Patients were eligible for these trials if they had fever >100°F, accompanied by at least one respiratory symptom (cough, nasal symptoms or sore throat) and at least one systemic symptom (myalgia, chills/sweats, malaise, fatigue or headache) and influenza virus was known to be circulating in the community. In addition, all patients enrolled in the trials were allowed to take fever-reducing medications.
Of 1355 patients enrolled in these two trials, 849 (63%) patients were influenza-infected (age range 18 to 65 years; median age 34 years; 52% male; 90% Caucasian; 31% smokers). Of the 849 influenza-infected patients, 95% were infected with influenza A, 3% with influenza B, and 2% with influenza of unknown type.
TAMIFLU was started within 40 hours of onset of symptoms. Subjects participating in the trials were required to self-assess the influenza-associated symptoms as "none", "mild", "moderate" or "severe". Time to improvement was calculated from the time of treatment initiation to the time when all symptoms (nasal congestion, sore throat, cough, aches, fatigue, headaches, and chills/sweats) were assessed as "none" or "mild". In both studies, at the recommended dose of TAMIFLU 75 mg twice daily for 5 days, there was a 1.3 day reduction in the median time to improvement in influenza-infected subjects receiving TAMIFLU compared to subjects receiving placebo. Subgroup analyses of these studies by gender showed no differences in the treatment effect of TAMIFLU in men and women.
In the treatment of influenza, no increased efficacy was demonstrated in subjects receiving treatment of 150 mg TAMIFLU twice daily for 5 days.
Geriatric Patients
Three double-blind placebo-controlled treatment trials were conducted in patients >/=65 years of age in three consecutive seasons. The enrollment criteria were similar to that of adult trials with the exception of fever being defined as >97.5°F. Of 741 patients enrolled, 476 (65%) patients were influenza-infected. Of the 476 influenza-infected patients, 95% were infected with influenza type A and 5% with influenza type B.
In the pooled analysis, at the recommended dose of TAMIFLU 75 mg twice daily for 5 days, there was a 1 day reduction in the median time to improvement in influenza-infected subjects receiving TAMIFLU compared to those receiving placebo (p=NS). However, the magnitude of treatment effect varied between studies.
Pediatric Patients
One double-blind placebo-controlled treatment trial was conducted in pediatric patients aged 1 to 12 years (median age 5 years), who had fever (>100°F) plus one respiratory symptom (cough or coryza) when influenza virus was known to be circulating in the community. Of 698 patients enrolled in this trial, 452 (65%) were influenza-infected (50% male; 68% Caucasian). Of the 452 influenza-infected patients, 67% were infected with influenza A and 33% with influenza B.
The primary endpoint in this study was the time to freedom from illness, a composite endpoint which required 4 individual conditions to be met. These were: alleviation of cough, alleviation of coryza, resolution of fever, and parental opinion of a return to normal health and activity. TAMIFLU treatment of 2 mg/kg twice daily, started within 48 hours of onset of symptoms, significantly reduced the total composite time to freedom from illness by 1.5 days compared to placebo. Subgroup analyses of this study by gender showed no differences in the treatment effect of TAMIFLU in males and females.
Prophylaxis of Influenza
The efficacy of TAMIFLU in preventing naturally occurring influenza illness has been demonstrated in three seasonal prophylaxis studies and a postexposure prophylaxis study in households. The primary efficacy parameter for all these studies was the incidence of laboratory confirmed clinical influenza. Laboratory confirmed clinical influenza was defined as oral temperature >/=99.0°F/37.2°C plus at least one respiratory symptom (cough, sore throat, nasal congestion) and at least one constitutional symptom (aches and pain, fatigue, headache, chills/sweats), all recorded within 24 hours, plus either a positive virus isolation or a fourfold increase in virus antibody titers from baseline.
In a pooled analysis of two seasonal prophylaxis studies in healthy unvaccinated adults (aged 13 to 65 years), TAMIFLU 75 mg once daily taken for 42 days during a community outbreak reduced the incidence of laboratory confirmed clinical influenza from 4.8% (25/519) for the placebo group to 1.2% (6/520) for the TAMIFLU group.
In a seasonal prophylaxis study in elderly residents of skilled nursing homes, TAMIFLU 75 mg once daily taken for 42 days reduced the incidence of laboratory confirmed clinical influenza from 4.4% (12/272) for the placebo group to 0.4% (1/276) for the TAMIFLU group. About 80% of this elderly population were vaccinated, 14% of subjects had chronic airway obstructive disorders, and 43% had cardiac disorders.
In a study of postexposure prophylaxis in household contacts (aged >/=13 years) of an index case, TAMIFLU 75 mg once daily administered within 2 days of onset of symptoms in the index case and continued for 7 days reduced the incidence of laboratory confirmed clinical influenza from 12% (24/200) in the placebo group to 1% (2/205) for the TAMIFLU group. Index cases did not receive TAMIFLU in the study.
CONTRAINDICATIONS
TAMIFLU is contraindicated in patients with known hypersensitivity to any of the components of the product.
PRECAUTIONS
General
There is no evidence for efficacy of TAMIFLU in any ill-ness caused by agents other than influenza viruses Types A and B.
Use of TAMIFLU should not affect the evaluation of individuals for annual influenza vaccination in accordance with guidelines of the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.
Efficacy of TAMIFLU in patients who begin treatment after 40 hours of symptoms has not been established.
Efficacy of TAMIFLU in the treatment of subjects with chronic cardiac disease and/or respiratory disease has not been established. No difference in the incidence of complications was observed between the treatment and placebo groups in this population. No information is available regarding treatment of influenza in patients with any medical condition sufficiently severe or unstable to be considered at imminent risk of requiring hospitalization.
Safety and efficacy of repeated treatment or prophylaxis courses have not been studied.
Efficacy of TAMIFLU for treatment or prophylaxis has not been established in immunocompromised patients.
Serious bacterial infections may begin with influenza-like symptoms or may coexist with or occur as complications during the course of influenza. TAMIFLU has not been shown to prevent such complications.
Hepatic Impairment
The safety and pharmacokinetics in patients with hepatic impairment have not been evaluated.
Renal Impairment
Dose adjustment is recommended for patients with a serum creatinine clearance <30 mL/min (see DOSAGE AND ADMINISTRATION ).
Information for Patients
Patients should be instructed to begin treatment with TAMIFLU as soon as possible from the first appearance of flu symptoms. Similarly, prevention should begin as soon as possible after exposure, at the recommendation of a physician.
Patients should be instructed to take any missed doses as soon as they remember, except if it is near the next scheduled dose (within 2 hours), and then continue to take TAMIFLU at the usual times.
TAMIFLU is not a substitute for a flu vaccination. Patients should continue receiving an annual flu vaccination according to guidelines on immunization practices.
Drug Interactions
Information derived from pharmacology and pharmacokinetic studies of oseltamivir suggests that clinically significant drug interactions are unlikely.
Oseltamivir is extensively converted to oseltamivir carboxylate by esterases, located predominantly in the liver. Drug interactions involving competition for esterases have not been extensively reported in literature. Low protein binding of oseltamivir and oseltamivir carboxylate suggests that the probability of drug displacement interactions is low.
In vitro studies demonstrate that neither oseltamivir nor oseltamivir carboxylate is a good substrate for P450 mixed-function oxidases or for glucuronyl transferases.
Cimetidine, a non-specific inhibitor of cytochrome P450 isoforms and competitor for renal tubular secretion of basic or cationic drugs, has no effect on plasma levels of oseltamivir or oseltamivir carboxylate.
Clinically important drug interactions involving competition for renal tubular secretion are unlikely due to the known safety margin for most of these drugs, the elimination characteristics of oseltamivir carboxylate (glomerular filtration and anionic tubular secretion) and the excretion capacity of these pathways. Coadministration of probenecid results in an approximate twofold increase in exposure to oseltamivir carboxylate due to a decrease in active anionic tubular secretion in the kidney. However, due to the safety margin of oseltamivir carboxylate, no dose adjustments are required when coadministering with probenecid.
Coadministration with amoxicillin does not alter plasma levels of either compound, indicating that competition for the anionic secretion pathway is weak.
In six subjects, multiple doses of oseltamivir did not affect the single-dose pharmacokinetics of acetaminophen.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term carcinogenicity tests with oseltamivir are underway but have not been completed. However, a 26-week dermal carcinogenicity study of oseltamivir carboxylate in FVB/Tg.AC transgenic mice was negative. The animals were dosed at 40, 140, 400 or 780 mg/kg/day in two divided doses. The highest dose represents the maximum feasible dose based on the solubility of the compound in the control vehicle. A positive control, tetradecanoyl phorbol-13-acetate administered at 2.5 µg per dose three times per week gave a positive response.
Oseltamivir was found to be non-mutagenic in the Ames test and the human lymphocyte chromosome assay with and without enzymatic activation and negative in the mouse micronucleus test. It was found to be positive in a Syrian Hamster Embryo (SHE) cell transformation test. Oseltamivir carboxylate was non-mutagenic in the Ames test and the L5178Y mouse lymphoma assay with and without enzymatic activation and negative in the SHE cell transformation test.
In a fertility and early embryonic development study in rats, doses of oseltamivir at 50, 250, and 1500 mg/kg/day were administered to females for 2 weeks before mating, during mating and until day 6 of pregnancy. Males were dosed for 4 weeks before mating, during and for 2 weeks after mating. There were no effects on fertility, mating performance or early embryonic development at any dose level. The highest dose was approximately 100 times the human systemic exposure (AUC 0-24h ) of oseltamivir carboxylate.
Pregnancy
Pregnancy Category C: There are insufficient human data upon which to base an evaluation of risk of TAMIFLU to the pregnant woman or developing fetus. Studies for effects on embryo-fetal development were conducted in rats (50, 250, and 1500 mg/kg/day) and rabbits (50, 150, and 500 mg/kg/day) by the oral route. Relative exposures at these doses were, respectively, 2, 13, and 100 times human exposure in the rat and 4, 8, and 50 times human exposure in the rabbit. Pharmacokinetic studies indicated that fetal exposure was seen in both species. In the rat study, minimal maternal toxicity was reported in the 1500 mg/kg/day group. In the rabbit study, slight and marked maternal toxicities were observed, respectively, in the 150 and 500 mg/kg/day groups. There was a dose-dependent increase in the incidence rates of a variety of minor skeletal abnormalities and variants in the exposed offspring in these studies. However, the individual incidence rate of each skeletal abnormality or variant remained within the background rates of occurrence in the species studied.
Because animal reproductive studies may not be predictive of human response and there are no adequate and well-controlled studies in pregnant women, TAMIFLU should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
In lactating rats, oseltamivir and oseltamivir carboxylate are excreted in the milk. It is not known whether oseltamivir or oseltamivir carboxylate is excreted in human milk. TAMIFLU should, therefore, be used only if the potential benefit for the lactating mother justifies the potential risk to the breast-fed infant.
Geriatric Use
The safety of TAMIFLU has been established in clinical studies which enrolled 741 subjects (374 received placebo and 362 received TAMIFLU). Some seasonal variability was noted in the clinical efficacy outcomes (see Description of Clinical Studies: Studies in Naturally Occurring Influenza: Treatment of Influenza: Geriatric Patients ).
Safety and efficacy have been demonstrated in elderly residents of nursing homes who took TAMIFLU for up to 42 days for the prevention of influenza. Many of these individuals had cardiac and/or respiratory disease, and most had received vaccine that season (see Description of Clinical Studies: Studies in Naturally Occurring Influenza: Prophylaxis of Influenza ).
Pediatric Use
The safety and efficacy of TAMIFLU in pediatric patients younger than 1 year of age have not been studied. TAMIFLU is not indicated for either treatment or prophylaxis of influenza in pediatric patients younger than 1 year of age because of uncertainties regarding the rate of development of the human blood-brain barrier and the unknown clinical significance of non-clinical animal toxicology data for human infants (see ANIMAL TOXICOLOGY ).
ANIMAL TOXICOLOGY
In a 2-week study in unweaned rats, administration of a single dose of 1000 mg/kg oseltamivir phosphate to 7-day-old rats resulted in deaths associated with unusually high exposure to the prodrug. However, at 2000 mg/kg, there were no deaths or other significant effects in 14-day-old unweaned rats. Further follow-up investigations of the unexpected deaths of 7-day-old rats at 1000 mg/kg revealed that the concentrations of the prodrug in the brains were approximately 1500-fold those of the brains of adult rats administered the same oral dose of 1000 mg/kg, and those of the active metabolite were approximately 3-fold higher. Plasma levels of the prodrug were 10-fold higher in 7-day-old rats as compared with adult rats. These observations suggest that the levels of oseltamivir in the brains of rats decrease with increasing age and most likely reflect the maturation stage of the blood-brain barrier. No adverse effects occurred at 500 mg/kg/day administered to 7- to 21-day-old rats. At this dosage, the exposure to prodrug was approximately 800-fold the exposure expected in a 1-year-old child.
ADVERSE REACTIONS
Treatment Studies in Adult Patients
A total of 1171 patients who participated in adult phase III controlled clinical trials for the treatment of influenza were treated with TAMIFLU. The most frequently reported adverse events in these studies were nausea and vomiting. These events were generally of mild to moderate degree and usually occurred on the first 2 days of administration. Less than 1% of subjects discontinued prematurely from clinical trials due to nausea and vomiting.
Adverse events that occurred with an incidence of >/=1% in 1440 patients taking placebo or TAMIFLU 75 mg twice daily in adult phase III treatment studies are shown in Table 3. This summary includes 945 healthy young adults and 495 "at risk" patients (elderly patients and patients with chronic cardiac or respiratory disease). Those events reported numerically more frequently in patients taking TAMIFLU compared with placebo were nausea, vomiting, bronchitis, insomnia, and vertigo.
Table 3. Most Frequent Adverse Events in Studies in Naturally Acquired InfluenzaTreatment Prophylaxis Adverse EventPlacebo
N=716Oseltamivir
75 mg bid
N=724Placebo
N=1434Oseltamivir
75 mg qd
N=1480Nausea (without vomiting)40 (5.6%) 72 (9.9%) 56 (3.9%) 104 (7.0%) Vomiting21 (2.9%) 68 (9.4%) 15 (1.0%) 31 (2.1%) Diarrhea70 (9.8%) 48 (6.6%) 38 (2.6%) 48 (3.2%) Bronchitis15 (2.1%) 17 (2.3%) 17 (1.2%) 11 (0.7%) Abdominal pain16 (2.2%) 16 (2.2%) 23 (1.6%) 30 (2.0%) Dizziness25 (3.5%) 15 (2.1%) 21 (1.5%) 24 (1.6%) Headache14 (2.0%) 13 (1.8%) 251 (17.5%) 298 (20.1%) Cough12 (1.7%) 9 (1.2%) 86 (6.0%) 83 (5.6%) Insomnia6 (0.8%) 8 (1.1%) 14 (1.0%) 18 (1.2%) Vertigo4 (0.6%) 7 (1.0%) 3 (0.2%) 4 (0.3%) Fatigue7 (1.0%) 7 (1.0%) 107 (7.5%) 117 (7.9%) Adverse events included are: all events reported in the treatment studies with frequency >/=1% in the oseltamivir 75 mg bid group.
Additional adverse events occurring in <1% of patients receiving TAMIFLU for treatment included unstable angina, anemia, pseudomembranous colitis, humerus fracture, pneumonia, pyrexia, and peritonsillar abscess.
Prophylaxis Studies
A total of 3434 subjects (adolescents, healthy adults and elderly) participated in phase III prophylaxis studies, of whom 1480 received the recommended dose of 75 mg once daily for up to 6 weeks. Adverse events were qualitatively very similar to those seen in the treatment studies, despite a longer duration of dosing (Table 3). Events reported more frequently in subjects receiving TAMIFLU compared to subjects receiving placebo in prophylaxis studies, and more commonly than in treatment studies, were aches and pains, rhinorrhea, dyspepsia and upper respiratory tract infections. However, the difference in incidence between TAMIFLU and placebo for these events was less than 1%. There were no clinically relevant differences in the safety profile of the 942 elderly subjects who received TAMIFLU or placebo, compared with the younger population.
Treatment Studies in Pediatric Patients
A total of 1032 pediatric patients aged 1 to 12 years (including 698 otherwise healthy pediatric patients aged 1 to 12 years and 334 asthmatic pediatric patients aged 6 to 12 years) participated in phase III studies of TAMIFLU given for the treatment of influenza. A total of 515 pediatric patients received treatment with TAMIFLU oral suspension.
Adverse events occurring in >1% of pediatric patients receiving TAMIFLU treatment are listed in Table 4. The most frequently reported adverse event was vomiting. Other events reported more frequently by pediatric patients treated with TAMIFLU included abdominal pain, epistaxis, ear disorder, and conjunctivitis. These events generally occurred once and resolved despite continued dosing. They did not cause discontinuation of drug in the vast majority of cases.
The adverse event profile in adolescents is similar to that described for adult patients and pediatric patients aged 1 to 12 years.
Table 4. Adverse Events Occurring On Treatment in >1% of Pediatric Patients Enrolled in Phase III Trials of TAMIFLU Treatment of Naturally Acquired InfluenzaAdverse EventPlacebo
N=517TAMIFLU
2 mg/kg twice daily
N=515Vomiting48 (9.3%) 77 (15.0%) Diarrhea55 (10.6%) 49 (9.5%) Otitis media58 (11.2%) 45 (8.7%) Abdominal pain20 (3.9%) 24 (4.7%) Asthma (including aggravated)19 (3.7%) 18 (3.5%) Nausea22 (4.3%) 17 (3.3%) Epistaxis13 (2.5%) 16 (3.1%) Pneumonia17 (3.3%) 10 (1.9%) Ear disorder6 (1.2%) 9 (1.7%) Sinusitis13 (2.5%) 9 (1.7%) Bronchitis11 (2.1%) 8 (1.6%) Conjunctivitis2 (0.4%) 5 (1.0%) Dermatitis10 (1.9%) 5 (1.0%) Lymphadenopathy8 (1.5%) 5 (1.0%) Tympanic membrane disorder6 (1.2%) 5 (1.0%) Observed During Clinical Practice for Treatment
The following adverse reactions have been identified during postmarketing use of TAMIFLU. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to TAMIFLU exposure.
General: Rash, swelling of the face or tongue, toxic epidermal necrolysis
Digestive: Hepatitis, liver function tests abnormal
Cardiac: Arrhythmia
Neurologic: Seizure, confusion
Metabolic: Aggravation of diabetes
OVERDOSAGE
At present, there has been no experience with overdose. Single doses of up to 1000 mg of TAMIFLU have been associated with nausea and/or vomiting.
DOSAGE AND ADMINISTRATION
TAMIFLU may be taken with or without food (see CLINICAL PHARMACOLOGY: Pharmacokinetics ). However, when taken with food, tolerability may be enhanced in some patients.
Standard Dosage - Treatment of Influenza:
Adults and Adolescents
The recommended oral dose of TAMIFLU for treatment of influenza in adults and adolescents 13 years and older is 75 mg twice daily for 5 days. Treatment should begin within 2 days of onset of symptoms of influenza.
Pediatric Patients
TAMIFLU is not indicated for treatment of influenza in pediatric patients younger than 1 year.
The recommended oral dose of TAMIFLU oral suspension for pediatric patients 1 year and older or adult patients who cannot swallow a capsule is:
Body Weight in kg Body Weight in lbs Recommended Dose
for 5 DaysNumber of
Bottles Needed to
Obtain the
Recommended
Dose</=15 kg </=33 lbs 30 mg twice daily 1 >15 kg to 23 kg >33 lbs to 51 lbs 45 mg twice daily 2 >23 kg to 40 kg >51 lbs to 88 lbs 60 mg twice daily 2 >40 kg >88 lbs 75 mg twice daily 3 An oral dosing dispenser with 30 mg, 45 mg, and 60 mg graduations is provided with the oral suspension; the 75 mg dose can be measured using a combination of 30 mg and 45 mg. It is recommended that patients use this dispenser. In the event that the dispenser provided is lost or damaged, another dosing syringe or other device may be used to deliver the following volumes: 2.5 mL (1/2 tsp) for children </=15 kg, 3.8 mL (3/4 tsp) for >15 to 23 kg, 5.0 mL (1 tsp) for >23 to 40 kg, and 6.2 mL (1 1/4 tsp) for >40 kg.
Standard Dosage - Prophylaxis of Influenza:
The safety and efficacy of TAMIFLU for prophylaxis of influenza in pediatric patients younger than 13 years of age have not been established.
The recommended oral dose of TAMIFLU for prophylaxis of influenza in adults and adolescents 13 years and older following close contact with an infected individual is 75 mg once daily for at least 7 days. Therapy should begin within 2 days of exposure. The recommended dose for prophylaxis during a community outbreak of influenza is 75 mg once daily. Safety and efficacy have been demonstrated for up to 6 weeks. The duration of protection lasts for as long as dosing is continued.
Special Dosage Instructions
Hepatic Impairment
The safety and pharmacokinetics in patients with hepatic impairment have not been evaluated.
Renal Impairment
For plasma concentrations of oseltamivir carboxylate predicted to occur following various dosing schedules in patients with renal impairment, see CLINICAL PHARMACOLOGY: Pharmacokinetics: Special Populations .
Treatment of Influenza
Dose adjustment is recommended for patients with creatinine clearance between 10 and 30 mL/min receiving TAMIFLU for the treatment of influenza. In these patients it is recommended that the dose be reduced to 75 mg of TAMIFLU once daily for 5 days. No recommended dosing regimens are available for patients undergoing routine hemodialysis and continuous peritoneal dialysis treatment with end-stage renal disease.
Prophylaxis of Influenza
For the prophylaxis of influenza, dose adjustment is recommended for patients with creatinine clearance between 10 and 30 mL/min receiving TAMIFLU. In these patients it is recommended that the dose be reduced to 75 mg of TAMIFLU every other day or 30 mg TAMIFLU oral suspension every day. No recommended dosing regimens are available for patients undergoing routine hemodialysis and continuous peritoneal dialysis treatment with end-stage renal disease.
Geriatric Patients
No dose adjustment is required for geriatric patients (see CLINICAL PHARMACOLOGY : Pharmacokinetics: Special Populations and PRECAUTIONS ).
Preparation of TAMIFLU Oral Suspension
It is recommended that TAMIFLU oral suspension be constituted by the pharmacist prior to dispensing to the patient:
- Tap the closed bottle several times to loosen the powder.
- Measure 23 mL of water in a graduated cylinder.
- Add the total amount of water for constitution to the bottle and shake the closed bottle well for 15 seconds.
- Remove the child-resistant cap and push bottle adapter into the neck of the bottle.
- Close bottle with child-resistant cap tightly. This will assure the proper seating of the bottle adapter in the bottle and child-resistant status of the cap.
NOTE: SHAKE THE TAMIFLU ORAL SUSPENSION WELL BEFORE EACH USE.
The constituted oral suspension should be used within 10 days of preparation; the pharmacist should write the date of expiration of the constituted suspension on a pharmacy label. The patient package insert and oral dispenser should be dispensed to the patient.
HOW SUPPLIED
TAMIFLU Capsules
Supplied as 75-mg (75 mg free base equivalent of the phosphate salt) grey/light yellow hard gelatin capsules. "Roche" is printed in blue ink on the grey body and "75 mg" is printed in blue ink on the light yellow cap. Available in blister packages of 10 (NDC 0004-0800-85).
Storage
Store the capsules at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86[ordm ]F). [See USP Controlled Room Temperature]
TAMIFLU for Oral Suspension
Supplied as a white powder blend for constitution to a white tutti-frutti-flavored suspension. Available in glass bottles containing 25 mL of suspension after constitution equivalent to 300 mg oseltamivir base. Each bottle is supplied with a bottle adapter and 1 oral dispenser (NDC 0004-0810-95).
Storage
Store dry powder at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature]
Store constituted suspension under refrigeration at 2° to 8°C (36° to 46°F). Do not freeze.
Manufactured by F. Hoffmann-La Roche Ltd., Basel, Switzerland
Distributed by Roche Laboratories Inc., Nutley, New Jersey 07110-1199
Licensor: Gilead Sciences, Inc., Foster City, California 94404
Revised: June 2004
Patient Information
TAMIFLU® (oseltamivir phosphate)
Rx onlyThis leaflet contains important information about TAMIFLU (TAM-ih-flew). Read it well before you begin treatment. This information does not take the place of talking with your health care professional about your medical condition or your treatment. This leaflet does not list all the benefits and risks of TAMIFLU. If you have any questions about TAMIFLU, ask your health care professional. Only your health care professional can determine if TAMIFLU is right for you.
What is TAMIFLU?
TAMIFLU attacks the influenza virus and stops it from spreading inside your body. TAMIFLU treats flu at its source, by attacking the virus that causes the flu, rather than simply masking symptoms.
TAMIFLU is for treating adults and children age 1 and older with the flu whose flu symptoms started within the last day or two. TAMIFLU can also reduce the chance of getting the flu in people age 13 and older who have a higher chance of getting the flu because they spend time with someone who has the flu. TAMIFLU can also reduce the chance of getting the flu if there is a flu outbreak in the community.
What is "Flu"?
"The flu" is an infection caused by the influenza virus. Flu symptoms include fever (usually 100°F to 103°F in adults, and sometimes higher in children) and problems such as cough, sore throat, runny or stuffy nose, headaches, muscle aches, fever, and extreme tiredness. Many people use the term "flu" to mean any combination of these symptoms, such as the common cold, but true influenza infection is often worse and may last longer than a cold.
Flu outbreaks happen about once a year, usually in the winter, when the influenza virus spreads widely in the community. Outside of those outbreaks, only a very tiny number of respiratory infections are caused by the influenza virus.
Should I get a flu shot?
TAMIFLU is not a substitute for a flu vaccination. You should continue to get a flu vaccination every year, according to your health care professional's advice.
Who should not take TAMIFLU?
Do not take TAMIFLU if you are allergic to the main ingredient, oseltamivir phosphate, or to any other ingredients of TAMIFLU. Before starting treatment, make sure your health care professional knows if you take any other medicines, or are pregnant, planning to become pregnant, or breastfeeding. TAMIFLU is normally not recommended for use during pregnancy or nursing, as the effects on the unborn child or nursing infant are unknown. TAMIFLU is not recommended for use in children younger than 1 year of age.
Tell your health care professional if you have any type of kidney disease, heart disease, respiratory disease, or any serious health condition.
How should I take TAMIFLU?
It is important that you begin your treatment with TAMIFLU as soon as possible from the first appearance of your flu symptoms or soon after you are exposed to the flu. If you feel worse or develop new symptoms during treatment with TAMIFLU, or if your flu symptoms do not start to get better, you should contact your health care professional.
If you have the flu : Take TAMIFLU twice a day for 5 days, once in the morning and once in the evening. You should complete the entire treatment of 10 doses (capsules or liquid), even if you feel better.
To prevent the flu : If someone in your home has the flu, take TAMIFLU once a day for at least 7 days or for as long as prescribed. You can take TAMIFLU for up to 6 weeks if you are exposed to the flu because of an outbreak in your community. Follow your health care professional's advice on how long to take TAMIFLU.
You can take TAMIFLU with food or without food. There is less chance of stomach upset if you take it with a light snack, milk, or a meal.
If you are taking TAMIFLU liquid, your pharmacist will give you a dosing dispenser marked with three possible doses. Follow your health care professional's instructions on which dose to take or how to combine them for the proper dose for you. In order to be sure you receive the proper dose, it is important that you use the dispenser provided. Review the instructions below on how to use the dispenser and ask your pharmacist if you have any questions. If you lose or damage the dispenser and cannot use it, contact your health care professional or pharmacist for advice on the proper dose.
If you forget to take your medicine, take the missed dose as soon as you remember, except if it is 2 hours or less before your next dose. Then continue to take TAMIFLU at the usual times. Do not take 2 doses at a time to make up for a missed dose. If you miss several doses, tell your health care professional and follow the advice given to you.
What are the possible side effects of TAMIFLU?
The most common side effects of TAMIFLU are nausea and vomiting. These are usually mild to moderate. They usually happen in the first 2 days of treatment. Taking TAMIFLU with food may reduce the chance of getting these side effects.
If you notice any side effects not mentioned in this leaflet, or if you have any concerns about the side effects you get, tell your health care professional.
How and where should I store TAMIFLU?
TAMIFLU capsules should be stored at room temperature below 77°F (25°C) and kept in a dry place. Keep this medication out of reach of children.
TAMIFLU suspension should be stored under refrigeration at 36° to 46°F (2° to 8°C). Do not freeze.
General advice about prescription medicines:
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use TAMIFLU for a condition for which it was not prescribed. Do not give TAMIFLU to other people, even if they have the same symptoms you have. It may not be right for them.
This leaflet summarizes the most important information about TAMIFLU. If you would like more information, talk with your health care professional. You can ask your pharmacist or health care professional for information about TAMIFLU that is written for health professionals.
DOSING INSTRUCTIONS FOR PATIENTS:
Please follow instructions carefully to ensure proper dosing of the oral suspension.
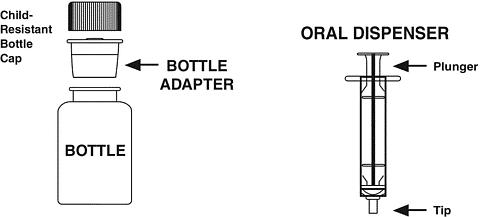
- Shake closed bottle well for about 5 seconds before each use.
- Remove child-resistant cap.
- Before inserting the tip of the oral dispenser into bottle adapter, push the plunger completely down toward the tip of the oral dispenser. Insert tip firmly into opening of the bottle adapter.
- Turn the entire unit (bottle and oral dispenser) upside down.
- Pull the plunger out slowly until the desired amount of medication is withdrawn into the oral dispenser (see figure). The 75 mg dose is obtained by filling the dispenser twice, once to the 30 mg graduation, and a second fill to the 45 mg graduation.
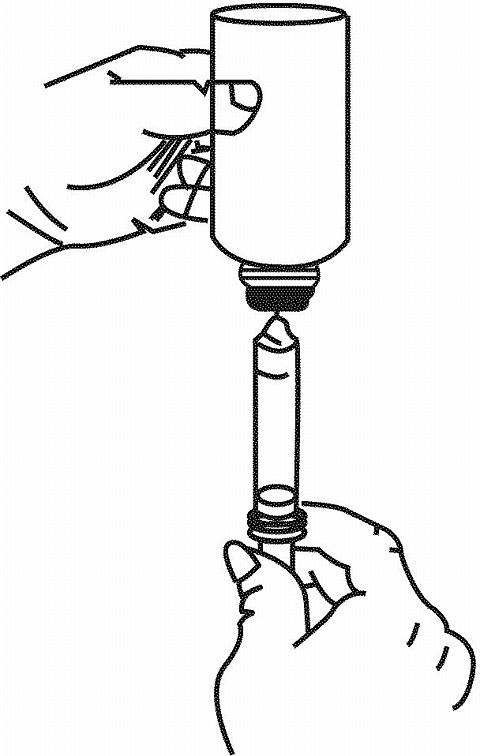
- Turn the entire unit right side up and remove the oral dispenser slowly from the bottle.
- Dispense directly into mouth. Do not mix with any liquid prior to dispensing.
- Close bottle with child-resistant cap after each use.
-
Disassemble oral dispenser, rinse under running tap water and air dry prior to next use.
Revised: June 2004
Subscribe to the "News" RSS Feed 
TOP ۞
