-
Thyrogen for Injection (Genzyme)
DESCRIPTION
Thyrogen® (thyrotropin alfa for injection) contains a highly purified recombinant form of human thyroid stimulating hormone (TSH), a glycoprotein which is produced by recombinant DNA technology. Thyrotropin alfa is synthesized in a genetically modified Chinese hamster ovary cell line.
Thyrotropin alfa is a heterodimeric glycoprotein comprised of two non-covalently linked subunits, an alpha subunit of 92 amino acid residues containing two N-linked glycosylation sites and a beta subunit of 118 residues containing one N-linked glycosylation site. The amino acid sequence of thyrotropin alfa is identical to that of human pituitary thyroid stimulating hormone.
Both thyrotropin alfa and naturally occurring human pituitary thyroid stimulating hormone are synthesized as a mixture of glycosylation variants. Unlike pituitary TSH, which is secreted as a mixture of sialylated and sulfated forms, thyrotropin alfa is sialylated but not sulfated. The biological activity of thyrotropin alfa is determined by a cell-based bioassay. In this assay, cells expressing a functional TSH receptor and a cAMP-responsive element coupled to a heterologous reporter gene, luciferase, enable the measurement of rhTSH activity by measuring the luciferase response. The specific activity of thyrotropin alfa is 4-12 IU/mg using this cell-based bioassay. The specific activity of thyrotropin alfa is determined relative to an internal Genzyme reference material that was calibrated against the World Health Organization (WHO) human pituitary derived TSH reference standard NIBSC 84/703 using an in vitro bioassay that measures the amount of cAMP produced by a bovine thyroid microsome preparation in response to rhTSH.
Thyrogen is supplied as a sterile, non-pyrogenic, white to off-white lyophilized product, intended for intramuscular (IM) administration after reconstitution with Sterile Water for Injection, USP. Each vial of Thyrogen contains 1.1 mg thyrotropin alfa (4-12 IU/mg), 36 mg Mannitol, 5.1 mg Sodium Phosphate, and 2.4 mg Sodium Chloride.
After reconstitution with 1.2 mL of Sterile Water for Injection, USP, the thyrotropin alfa concentration is 0.9 mg/mL. The pH of the reconstituted solution is approximately 7.0.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Thyrotropin alfa (recombinant human thyroid stimulating hormone) is a heterodimeric glycoprotein produced by recombinant DNA technology. It has comparable biochemical properties to the human pituitary TSH. Binding of thyrotropin alfa to TSH receptors on normal thyroid epithelial cells or on well-differentiated thyroid cancer tissue stimulates iodine uptake and organification, and synthesis and secretion of thyroglobulin (Tg), triiodothyronine (T 3 ) and thyroxine (T 4 ).
In patients with thyroid cancer, a near total or total thyroidectomy is performed and patients are placed on synthetic thyroid hormone supplements to replace endogenous hormone and to suppress serum levels of TSH in order to avoid TSH-stimulated tumor growth. Thereafter, patients are followed up for the presence of remnants or of residual or recurrent cancer by thyroglobulin (Tg) testing while they remain on thyroid hormone suppressive therapy and are euthyroid, or by Tg testing and radioiodine imaging after thyroid hormone withdrawal. Thyrogen is an exogenous source of human TSH that offers an additional diagnostic tool in the follow-up of patients with a history of well-differentiated thyroid cancer.
Pharmacokinetics
The pharmacokinetics of Thyrogen were studied in 16 patients with well-differentiated thyroid cancer given a single 0.9 mg IM dose. Mean peak concentrations of 116 ± 38 mU/L were reached between 3 and 24 hours after injection (median of 10 hours). The mean apparent elimination half-life was 25 ± 10 hours. The organ(s) of TSH clearance in man have not been identified, but studies of pituitary-derived TSH suggest the involvement of the liver and kidneys.
Clinical Trials
Two phase 3 clinical trials were conducted in 358 evaluable patients with well-differentiated thyroid cancer to compare 48-hour radioiodine ( 131 I) whole body scans obtained after Thyrogen to whole body scans after thyroid hormone withdrawal. One of these trials also compared Tg levels obtained after Thyrogen to those on thyroid hormone suppressive therapy, and to those after thyroid hormone withdrawal. All Tg testing was performed in a central laboratory using a radioimmunoassay (RIA) with a functional sensitivity of 2.5 ng/mL. Only successfully ablated patients (defined as patients who have undergone total or near total thyroidectomy with or without radioiodine ablation, and with < 1% uptake in the thyroid bed on a scan after thyroid hormone withdrawal) without detectable anti-thyroglobulin antibodies were included in the Tg data analysis. The maximum Thyrogen Tg value was obtained 72 hours after the final Thyrogen injection, and this value was used in the analysis (see DOSAGE AND ADMINISTRATION ).
Radioiodine Whole Body Scan Results
The following table summarizes the scan data in patients with positive scans after withdrawal of thyroid hormone from the phase 3 studies:
# scan pairs by
disease
category#(%) scan pairs
in which Thyrogen
scan detected
disease seen on
withdrawal scan#(%) scan pairs
in which Thyrogen
scan did not detect
disease seen on
withdrawal scanFirst Phase 3 Study (0.9 mg IM qd × 2)positive for remnant or cancer in thyroid bed48 39(81) 9(19) metastatic disease15 11(73) 4(27) total positive withdrawal scans *63 50(79) 13(21) Second Phase 3 Study (0.9 mg IM qd × 2)positive for remnant or cancer in thyroid bed35 30(86) 5(14) metastatic disease9 6(67) 3(33) total positive withdrawal scans *44 36(82) 8(18) Second Phase 3 Study (0.9 mg IM q 72 hrs × 3)positive for remnant or cancer in thyroid bed41 35(85) 6(15) metastatic disease14 12(86) 2(14) total positive withdrawal scans *55 47(85) 8(15) * Across all studies, uptake was detected on the Thyrogen scan but not observed on the scan after thyroid hormone withdrawal in 5 patients with remnant or cancer in the thyroid bed. Across the two clinical studies, the Thyrogen scan failed to detect remnant and/or cancer localized to the thyroid bed in 16% (20/124) of patients in whom it was detected by a scan after thyroid hormone withdrawal. In addition, the Thyrogen scan failed to detect metastatic disease in 24% (9/38) of patients in whom it was detected by a scan after thyroid hormone withdrawal.
Thyroglobulin (Tg) Results:
Thyrogen Tg Testing Alone and in Combination with Radioiodine Imaging: Comparison with Results after Thyroid Hormone Withdrawal:
In Tg antibody negative patients with a thyroid remnant or cancer as defined by a withdrawal Tg >/= 2.5 ng/mL or a positive scan (after thyroid hormone withdrawal or after radioiodine therapy), the Thyrogen Tg was >/= 2.5 ng/mL in 69% (40/58) of patients after 2 doses of Thyrogen, and in 80% (53/66) of patients after 3 doses of Thyrogen. Across both dosage groups, 45% had a Tg >/= 2.5 ng/mL on thyroid hormone suppressive therapy.
In these same patients, adding the whole body scan increased the detection rate of thyroid remnant or cancer to 84% (49/58) of patients after 2 doses of Thyrogen and 94% (62/66) of patients after 3 doses of Thyrogen.
Thyrogen Tg Testing Alone and in Combination with Radioiodine Imaging in Patients with Confirmed Metastatic Disease:
Metastatic disease was confirmed by a post-treatment scan or by lymph node biopsy in 35 patients. Thyrogen Tg was >/= 2.5 ng/mL in all 35 patients while Tg on thyroid hormone suppressive therapy was >/= 2.5 ng/mL in 79% of these patients.
In this same cohort of 35 patients with confirmed metastatic disease, the Thyrogen Tg levels were below 10 ng/mL in 27% (3/11) of patients after 2 doses of Thyrogen and in 13% (3/24) of patients after 3 doses of Thyrogen. The corresponding thyroid hormone withdrawal Tg levels in these 6 patients were 15.6-137 ng/mL. The Thyrogen scan detected metastatic disease in 1 of these 6 patients (see INDICATIONS AND USAGE , Considerations in the Use of Thyrogen ).
As with thyroid hormone withdrawal, the intra-patient reproducibility of Thyrogen testing with regard to both Tg stimulation and radioiodine imaging has not been studied.
Quality of Life:
Following Thyrogen, no change was observed in any of the 8 domains of the SF-36 Health Survey, a patient-administered quality-of-life measurement instrument. Following thyroid hormone withdrawal, statistically significant negative changes in quality of life parameters were observed in 4 of the 8 SF-36 domains. These 4 domains were: physical functioning, physical role, bodily pain and emotional role. No change was observed in the following scales: general health, vitality, social functioning and mental health.
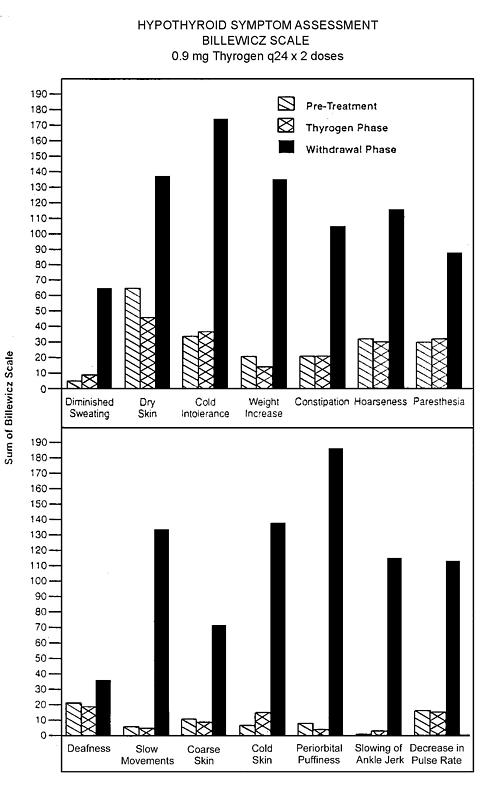
Hypothyroid Signs and Symptoms:
Thyrogen administration was not associated with the signs and symptoms of hypothyroidism that accompanied thyroid hormone withdrawal as measured by the Billewicz scale. Statistically significant worsening in all signs and symptoms were observed during the hypothyroid phase (p<0.01).
INDICATIONS AND USAGE
Thyrogen (thyrotropin alfa for injection) is indicated for use as an adjunctive diagnostic tool for serum thyroglobulin (Tg) testing with or without radioiodine imaging in the follow-up of patients with well-differentiated thyroid cancer.
Potential Clinical Uses:
- Thyrogen Tg testing may be used in patients with an undetectable Tg on thyroid hormone suppressive therapy to exclude the diagnosis of residual or recurrent thyroid cancer (see CLINICAL PHARMACOLOGY , Clinical Trials , Thyroglobulin (Tg) Results ).
- Thyrogen testing may be used in patients requiring serum Tg testing and radioiodine imaging who are unwilling to undergo thyroid hormone withdrawal testing and whose treating physician believes that use of a less sensitive test is justified.
- Thyrogen testing may be used in patients who are either unable to mount an adequate endogenous TSH response to thyroid hormone withdrawal or in whom withdrawal is medically contraindicated.
Considerations in the Use of Thyrogen:
- Even when Thyrogen-stimulated Tg testing is performed in combination with radioiodine imaging, there remains a meaningful risk of missing a diagnosis of thyroid cancer or of underestimating the extent of disease. Therefore, thyroid hormone withdrawal Tg testing with radioiodine imaging remains the standard diagnostic modality to assess the presence, location and extent of thyroid cancer.
- Thyrogen Tg levels are generally lower than, and do not correlate with Tg levels after thyroid hormone withdrawal (see CLINICAL PHARMACOLOGY , Thyroglobulin (Tg) Results ).
- A newly detectable Tg level or a Tg level rising over time after Thyrogen, or a high index of suspicion of metastatic disease, even in the setting of a negative or low-stage Thyrogen radioiodine scan, should prompt further evaluation such as thyroid hormone withdrawal to definitively establish the location and extent of thyroid cancer. On the other hand, none of the 31 patients studied with undetectable Thyrogen Tg levels (< 2.5 ng/mL) had metastatic disease. Therefore, an undetectable Thyrogen Tg level suggests the absence of clinically significant disease (see CLINICAL PHARMACOLOGY , Clinical Trials ).
- The decisions whether to perform a Thyrogen radioiodine scan in conjunction with a Thyrogen serum Tg test and whether and when to withdraw a patient from thyroid hormone are complex. Pertinent factors in these decisions include the sensitivity of the Tg assay used, the Thyrogen Tg level obtained, and the index of suspicion of recurrent or persistent local or metastatic disease. In the clinical trials, combination Tg and scan testing did enhance the diagnostic accuracy of Thyrogen in some cases (see CLINICAL PHARMACOLOGY , Clinical Trials ).
- Thyrogen is not recommended to stimulate radioiodine uptake for the purposes of ablative radiotherapy of thyroid cancer.
- The signs and symptoms of hypothyroidism which accompany thyroid hormone withdrawal are avoided with Thyrogen (see CLINICAL PHARMACOLOGY , Clinical Trials , Quality of Life , Hypothyroid Signs and Symptoms ).
PRECAUTIONS
(see INDICATIONS AND USAGE , Considerations in the Use of Thyrogen )
General
The use of Thyrogen (thyrotropin alfa for injection) should be directed by physicians knowledgeable in the management of patients with thyroid cancer.
Thyroglobulin (Tg) antibodies may confound the Tg assay and render Tg levels uninterpretable. Therefore, in such cases, even with a negative or low-stage Thyrogen radioiodine scan, consideration should be given to evaluating patients further with, for example, a confirmatory thyroid hormone withdrawal scan to determine the location and extent of thyroid cancer.
Thyrogen should be administered intramuscularly only. It should not be administered intravenously.
TSH antibodies have not been reported in patients treated with Thyrogen in the clinical trials, although only 27 patients received Thyrogen on more than one occasion.
Caution should be exercised when Thyrogen is administered to patients who have been previously treated with bovine TSH and, in particular, to those patients who have experienced hypersensitivity reactions to bovine TSH.
Thyrogen is known to cause a transient but significant rise in serum thyroid hormone concentration. Therefore, caution should be exercised in patients with a known history of heart disease and with significant residual thyroid tissue (see ADVERSE REACTIONS ).
Drug-Drug Interactions
Formal interaction studies between Thyrogen and other medicinal products have not been performed. In clinical trials, no interactions were observed between Thyrogen and the thyroid hormones triiodothyronine (T 3 ) and thyroxine (T 4 ) when administered concurrently.
The use of Thyrogen allows for radioiodine imaging while patients are euthyroid on triiodothyronine (T 3 ) and/or thyroxine (T 4 ). Data on radioiodine 131 I kinetics indicate that the clearance of radioiodine is approximately 50% greater in euthyroid patients than in hypothyroid patients, who have decreased renal function. Thus radioiodine retention is less in euthyroid patients at the time of imaging and this factor should be considered when selecting the activity of radio-iodine for use in radioiodine imaging.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term toxicity studies in animals have not been performed with Thyrogen to evaluate the carcinogenic potential of the drug. Thyrogen was not mutagenic in the bacterial reverse mutation assay. Studies have not been performed with Thyrogen to evaluate the effects on fertility.
Pregnancy Category C
Animal reproduction studies have not been conducted with Thyrogen.
It is also not known whether Thyrogen can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Thyrogen should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether the drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Thyrogen is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 16 years have not been established.
Geriatric Use
Results from controlled trials indicate no difference in the safety and efficacy of Thyrogen between adult patients less than 65 years and those greater than 65 years of age.
ADVERSE REACTIONS
Adverse reaction data are derived from the two clinical trials in which 381 patients were treated with Thyrogen (thyrotropin alfa for injection) and from post-marketing surveillance.
The most common adverse events (> 5%) reported in clinical trials were: nausea (10.5%) and headache (7.3%). Events reported in >/= 1% of patients in the trials are summarized in the following table:
Summary of Adverse Events During
Clinical Studies (>/= 1%)% of Patients with Adverse Events (n)
(n = 381)Body as a WholeHeadache7.3%(28) Asthenia3.4%(13) Chills1.0%(4) Fever1.0%(4) Flu Syndrome1.0%(4) Digestive SystemNausea10.5%(40) Vomiting2.1%(8) Nausea and Vomiting1.3%(5) Nervous SystemDizziness1.6%(6) Paresthesia1.6%(6) There have been several reports of hypersensitivity reactions including urticaria, rash, pruritus, flushing and respiratory difficulties requiring treatment. However, in clinical trials no patients have developed antibodies to thyrotropin alfa, either after single dose or repeated (27 patients) use of the product.
Four patients out of 55 (7.3%) with CNS metastases who were followed in a special treatment protocol experienced acute hemiplegia, hemiparesis or pain one to three days after Thyrogen administration. The symptoms were attributed to local edema and/or focal hemorrhage at the site of the cerebral or spinal cord metastases. In addition, one case each of acute visual loss and of laryngeal edema with respiratory distress, requiring tracheotomy, with onset of symptoms within 24 hours after Thyrogen administration, have been reported in patients with metastases to the optic nerve and paratracheal areas, respectively. In addition, sudden, rapid and painful enlargement of locally recurring papillary carcinoma has been reported within 12-48 hours of Thyrogen administration. The enlargement was accompanied by dyspnea, stridor or dysphonia. Rapid clinical improvement occurred following glucocorticoid therapy. It is recommended that pretreatment with glucocorticoids be considered for patients in whom local tumor expansion may compromise vital anatomic structures.
A 77 year-old non-thyroidectomized patient with a history of heart disease and spinal metastases who received 4 Thyrogen injections over 6 days in a special treatment protocol experienced a fatal MI 24 hours after he received the last Thyrogen injection. The event was likely related to Thyrogen-induced hyperthyroidism.
OVERDOSAGE
There has been no reported experience of overdose in humans. However, in clinical trials, three patients experienced symptoms after receiving Thyrogen doses higher than those recommended. Two patients had nausea after a 2.7 mg IM dose, and in one of these patients, the event was accompanied by weakness, dizziness and headache. Another patient experienced nausea, vomiting and hot flashes after a 3.6 mg IM dose.
In addition, one patient experienced symptoms after receiving Thyrogen intravenously. This patient received 0.3 mg Thyrogen as a single intravenous bolus and, 15 minutes later experienced severe nausea, vomiting, diaphoresis, hypotension (BP decreased from 115/66 mm Hg to 81/44 mm Hg) and tachycardia (pulse increased from 75 to 117 bpm).
DOSAGE AND ADMINISTRATION
Thyrogen 0.9 mg intramuscularly may be administered every 24 hours for two doses or every 72 hours for three doses.
After reconstitution with 1.2 mL Sterile Water for Injection, a 1.0 mL solution (0.9 mg thyrotropin alfa) is administered by intramuscular injection to the buttock.
For radioiodine imaging, radioiodine administration should be given 24 hours following the final Thyrogen injection. Scanning should be performed 48 hours after radioiodine administration (72 hours after the final injection of Thyrogen).
The following parameters utilized in the second Phase 3 study are recommended for radioiodine scanning with Thyrogen:
- A diagnostic activity of 4 mCi (148 MBq) 131 I should be used.
- Whole body images should be acquired for a minimum of 30 minutes and/or should contain a minimum of 140,000 counts.
- Scanning times for single (spot) images of body regions should be 10-15 minutes or less if the minimum number of counts is reached sooner (i.e., 60,000 for a large field of view camera, 35,000 counts for a small field of view).
For serum Tg testing, the serum sample should be obtained 72 hours after the final injection of Thyrogen.
INSTRUCTIONS FOR USE
Thyrogen (thyrotropin alfa for injection) is for intramuscular injection to the buttock. The powder should be reconstituted immediately prior to use with 1.2 mL of sterile Water for Injection, USP. Each vial of Thyrogen and each vial of diluent, if provided, is intended for single use. Discard unused portion of the diluent.
Thyrogen should be stored at 2-8°C (36-46°F). Each vial, after reconstitution with 1.2 mL of the accompanying Sterile Water for Injection, USP, should be inspected visually for particulate matter or discoloration before use. Any vials exhibiting particulate matter or discoloration should not be used.
If necessary, the reconstituted solution can be stored for up to 24 hours at a temperature between 2°C and 8°C, while avoiding microbial contamination.
DO NOT USE Thyrogen after the expiration date on the vial. Protect from light.
HOW SUPPLIED
Thyrogen (thyrotropin alfa for injection) is supplied as a sterile, non-pyrogenic, lyophilized product. It is available either in a two-vial kit or a four-vial kit. The two-vial kit contains two 1.1 mg vials of Thyrogen® (thyrotropin alfa for injection). The four-vial kit contains two 1.1 mg vials of Thyrogen®, as well as two 10 mL vials of Sterile Water for Injection, USP.
NDC 58468-1849-4 (4-vial kit)
NDC 58468-0030-2 (2-vial kit)
Store at 2-8°C.
Rx ONLY
Thyrogen® (thyrotropin alfa for injection)
Genzyme Corporation 500 Kendall Street Cambridge, MA 02142 GENERAL(800) 745-4447 therapeutics4728 (12/04)
Subscribe to the "News" RSS Feed 
TOP ۞
