-
Trelstar LA Suspension (Watson)
DESCRIPTION
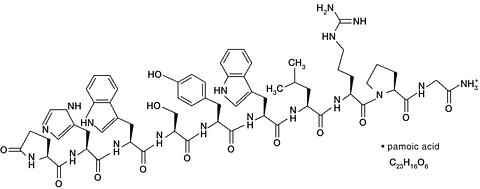
TRELSTAR LA contains a pamoate salt of triptorelin, and triptorelin is a synthetic decapeptide agonist analog of luteinizing hormone releasing hormone (LHRH or GnRH) with greater potency than the naturally occurring LHRH. The chemical name of triptorelin pamoate is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-tryptophyl-L-leucyl-L-arginyl-L-prolylglycine amide (pamoate salt); the empirical formula is C 64 H 82 N 18 O 13 · C 23 H 16 O 6 and the molecular weight is 1699.9. The structural formula is shown below.
TRELSTAR LA is a sterile, lyophilized biodegradable microgranule formulation supplied as a single-dose vial containing triptorelin pamoate (11.25 mg as the peptide base), 145 mg poly- d,l -lactide-co-glycolide, 85 mg mannitol, USP, 30 mg carboxymethylcellulose sodium, USP, 2 mg polysorbate 80, NF. When 2 mL sterile water for injection is added to the vial containing TRELSTAR LA and mixed, a suspension is formed which is intended as an intramuscular injection to be administered every 84 days (ie, every 12 weeks). TRELSTAR LA is available in 2 packaging configurations: (a) TRELSTAR LA vial alone or (b) TRELSTAR LA vial plus a separate pre-filled syringe that contains sterile water for injection, USP, 2 mL, pH 6 to 8.5 (Clip'n'Ject®).
CLINICAL PHARMACOLOGY
Mechanism of Action
Triptorelin is a potent inhibitor of gonadotropin secretion when given continuously and in therapeutic doses. Following the first administration, there is a transient surge in circulating levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, and estradiol (see ADVERSE REACTIONS ). After chronic and continuous administration, usually 2 to 4 weeks after initiation of therapy, a sustained decrease in LH and FSH secretion and marked reduction of testicular and ovarian steroidogenesis is observed. In men, a reduction of serum testosterone concentration to a level typically seen in surgically castrated men is obtained. Consequently, the result is that tissues and functions that depend on these hormones for maintenance become quiescent. These effects are usually reversible after cessation of therapy.
Following a single intramuscular (IM) injection of TRELSTAR LA to men with advanced prostate cancer, serum testosterone levels first increased, peaking on days 2-3, and declined thereafter to low levels by weeks 3-4.
Pharmacokinetics
Results of pharmacokinetic investigations conducted in healthy men indicate that after intravenous (IV) bolus administration, triptorelin is distributed and eliminated according to a 3-compartment model and corresponding half-lives are approximately 6 minutes, 45 minutes, and 3 hours.
Absorption: Triptorelin pamoate is not active when given orally. The pharmacokinetic parameters following a single IM injection of 11.25 mg of TRELSTAR LA to 13 patients with prostate cancer are listed in Table 1. Triptorelin did not accumulate over 9 months of treatment.
TABLE 1. PHARMACOKINETIC PARAMETERS
(MEAN ± SD) FOLLOWING
INTRAMUSCULAR ADMINISTRATION OF
TRELSTAR LA TO PATIENTS WITH PROSTATE CANCERDose
(No. of subjects)C max(0-85d)
(ng/mL)T max(1-85d)
(h)AUC (1-85d)
(h·ng/mL)11.25 mg
(n=13)38.5 ± 10.5 2.9 ± 1.3 2268.0 ± 444.6 Distribution: The volume of distribution following a single IV bolus dose of 0.5 mg of triptorelin peptide was 30-33 L in healthy male volunteers. There is no evidence that triptorelin, at clinically relevant concentrations, binds to plasma proteins.
Metabolism: The metabolism of triptorelin in humans is unknown, but is unlikely to involve hepatic microsomal enzymes (cytochrome P-450). However, the effect of triptorelin on the activity of other drug metabolizing enzymes is unknown. Thus far, no metabolites of triptorelin have been identified. Pharmacokinetic data suggest that C-terminal fragments produced by tissue degradation are either completely degraded in the tissues, or rapidly degraded in plasma, or cleared by the kidneys.
Excretion: Triptorelin is eliminated by both the liver and the kidneys. Following IV administration of 0.5 mg triptorelin peptide to 6 healthy male volunteers with a creatinine clearance of 149.9 mL/min, 41.7% of the dose was excreted in urine as intact peptide with a total triptorelin clearance of 211.9 mL/min. This percentage increased to 62.3% in patients with liver disease who have a lower creatinine clearance (89.9 mL/min). It has also been observed that the nonrenal clearance of triptorelin (patient anuric, Cl creat =0) was 76.2 mL/min, thus indicating that the nonrenal elimination of triptorelin is mainly dependent on the liver (see Special Populations ).
Special Populations:
Renal and Hepatic Impairment: After an IV bolus injection of 0.5 mg triptorelin peptide, the two distribution half-lives were unaffected by renal and hepatic impairment, but renal insufficiency led to a decrease in total triptorelin clearance proportional to the decrease in creatinine clearance as well as an increase in volume of distribution and consequently an increase in elimination half-life (Table 2). The decrease in triptorelin clearance was more pronounced in subjects with liver insufficiency, but the half-life was prolonged similarly in subjects with renal insufficiency, since the volume of distribution was only minimally increased. Patients with renal or hepatic impairment had 2- to 4-fold higher exposure (AUC values) than young healthy males.
Age and Race: The effects of age and race on triptorelin pharmacokinetics have not been systematically studied. However, pharmacokinetic data obtained in young healthy male volunteers aged 20 to 22 years with an elevated creatinine clearance (approximately 150 mL/min) indicates that triptorelin was eliminated twice as fast in this young population (see Special Populations , Renal and Hepatic Impairment ) as compared to patients with moderate renal insufficiency. This is related to the fact that triptorelin clearance is partly correlated to total creatinine clearance, which is well known to decrease with age.
Pharmacokinetic Drug-Drug Interactions: No pharmacokinetic drug-drug interaction studies have been conducted with triptorelin (see PRECAUTIONS , Drug Interactions ).
TABLE 2. PHARMACOKINETIC PARAMETERS (MEAN ±SD) IN HEALTHY VOLUNTEERS AND SPECIAL POPULATIONS GroupCmax
(ng/mL)AUC inf
(h·ng/mL)Cl p
(mL/min)Cl renal
(mL/min)t 1/2
(h)Cl creat
(mL/min)6 healthy male volunteers48.2±11.8 36.1±5.8 211.9±31.6 90.6±35.3 2.81±1.21 149.9±7.3 6 males with moderate renal impairment45.6±20.5 69.9±24.6 120.0±45.0 23.3±17.6 6.56±1.25 39.7±22.5 6 males with severe renal impairment46.5±14.0 88.0±18.4 88.6±19.7 4.3±2.9 7.65±1.25 8.9±6.0 6 males with liver disease54.1±5.3 131.9±18.1 57.8±8.0 35.9±5.0 7.58±1.17 89.9±15.1 Clinical Trials
TRELSTAR LA was studied in a randomized, active control trial of 346 men with advanced prostate cancer in South Africa. The clinical trial population consisted of 48% Caucasian, 38% Black, and 15% Other. Men were between 45 and 96 years of age (71 mean). Patients received either TRELSTAR LA (n = 174) every 84 days for a total of up to 3 doses (maximum treatment period of 252 days) or Trelstar Depot (n = 172) every 28 days for a total of up to 9 doses. The primary efficacy endpoints were both achievement of castration by Day 29 and maintenance of castration from Day 57 through Day 253.
Castration levels of serum testosterone (<1.735 nmol/L) were achieved at Day 29 in 167 of 171 (97.7%) of patients treated with TRELSTAR LA .
Maintenance of castration levels of serum testosterone from Day 57 through Day 253 was found in 94.4% of patients treated with TRELSTAR LA .
INDICATIONS AND USAGE
TRELSTAR LA is indicated in the palliative treatment of advanced prostate cancer. It offers an alternative treatment for prostate cancer when orchiectomy or estrogen administration are either not indicated or unacceptable to the patient.
CONTRAINDICATIONS
TRELSTAR LA is contraindicated in individuals with a known hypersensitivity to triptorelin or any other component of the product, other LHRH agonists or LHRH.
TRELSTAR LA is contraindicated in women who are or may become pregnant while receiving the drug. TRELSTAR LA may cause fetal harm when administered to a pregnant woman.
WARNINGS
Rare reports of anaphylactic shock and angioedema related to triptorelin administration have been reported. In the event of a reaction, therapy with TRELSTAR LA should be discontinued immediately and the appropriate supportive and symptomatic care should be administered.
Initially, triptorelin, like other LHRH agonists, causes a transient increase in serum testosterone levels. As a result, isolated cases of worsening of signs and symptoms of prostate cancer during the first weeks of treatment have been reported with LHRH agonists. Patients may experience worsening of symptoms or onset of new symptoms, including bone pain, neuropathy, hematuria, or urethral or bladder outlet obstruction. Cases of spinal cord compression, which may contribute to paralysis with or without fatal complications, have been reported with LHRH agonists.
If spinal cord compression or renal impairment develops, standard treatment of these complications should be instituted, and in extreme cases an immediate orchiectomy considered.
PRECAUTIONS
General: Patients with metastatic vertebral lesions and/or with upper or lower urinary tract obstruction should be closely observed during the first few weeks of therapy (see WARNINGS ). Hypersensitivity and anaphylactic reactions have been reported with triptorelin as with other LHRH agonists (see CONTRAINDICATIONS and WARNINGS ).
Laboratory Tests: Response to TRELSTAR LA should be monitored by measuring serum levels of testosterone and prostate-specific antigen. Testosterone levels should be measured immediately prior to or immediately after dosing.
Drug Interactions: No drug-drug interaction studies involving triptorelin have been conducted. In the absence of relevant data and as a precaution, hyperprolactinemic drugs should not be prescribed concomitantly with TRELSTAR LA since hyperprolactinemia reduces the number of pituitary GnRH receptors.
Drug/Laboratory Test Interactions: Chronic or continuous administration of triptorelin in therapeutic doses results in suppression of the pituitary-gonadal axis. Diagnostic tests of the pituitary-gonadal function conducted during treatment and after cessation of therapy may therefore be misleading.
Pregnancy, Teratogenic Effects: Pregnancy Category X (see CONTRAINDICATIONS ). TRELSTAR LA is contraindicated in women who are or may become pregnant while receiving the drug. Studies in pregnant rats administered triptorelin at doses of 2, 10, and 100 mcg/kg/day (approximately equivalent to 0.2, 0.8, and 8 times the recommended human therapeutic dose based on body surface area) during the period of organogenesis displayed maternal toxicity and embryotoxicity, but no fetotoxicity or teratogenicity. Similarly, no teratogenic effects were observed when mice were administered doses of 2, 20, and 200 mcg/kg/day (approximately equivalent to 0.1, 0.7, and 7 times the recommended human therapeutic dose based on body surface area). If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, she should be apprised of the potential hazard to the fetus (see PRECAUTIONS , and Pregnancy ).
Carcinogenesis, Mutagenesis, Impairment of Fertility: In rats, doses of 120, 600, and 3000 mcg/kg given every 28 days (approximately 0.3, 2, and 8 times the recommended human therapeutic dose based on body surface area) resulted in increased mortality with a drug treatment period of 13-19 months. The incidence of benign and malignant pituitary tumors and histiosarcomas were increased in a dose related manner. No oncogenic effect was observed in mice administered triptorelin for 18 months at doses up to 6000 mcg/kg every 28 days (approximately 8 times the human therapeutic dose based on body surface area).
Mutagenicity studies performed with triptorelin using bacterial and mammalian systems (in vitro Ames test and chromosomal aberration test in CHO cells and an in vivo mouse micronucleus test) provided no evidence of mutagenic potential.
After 60 days of treatment followed by a minimum of four estrus cycles prior to mating, triptorelin, at doses of 2, 20, and 200 mcg/kg/day in saline (approximately 0.2, 2.0, and 16 times the recommended human therapeutic dose based on body surface area) or 20 mcg/kg/day in slow release microspheres, had no effect on the fertility or general reproductive performance of female rats. Treatment did not elicit embryotoxicity, teratogenicity, or any effects on the development of the offspring (F 1 generation) or their reproductive performance.
No studies were conducted to assess the effect of triptorelin on male fertility.
Geriatric Use: Prostate cancer occurs primarily in an older patient population. Clinical studies with TRELSTAR LA have been conducted primarily in patients >65 years old.
Use in Women: TRELSTAR LA has not been studied in women and is not indicated for use in women.
Nursing Mothers: It is not known whether TRELSTAR LA is excreted in human milk. Because many drugs are excreted in human milk and because the effects of TRELSTAR LA on lactation and/or the breastfed child have not been determined, TRELSTAR LA should not be used by nursing mothers.
Pediatric Use: TRELSTAR LA has not been studied in pediatric patients and is not indicated for use in pediatric patients.
ADVERSE REACTIONS
In the majority of patients, testosterone levels increased above baseline during the first week following the initial injection, declining thereafter to baseline levels or below by the end of the second week of treatment. The transient increase in testosterone levels may be associated with temporary worsening of disease signs and symptoms, including bone pain, hematuria, and bladder outlet obstruction. Isolated cases of spinal cord compression with weakness or paralysis of the lower extremities have occurred (see WARNINGS ).
In a controlled, comparative clinical trial, the following adverse reactions were reported to have a possible or probable relationship to therapy as ascribed by the treating physician in 1% or more of the patients receiving triptorelin (Table 3). Often, causality is difficult to assess in patients with metastatic prostate cancer. Reactions considered not drug-related or unlikely to be related are excluded.
TABLE 3. TREATMENT-RELATED ADVERSE
EVENTS REPORTED BY 1% OR MORE OF
PATIENTS DURING TREATMENT WITH TRELSTAR LATRELSTAR LA
(n=174)Adverse EventN % Application SiteInjection site pain7 4.0 Body As A WholeHot Flushes *127 73.0 Leg pain9 5.2 Pain6 3.4 Back pain5 2.9 Fatigue4 2.3 Chest pain3 1.7 Asthenia2 1.1 Peripheral edema2 1.1 CardiovascularHypertension7 4.0 Dependent edema4 2.3 Central and Peripheral Nervous SystemHeadache12 6.9 Dizziness5 2.9 Leg cramps3 1.7 EndocrineBreast pain4 2.3 Gynecomastia3 1.7 GastrointestinalNausea5 2.9 Constipation3 1.7 Dyspepsia3 1.7 Diarrhea2 1.1 Abdominal pain2 1.1 Liver and Biliary SystemAbnormal hepatic function2 1.1 Metabolic and NutritionalEdema in legs11 6.3 Increased alkaline phosphatase3 1.7 Musculoskeletal SystemSkeletal pain23 13.2 Arthralgia4 2.3 Myalgia2 1.1 PsychiatricDecreased libido *4 2.3 Impotence *4 2.3 Insomnia3 1.7 Anorexia3 1.7 Respiratory SystemCoughing3 1.7 Dyspnea2 1.1 Pharyngitis2 1.1 Skin and AppendagesRash3 1.7 Urinary SystemDysuria8 4.6 Urinary retention2 1.1 Vision DisordersEye pain2 1.1 Conjunctivitis2 1.1 * Expected pharmacologic consequences of testosterone suppression. Changes in Laboratory Values During Treatment: The following abnormalities in laboratory values not present at baseline were observed in 10% or more of patients at the Day 253 visit: decreased hemoglobin and RBC count and increased glucose, BUN, SGOT, SGPT, and alkaline phosphatase. The relationship of these changes to drug treatment is difficult to assess in this population.
OVERDOSAGE
There is no experience of overdosage in clinical trials. In single dose toxicity studies in mice and rats, the subcutaneous LD 50 of triptorelin was 400 mg/kg in mice and 250 mg/kg in rats, approximately 7000 and 4000 times, respectively, the usual human dose. If overdosage occurs however, therapy should be discontinued immediately and the appropriate supportive and symptomatic treatment administered.
DOSAGE AND ADMINISTRATION
TRELSTAR LA Must Be Administered Under the Supervision of a Physician.
The recommended dose of TRELSTAR LA is 11.25 mg incorporated in a long acting formulation administered every 84 days as a single intramuscular injection administered in either buttock. The lyophilized microgranules are to be reconstituted in sterile water. No other diluent should be used. Reconstitute in accord with the following:
For TRELSTAR LA:
- Using a syringe fitted with a sterile 20-gauge needle, withdraw 2 mL sterile water for injection, USP, and after removing the flip-off seal from the vial, inject into the vial.
- Shake well to thoroughly disperse particles to obtain a uniform suspension. The suspension will appear milky.
- Slowly withdraw the entire contents of the reconstituted suspension into the syringe.
- Inject the patient in either buttock with the contents of the syringe.
For the TRELSTAR LA Clip'n'Ject® single-dose delivery system see, adjacent INSTRUCTIONS FOR CLIP ' N ' JECT® USE section.
The suspension should be discarded if not used immediately after reconstitution.
As with other drugs administered by intramuscular injection, the injection site should be altered periodically.
HOW SUPPLIED
TRELSTAR LA (NDC 52544-154-02) is supplied in a single-dose vial with a flip-off seal containing sterile lyophilized triptorelin pamoate microgranules equivalent to 11.25 mg triptorelin peptide base, incorporated in a biodegradable copolymer of lactic and glycolic acids. A single dose vial of TRELSTAR LA contains triptorelin pamoate (11.25 mg as peptide base units), poly- d,l -lactide-co-glycolide (145 mg), mannitol, USP (85 mg), carboxymethylcellulose sodium, USP (30 mg), and polysorbate 80, NF (2 mg).
TRELSTAR LA (NDC 52544-154-76) is also supplied in the TRELSTAR LA Clip'n'Ject® single-dose delivery system consisting of a vial with a flip-off seal containing sterile lyophilized triptorelin pamoate microgranules equivalent to 11.25 mg of triptorelin peptide base, incorporated in a biodegradable copolymer of lactic and glycolic acids, and a pre-filled syringe containing sterile water for injection, USP, 2 mL, pH 6 to 8.5.
When mixed with sterile water for injection, TRELSTAR LA is administered every 84 days as a single intramuscular injection.
Store at 20-25°C (68-77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Do not freeze.
Rx only
Product No. 1120-02
Revised: December 2004
U.S. Patent Nos.: 5,134,122; 5,225,205; 5,192,741.
Clip'n'Ject is manufactured by
and is a registered trademark of
West Pharmaceutical Services, Inc.
Lionville, PA 19341 USA
Tyvek® is a registered trademark of
E.I. du Pont de Nemours and Company
Manufactured for:
Watson Pharma, Inc.
A Subsidiary of Watson Pharmaceuticals, Inc.
Corona, CA 92880 USA
by: Debio RP
CH-1920 Martigny, Switzerland
6800112052544A1
INSTRUCTIONS FOR CLIP ' N ' JECT® USE
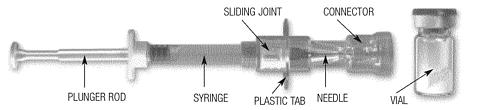
Clip ' n ' Ject® Preparation
Wash hands with soap and hot water immediately prior to preparing injection. Place the package containing the Clip'n'Ject® system and the drug product vial on a clean, flat surface that is covered with a sterile pad or cloth and peel the Tyvek® cover away from the blister package. Place the vial, connector, alcohol swab, and plunger rod on the prepared surface. Remove the flip-off button from the top of the vial and cleanse the rubber portion of the vial cap with the alcohol swab. Discard the alcohol swab.
Clip ' n ' Ject® Activation
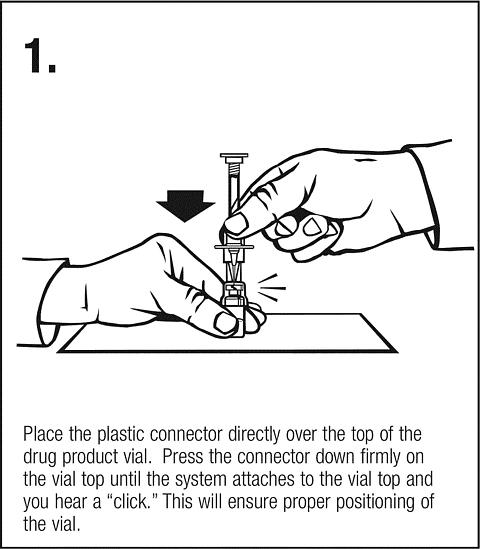
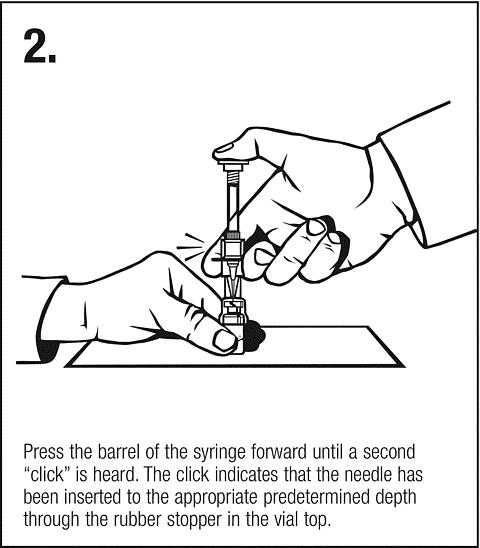
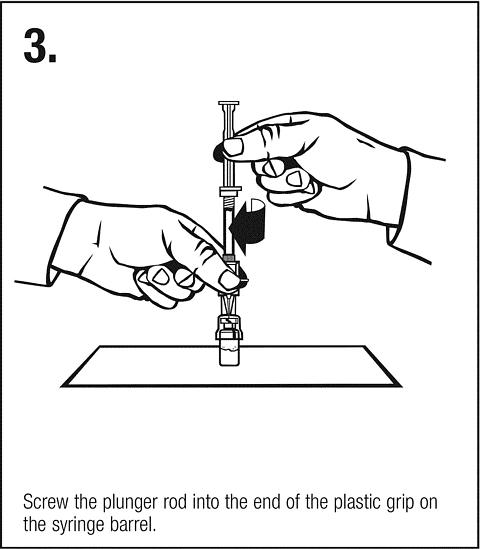
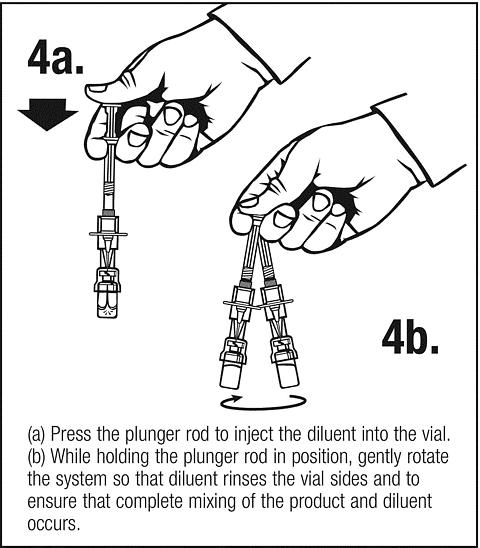
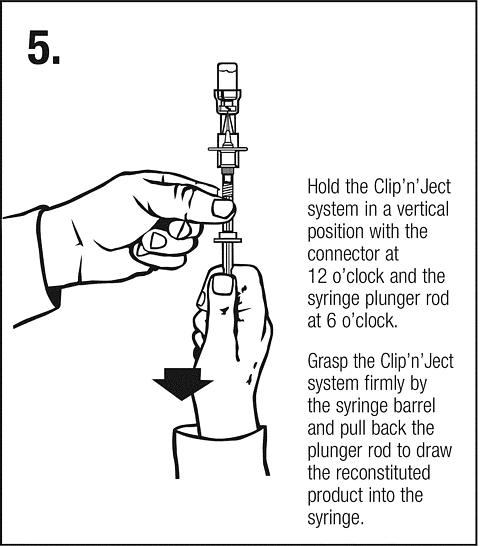
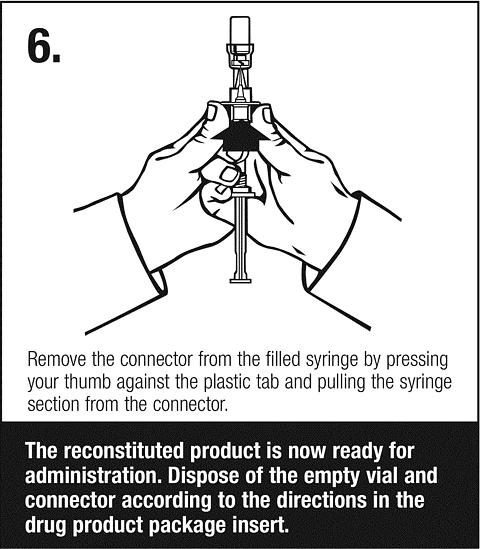
Follow safe disposal procedures for all components.
Subscribe to the "News" RSS Feed 
TOP ۞
