-
Zostavax Zoster Vaccine Live (Merck)
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZOSTAVAX1 safely and effectively.See full prescribing information for ZOSTAVAX.
ZOSTAVAX Zoster Vaccine Live
Lyophilized preparation for subcutaneous injection
Initial U.S. Approval: 2006
----------------------------INDICATIONS AND USAGE----------------------------
ZOSTAVAX is a live attenuated virus vaccine indicated for prevention of herpes zoster (shingles) in individuals 60 years of age and older (1). ZOSTAVAX is not indicated for the treatment of zoster or postherpetic neuralgia (PHN) (1).
----------------------- DOSAGE AND ADMINISTRATION------------------------
A lyophilized preparation for reconstitution containing not less than 19,400 plaque-forming units [PFU] per 0.65 mL dose supplied as single dose vials (2.1, 3, 16).
--------------------- DOSAGE FORMS AND STRENGTHS ---------------------
A lyophilized preparation for reconstitution containing not less than 19,400 plaque-forming units [PFU] per 0.65 mL dose supplied as single dose vials (2.1, 3, 16).
-------------------------------CONTRAINDICATIONS -------------------------------- History of anaphylactic/anaphylactoid reaction to gelatin, neomycin, or any other component of the vaccine (4.1).
- History of primary or acquired immunodeficiency states (4.2).
- On immunosuppressive therapy (4.2).
- ZOSTAVAX is not indicated in women of child-bearing age and should not be administered to pregnant females (4.3, 8.1, 17.1).
INDICATIONS AND USAGE
ZOSTAVAX is a live attenuated virus vaccine indicated for prevention of herpes zoster (shingles) in individuals 60 years of age and older.
ZOSTAVAX is not indicated for the treatment of zoster or postherpetic neuralgia (PHN).DOSAGE AND ADMINISTRATION
Recommended Dose and Schedule
ZOSTAVAX should be administered as a single 0.65 mL dose subcutaneously in the deltoid region of the upper arm.
Do not inject intravascularly or intramuscularly. Use only sterile syringes free of preservatives, antiseptics, and detergents for each injection and/or reconstitution of ZOSTAVAX. Preservatives, antiseptics and detergents may inactivate the vaccine virus.
Preparation for Administration
ZOSTAVAX is stored frozen and should be reconstituted immediately upon removal from the freezer.
The diluent should be stored separately at room temperature or in the refrigerator.
Use separate sterile needles for reconstitution and administration of ZOSTAVAX.
To reconstitute the vaccine: Use only the diluent supplied. Withdraw the entire contents of the diluent into a syringe. Inject all of the diluent in the syringe into the vial of lyophilized vaccine and gently agitate to mix thoroughly.
ZOSTAVAX when reconstituted is a semi-hazy to translucent, off-white to pale yellow liquid.
Withdraw the entire contents of reconstituted vaccine into a syringe and inject the total volume subcutaneously.
THE VACCINE SHOULD BE ADMINISTERED IMMEDIATELY AFTER RECONSTITUTION, TO MINIMIZE LOSS OF POTENCY.
DISCARD RECONSTITUTED VACCINE IF IT IS NOT USED WITHIN 30 MINUTES.
DO NOT FREEZE RECONSTITUTED VACCINE.
Needles should be disposed of properly and should not be recapped.DOSAGE FORMS AND STRENGTHS
ZOSTAVAX is a lyophilized preparation of live, attenuated varicella-zoster virus (Oka/Merck) to be reconstituted with sterile diluent to give a single dose suspension with a minimum of 19,400 PFU (plaque forming units) when stored at room temperature for up to 30 minutes.
CONTRAINDICATIONS
Hypersensitivity
Do not administer ZOSTAVAX to individuals with a history of anaphylactic/anaphylactoid reaction to gelatin, neomycin or any other component of the vaccine. Neomycin allergy manifested as contact dermatitis is not a contraindication to receiving this vaccine.1
Immunosuppression
Do not administer ZOSTAVAX to individuals with a history of primary or acquired immunodeficiency states including leukemia; lymphomas of any type, or other malignant neoplasms affecting the bone marrow or lymphatic system; or AIDS or other clinical manifestations of infection with human immunodeficiency viruses. ZOSTAVAX is a live attenuated varicella-zoster vaccine and administration may result in disseminated disease in individuals who are immunosuppressed. Do not administer ZOSTAVAX to individuals on immunosuppressive therapy.
Pregnancy
ZOSTAVAX is not indicated in women of child-bearing age and should not be administered to pregnant females [see Pregnancy (8.1)].WARNINGS AND PRECAUTIONS
Transmission of Vaccine Virus
Transmission of vaccine virus may occur rarely between vaccinees and susceptible contacts.
Primary Varicella Disease
ZOSTAVAX is not indicated for prevention of primary varicella infection (Chickenpox).
Preventing and Managing Allergic Vaccine Reactions As with any vaccine, adequate treatment provisions, including epinephrine injection (1:1000), should be available for immediate use should an anaphylactic/anaphylactoid reaction occur.
Limitations of Vaccine Effectiveness The duration of protection beyond 4 years after vaccination with ZOSTAVAX is unknown. The need for revaccination has not been defined.
Vaccination with ZOSTAVAX may not result in protection of all vaccine recipients.
Concurrent Illness
Vaccination should be deferred in patients with active untreated tuberculosis. Deferral should be considered in acute illness, for example, in the presence of fever.ADVERSE REACTIONS
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
Shingles Prevention Study
In clinical trials, ZOSTAVAX has been evaluated for safety in approximately 21,000 adults. In the largest of these trials, the Shingles Prevention Study (SPS), subjects received a single dose of either ZOSTAVAX (n=19,270) or placebo (n=19,276). The racial distribution across both vaccination groups was similar: White (95%); Black (2.0%); Hispanic (1.0%) and Other (1.0%) in both vaccination groups.
The gender distribution was 59% male and 41% female in both vaccination groups. The age distribution of subjects enrolled, 59-99 years, was similar in both vaccination groups.
The Adverse Event Monitoring Substudy of the SPS, designed to provide detailed data on the safety profile of the zoster vaccine (n=3,345 received ZOSTAVAX and n=3,271 received placebo) used vaccination report cards (VRC) to record adverse events occurring from Days 0 to 42 postvaccination (97% of subjects completed VRC in both vaccination groups). In addition, monthly surveillance for hospitalization was conducted through the end of the study, 2 to 5 years postvaccination.
The remainder of subjects in the SPS (n=15,925 received ZOSTAVAX and n=16,005 received placebo) were actively followed for safety outcomes through Day 42 postvaccination and passively followed for safety after Day 42.
Serious Adverse Events Occurring 0�42 Days Postvaccination
In the overall SPS study population, serious adverse events occurred at a similar rate (1.4%) in subjects vaccinated with ZOSTAVAX or placebo.
In the AE Monitoring Substudy, the rate of SAEs was increased in the group of subjects who received ZOSTAVAX as compared to the group of subjects who received placebo (Table 1).
Table 1
Number of Subjects with =1 Serious Adverse Events
(0-42 Days Postvaccination) in the Shingles Prevention Study
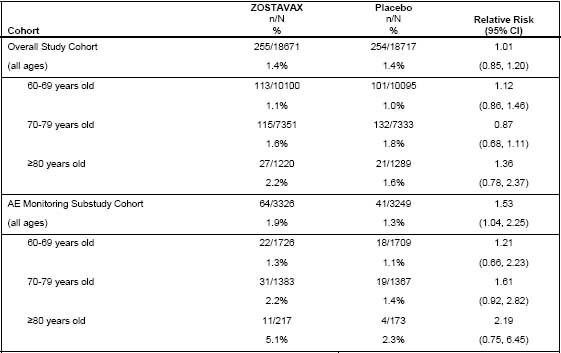
N=number of subjects in cohort with safety follow-up
n=number of subjects reporting an SAE 0-42 Days postvaccination
Among reported serious adverse events in the SPS (Days 0 to 42 postvaccination), serious cardiovascular events occurred more frequently in subjects who received ZOSTAVAX (20 [0.6%]) than in subjects who received placebo (12 [0.4%]) in the AE Monitoring Substudy. The frequencies of serious cardiovascular events were similar in subjects who received ZOSTAVAX (81 [0.4%]) and in subjects who received placebo (72 [0.4%]) in the entire study cohort (Days 0 to 42 postvaccination).
Serious Adverse Events Occurring Over the Entire Course of the Study
Rates of hospitalization were similar among subjects who received ZOSTAVAX and subjects who received placebo in the AE Monitoring Substudy, throughout the entire study.
Fifty-one individuals (1.5%) receiving ZOSTAVAX were reported to have congestive heart failure (CHF) or pulmonary edema compared to 39 individuals (1.2%) receiving placebo in the AE Monitoring
Substudy; 58 individuals (0.3%) receiving ZOSTAVAX were reported to have congestive heart failure (CHF) or pulmonary edema compared to 45 (0.2%) individuals receiving placebo in the overall study.
In the SPS, all subjects were monitored for vaccine-related SAEs. Investigator-determined, vaccinerelated serious adverse experiences were reported for 2 subjects vaccinated with ZOSTAVAX (asthma exacerbation and polymyalgia rheumatica) and 3 subjects who received placebo (Goodpasture�s syndrome, anaphylactic reaction, and polymyalgia rheumatica). Deaths
The incidence of death was similar in the groups receiving ZOSTAVAX or placebo during the Days 0- 42 postvaccination period; 14 deaths occurred in the group of subjects who received ZOSTAVAX and 16 deaths occurred in the group of subjects who received placebo. The most common reported cause of death was cardiovascular disease (10 in the group of subjects who received ZOSTAVAX, 8 in the group of subjects who received placebo). The overall incidence of death occurring at any time during the study was similar between vaccination groups: 793 deaths (4.1%) occurred in subjects who received ZOSTAVAX and 795 deaths (4.1%) in subjects who received placebo.
Most Common Adverse Reactions
Adverse Events Reported in the AE Monitoring Substudy of the SPS
Injection-site and systemic adverse events reported at an incidence =1% are shown in Table 2. Most of these adverse events were reported as mild in intensity. The overall incidence of vaccine-related injection-site adverse reactions was significantly greater for subjects vaccinated with ZOSTAVAX versus subjects who received placebo (48% for ZOSTAVAX and 17% for placebo).
Table 2
Injection-Site and Systemic Adverse Experiences Reported by Vaccine Report Card in =1% of Adults Who Received ZOSTAVAX or Placebo (0-42 Days Postvaccination) in the AE Monitoring Substudy of the Shingles Prevention Study
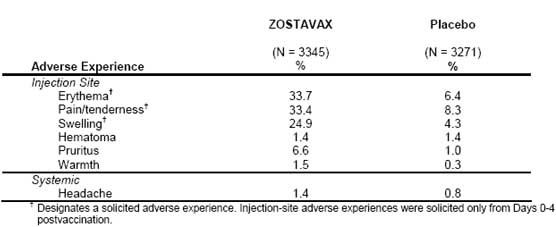 The numbers of subjects with elevated temperature (=38.3�C [=101.0�F]) within 42 days
postvaccination were similar in the ZOSTAVAX and the placebo vaccination groups [27 (0.8%) vs. 27
(0.9%), respectively].
The numbers of subjects with elevated temperature (=38.3�C [=101.0�F]) within 42 days
postvaccination were similar in the ZOSTAVAX and the placebo vaccination groups [27 (0.8%) vs. 27
(0.9%), respectively].
The following adverse experiences in the AE Monitoring Substudy of the SPS (Days 0 to 42 postvaccination) were reported at an incidence =1% and greater in subjects who received ZOSTAVAX than in subjects who received placebo, respectively: respiratory infection (65 [1.9%] vs. 55 [1.7%]), fever (59 [1.8%] vs. 53 [1.6%]), flu syndrome (57 [1.7%] vs. 52 [1.6%]), diarrhea (51 [1.5%] vs. 41 [1.3%]), rhinitis (46 [1.4%] vs. 36 [1.1%]), skin disorder (35 [1.1%] vs. 31 [1.0%]), respiratory disorder (35 [1.1%] vs. 27 [0.8%]), asthenia (32 [1.0%] vs. 14 [0.4%]). 6.1.2 VZV Rashes Following Vaccination
Within the 42-day post vaccination reporting period in the SPS, non-injection-site zoster-like rashes were reported by 53 subjects (17 for ZOSTAVAX and 36 for placebo). Of 41 specimens that were adequate for Polymerase Chain Reaction (PCR) testing, wild-type VZV was detected in 25 (5 for ZOSTAVAX, 20 for placebo) of these specimens. The Oka/Merck strain of VZV was not detected from any of these specimens.
Of reported varicella-like rashes (n=59), 10 had specimens that were available and adequate for PCR testing. VZV was not detected in any of these specimens. In clinical trials in support of the initial licensure of the frozen formulation of ZOSTAVAX, the reported rates of noninjection-site zoster-like and varicella-like rashes within 42 days postvaccination were also low in both zoster vaccine and placebo recipients. Of 17 reported varicella-like rashes and noninjectionsite, zoster-like rashes, 10 specimens were available and adequate for PCR testing. The Oka/Merck strain was identified by PCR analysis from the lesion specimens of two subjects who reported varicellalike rashes (onset on Day 8 and 17). 6.2 Post-Marketing Experience
Reporting Adverse Events
The U.S. Department of Health and Human Services has established a Vaccine Adverse Event Reporting System (VAERS) to accept all reports of suspected adverse events after the administration of any vaccine. For information or a copy of the vaccine reporting form, call the VAERS toll-free number at 1-800-822-7967 or report online to www.vaers.hhs.gov.2DRUG INTERACTIONS
Concurrent administration of ZOSTAVAX and antiviral medications known to be effective against VZV has not been evaluated.
Concomitant Administration with Other Vaccines For administration of ZOSTAVAX with trivalent inactivated influenza vaccine, [see Clinical Studies (14)].USE IN SPECIFIC POPULATIONS
Pregnancy Pregnancy Category C: Animal reproduction studies have not been conducted with ZOSTAVAX. It is also not known whether ZOSTAVAX can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. However, naturally occurring VZV infection is known to sometimes cause fetal harm. ZOSTAVAX is not indicated in women of child-bearing age and should not be administered to pregnant females. Vaccinees and health care providers are encouraged to report any exposure to ZOSTAVAX during pregnancy by calling (800) 986-8999.
Nursing Mothers
ZOSTAVAX is not indicated in women who are nursing. It is not known whether VZV is secreted in human milk. Therefore, because some viruses are secreted in human milk, caution should be exercised if ZOSTAVAX is administered to a nursing woman.
Pediatric Use
ZOSTAVAX is not indicated for prevention of primary varicella infection (Chickenpox) and should not be used in children and adolescents.
Geriatric Use
The median age of subjects enrolled in the largest (N=38,546) clinical study of ZOSTAVAX was 69 years (range 59-99 years). Of the 19,270 subjects who received ZOSTAVAX, 10,378 were 60-69 years of age, 7,629 were 70-79 years of age, and 1,263 were 80 years of age or older.DESCRIPTION
ZOSTAVAX is a lyophilized preparation of the Oka/Merck strain of live, attenuated varicella-zoster virus (VZV). ZOSTAVAX, when reconstituted as directed, is a suspension for subcutaneous administration. Each 0.65-mL dose contains a minimum of 19,400 PFU (plaque-forming units) of Oka/Merck strain of VZV when reconstituted and stored at room temperature for up to 30 minutes.
Each dose contains 31.16 mg of sucrose, 15.58 mg of hydrolyzed porcine gelatin, 3.99 mg of sodium chloride, 0.62 mg of monosodium L-glutamate, 0.57 mg of sodium phosphate dibasic, 0.10 mg of potassium phosphate monobasic, 0.10 mg of potassium chloride; residual components of MRC-5 cells including DNA and protein; and trace quantities of neomycin and bovine calf serum. The product contains no preservatives.CLINICAL PHARMACOLOGY
Mechanism of Action
The risk of developing zoster appears to be related to a decline in VZV-specific immunity. ZOSTAVAX was shown to boost VZV-specific immunity, which is thought to be the mechanism by which it protects against zoster and its complications. [See Clinical Studies (14).] Herpes zoster (HZ), commonly known as shingles or zoster, is a manifestation of the reactivation of varicella zoster virus (VZV), which, as a primary infection, produces chickenpox (varicella). Following initial infection, the virus remains latent in the dorsal root or cranial sensory ganglia until it reactivates, producing zoster. Zoster is characterized by a unilateral, painful, vesicular cutaneous eruption with a dermatomal distribution.
Pain associated with zoster may occur during the prodrome, the acute eruptive phase, and the postherpetic phase of the infection. Pain occurring in the postherpetic phase of infection is commonly referred to as postherpetic neuralgia (PHN).
Serious complications, such as PHN, scarring, bacterial superinfection, allodynia, cranial and motor neuron palsies, pneumonia, encephalitis, visual impairment, hearing loss, and death can occur as the result of zoster.NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
ZOSTAVAX has not been evaluated for its carcinogenic or mutagenic potential, or its potential to impair fertility.CLINICAL STUDIES
Efficacy of ZOSTAVAX was evaluated in the Shingles Prevention Study (SPS), a placebo-controlled, double-blind clinical trial in which 38,546 subjects 60 years of age or older were randomized to receive a single dose of either ZOSTAVAX (n=19,270) or placebo (n=19,276). Subjects were followed for the development of zoster for a median of 3.1 years (range 31 days to 4.90 years). The study excluded people who were immunocompromised or using corticosteroids on a regular basis, anyone with a previous history of HZ, and those with conditions that might interfere with study evaluations, including people with cognitive impairment, severe hearing loss, those who were non-ambulatory and those whose survival was not considered to be at least 5 years. Randomization was stratified by age, 60-69 and =70 years of age. Suspected zoster cases were confirmed by Polymerase Chain Reaction (PCR) [93%], viral culture [1%], or in the absence of viral detection, as determined by the Clinical Evaluation Committee [6%]. Individuals in both vaccination groups who developed zoster were given famciclovir, and, as necessary, pain medications. The primary efficacy analysis included all subjects randomized in the study who were followed for at least 30 days postvaccination and did not develop an evaluable case of HZ within the first 30 days postvaccination (Modified Intent-To-Treat [MITT] analysis). ZOSTAVAX significantly reduced the risk of developing zoster when compared with placebo (Table 3). Vaccine efficacy for the prevention of HZ was highest for those subjects 60-69 years of age and declined with increasing age.
Table 3
Efficacy of ZOSTAVAX on HZ Incidence Compared with Placebo in the Shingles Prevention Study*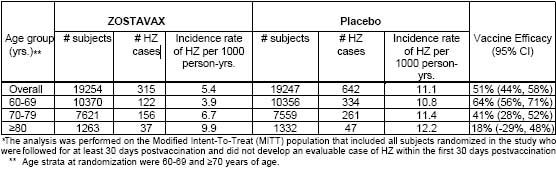
Forty-five subjects were excluded from the MITT analysis (16 in the group of subjects who received ZOSTAVAX and 29 in the group of subjects who received placebo), including 24 subjects with evaluable HZ cases that occurred in the first 30 days postvaccination (6 evaluable HZ cases in the group of subjects who received ZOSTAVAX and 18 evaluable HZ cases in the group of subjects who received placebo).
Suspected HZ cases were followed prospectively for the development of HZ-related complications. Table 4 compares the rates of PHN defined as HZ-associated pain (rated as 3 or greater on a 10-point scale by the study subject and occurring or persisting at least 90 days) following the onset of rash in evaluable cases of HZ.
Table 4
Postherpetic Neuralgia (PHN)* in the Shingles Prevention Study**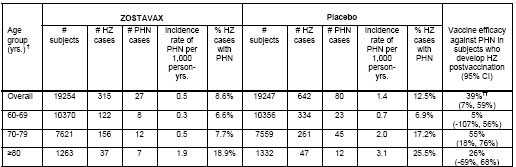 * PHN was defined as HZ-associated pain rated as =3 (on a 0-10 scale), persisting or appearing more than 90 days after onset of
HZ rash using Zoster Brief Pain Inventory (ZBPI)3.
* PHN was defined as HZ-associated pain rated as =3 (on a 0-10 scale), persisting or appearing more than 90 days after onset of
HZ rash using Zoster Brief Pain Inventory (ZBPI)3.
** The table is based on the Modified Intent-To-Treat (MITT) population that included all subjects randomized in the study who were followed for at least 30 days postvaccination and did not develop an evaluable case of HZ within the first 30 days postvaccination.
� Age strata at randomization were 60-69 and =70 years of age.
�� Age-adjusted estimate based on the age strata (60-69 and =70 years of age) at randomization.
The median duration of clinically significant pain (defined as =3 on a 0-10 point scale) among HZ cases in the group of subjects who received ZOSTAVAX as compared to the group of subjects who received placebo was 20 days vs. 22 days based on the confirmed HZ cases.
Overall, the benefit of ZOSTAVAX in the prevention of PHN can be primarily attributed to the effect of the vaccine on the prevention of herpes zoster. Vaccination with ZOSTAVAX in the SPS reduced the incidence of PHN in individuals 70 years of age and older who developed zoster postvaccination. Other prespecified zoster-related complications were reported less frequently in subjects who received ZOSTAVAX compared to subjects who received placebo. Among HZ cases, zoster-related complications were reported at similar rates in both vaccination groups (Table 5).
Table 5
Specific complications* of zoster among HZ cases in the Shingles Prevention Study
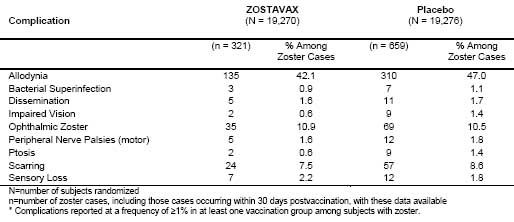 Visceral complications reported by fewer than 1% of subjects with zoster included 3 cases of
pneumonitis and 1 case of hepatitis in the placebo group, and 1 case of meningoencephalitis in the
vaccine group.
Visceral complications reported by fewer than 1% of subjects with zoster included 3 cases of
pneumonitis and 1 case of hepatitis in the placebo group, and 1 case of meningoencephalitis in the
vaccine group.
Immune responses to vaccination were evaluated in a subset of subjects enrolled in the Shingles Prevention Study (N=1395). VZV antibody levels (Geometric Mean Titers, GMT), as measured by glycoprotein enzyme-linked immunosorbent assay (gpELISA) 6 weeks postvaccination, were increased 1.7-fold (95% CI: [1.6 to 1.8]) in the group of subjects who received ZOSTAVAX compared to subjects who received placebo; the specific antibody level that correlates with protection from zoster has not been established.
In a double-blind, controlled substudy, 374 adults in the US, 60 years of age and older (median age = 66 years), were randomized to receive trivalent inactivated influenza vaccine (TIV) and ZOSTAVAX concurrently (N=188), or TIV alone followed 4 weeks later by ZOSTAVAX alone (N=186). The antibody responses to both vaccines at 4 weeks postvaccination were similar in both groups.REFERENCES
1. Reitschel RL, Bernier R. Neomycin sensitivity and the MMR vaccine. JAMA 1981;245(6):571.
2. Atkinson WL, Pickering LK, Schwartz B, Weniger BG, Iskander JK, Watson JC. General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). MMWR 2002;51(RR02):1-36.
3. Coplan PM, Schmader K, Nikas A, Chan ISF, Choo P, Levin MJ, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: Adaptation of the brief pain inventory. J Pain 2004;5(6):344-56.HOW SUPPLIED/STORAGE AND HANDLING
No. 4963-00 � ZOSTAVAX is supplied as follows: (1) a package of 1 single-dose vial of lyophilized vaccine, NDC 0006-4963-00 (package A); and (2) a separate package of 10 vials of diluent (package B).
No. 4963-41 � ZOSTAVAX is supplied as follows: (1) a package of 10 single-dose vials of lyophilized vaccine, NDC 0006-4963-41 (package A); and (2) a separate package of 10 vials of diluent (package B).
Handling and Storage
During shipment, to ensure that there is no loss of potency, the vaccine must be maintained at a temperature of -15�C (+5�F) or colder.
ZOSTAVAX SHOULD BE STORED FROZEN at an average temperature of -15�C (+5�F) or colder until it is reconstituted for injection. Any freezer, including frost-free, that has a separate sealed freezer door and reliably maintains an average temperature of -15�C or colder is acceptable for storing ZOSTAVAX.
For information regarding stability under conditions other than those recommended, call 1-800- MERCK-90.
Before reconstitution, protect from light.
The diluent should be stored separately at room temperature (20 to 25�C, 68 to 77�F), or in the refrigerator (2 to 8�C, 36 to 46�F).PATIENT COUNSELING INFORMATION
Instructions
The health care provider should question the vaccine recipient about reactions to previous vaccines. The health care provider should also inform the vaccine recipient of the benefits and risks of ZOSTAVAX. Patients should be provided with a copy of the Patient Information about ZOSTAVAX at the end of this insert, and be given an opportunity to discuss any questions or concerns. Vaccinees should also be informed of the potential risk of transmitting the vaccine virus to varicellasusceptible individuals, including pregnant women who have not had chickenpox. Patients should be instructed to report any adverse reactions to their health care provider.COPYRIGHT © Merck & CO., Inc.
All rights reserved
Subscribe to the "News" RSS Feed 
TOP ۞
