-
Activella Tablets (Novo Nordisk)
DESCRIPTION
Activella® is a single tablet containing an estrogen, estradiol (E 2 ), and a progestin, norethindrone acetate (NETA), for oral administration. Each tablet contains 1 mg estradiol and 0.5 mg norethindrone acetate and the following excipients: lactose monohydrate, starch (corn), copovidone, talc, magnesium stearate, hypromellose and triacetin.
Estradiol (E 2 ) is a white or almost white crystalline powder. Its chemical name is estra-1, 3, 5 (10)-triene-3, 17(beta)-diol hemihydrate with the empirical formula of C 18 H 24 O 2 , 1/2 H 2 O and a molecular weight of 281.4. The structural formula of E 2 is as follows:
Norethindrone acetate (NETA) is a white or yellowish-white crystalline powder. Its chemical name is 17(beta)-acetoxy-19-nor-17(alpha)-pregn-4-en-20-yn-3-one with the empirical formula of C 22 H 28 O 3 and a molecular weight of 340.5. The structural formula of NETA is as follows:
CLINICAL PHARMACOLOGY
Estrogen drug products act by regulating the transcription of a limited number of genes. Estrogens diffuse through cell membranes and bind to and activate the nuclear estrogen receptor, a DNA-binding protein that is found in estrogen-responsive tissues. The activated estrogen receptor binds to specific DNA sequences, or hormone-response elements, that enhance the transcription of adjacent genes and in turn lead to the observed effects. Estrogen receptors have been identified in tissues of the reproductive tract, breast, pituitary, hypothalamus, liver, and bone in women.
Estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol, at the receptor level. The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 µg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion in peripheral tissues of androstenedione which is secreted by the adrenal cortex, to estrone. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) through a negative feedback mechanism, and estrogen replacement therapy acts to reduce the elevated levels of these hormones seen in postmenopausal women. Progestin compounds enhance cellular differentiation and generally oppose the actions of estrogens by decreasing estrogen receptor levels, increasing local metabolism of estrogens to less active metabolites, or inducing gene products that blunt cellular responses to estrogen.
Progestins exert their effects in target cells by binding to specific progesterone receptors that interact with progesterone response elements in target genes. Progesterone receptors have been identified in the female reproductive tract, breast, pituitary, hypothalamus, and central nervous system. Progestins produce similar endometrial changes to those of the naturally occurring hormone progesterone.
The use of unopposed estrogen therapy has been associated with an increased risk of endometrial hyperplasia, a possible precursor of endometrial adenocarcinoma. The addition of a progestin, in adequate doses and appropriate duration, to an estrogen replacement regimen reduces the incidence of endometrial hyperplasia, and the attendant risk of carcinoma in women with intact uterus.
PHARMACOKINETICS
ABSORPTION
Estradiol is well absorbed through the gastrointestinal tract. Following oral administration of Activella® (estradiol/norethindrone acetate tablets), peak plasma estradiol concentrations are reached slowly within 5-8 hours. When given orally, estradiol is extensively metabolized (first-pass effect) to estrone sulfate, with smaller amounts of other conjugated and unconjugated estrogens. After oral administration, norethindrone acetate is rapidly absorbed and transformed to norethindrone. It undergoes first-pass metabolism in the liver and other enteric organs, and reaches a peak plasma concentration within 0.5-1.5 hours. The oral bioavailability of estradiol and norethindrone following administration of Activella® when compared to a combination oral solution is 53% and 100%, respectively. The pharmacokinetic parameters of estradiol (E 2 ). estrone (E 1 ), and norethindrone (NET) following single oral administration of Activella® in 25 volunteers are summarized in TABLE 1.
TABLE 1
PHARMACOKINETIC PARAMETERS
AFTER A SINGLE DOSE OF
ACTIVELLA® IN HEALTHY
POSTMENOPAUSAL WOMENActivella®
(n=25)
Mean c ± SDEstradiol a (E 2 )AUC (0-72h)(pg/ml*h)1053 ± 310 C max (pg/ml)34.6 ± 10.8 t max (h)6.8 ± 2.9 t 1/2 (h) d13.2 ± 4.7 Estrone a (E 1 )AUC (0-72h)(pg/ml*h)5223 ± 1618 C max (pg/ml)251.1 ± 91.0 t max (h)5.7 ± 1.4 t 1/2 (h) d12.2 ± 4.6 Norethindrone (NET)AUC (0-72h)(pg/ml*h)23681 ± 9023 b C max (pg/ml)5308 ± 1510 t max (h)1.0 ± 0.0 t 1/2 (h)11.4 ± 2.7 AUC = area under the curve,C max = maximum plasma concentration,t max = time at maximum plasma concentration,t 1/2 = half-life,SD = standard deviationa baseline unadjusted data;b (n=23);c arithmetic mean;d baseline adjusted data
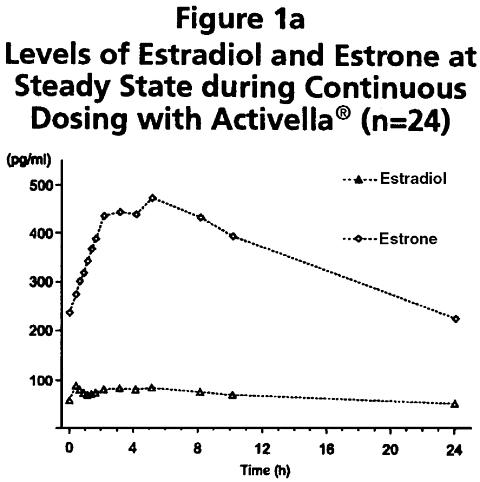
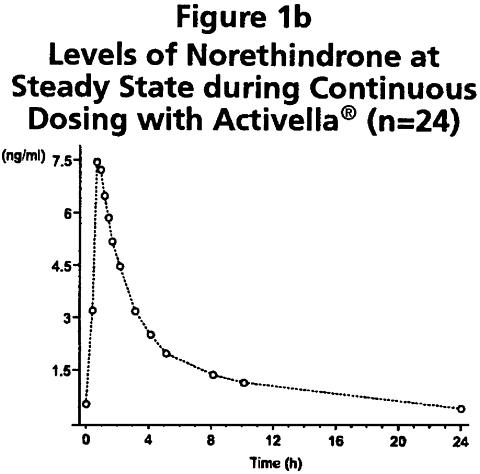
Following continuous dosing with once-daily administration of Activella® (estradiol/norethindrone acetate tablets), serum levels of estradiol, estrone, and norethindrone reached steady-state within two weeks with an accumulation of 33-47% above levels following single dose adminstration. Unadjusted circulating levels of E 2 , E 1 , and NET during Activella® treatment at steady state (dosing at time 0) are provided in Figures 1a and 1b.
DISTRIBUTION
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estradiol circulates in the blood bound to sex-hormone-binding globulin (SHBG) (37%) and to albumin (61%), while only approximately 1-2% is unbound. Norethindrone also binds to a similar extent to SHBG (36%) and to albumin (61%).
METABOLISM AND EXCRETION
Estradiol: Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is the major urinary metabolite.
Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the gut followed by reabsorption. In postmenopausal women, a significant portion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens. The half-life of estradiol following single dose administration of Activella® (estradiol/norethindrone acetate tablets) is 12-14 hours.
Norethindrone Acetate: The most important metabolites of norethindrone are isomers of 5(alpha)-dihydro-norethindrone and tetrahydro-norethindrone, which are excreted mainly in the urine as sulfate or glucuronide conjugates. The terminal half-life of norethindrone is about 8-11 hours.
DRUG-DRUG INTERACTIONS
Coadministration of estradiol with norethindrone acetate did not elicit any apparent influence on the pharmacokinetics of norethindrone. Similarly, no relevant interaction of norethindrone on the pharmacokinetics of estradiol was found within the NETA dose range investigated in a single dose study.
FOOD-DRUG INTERACTIONS
A single-dose study in 24 healthy postmenopausal women was conducted to investigate any potential impact of administration of Activella® with and without food. Administration of Activella® with food did not modify the bioavailability of estradiol, although increases in AUC 0-72 of 19% and decreases in C max of 36% for norethindrone were seen.
CLINICAL STUDIES
VASOMOTOR SYMPTOMS
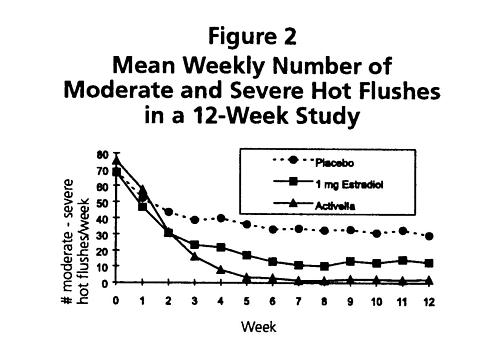
Activella® is effective in reducing the number of moderate-to-severe vasomotor symptoms in postmenopausal women. In a 12-week radomized clinical trial involving 92 subjects, Activella® was compared to 1 mg of estradiol and to placebo. The mean number and intensity of hot flushes were significantly reduced from baseline to week 12 in both the Activella® and the 1 mg estradiol group compared to placebo (see Figure 2).
ENDOMETRIAL HYPERPLASIA
Activella® (estradiol/norethindrone acetate tablets) reduced the incidence of estrogen-induced endometrial hyperplasia at 1 year in a randomized, controlled clinical trial. This trial enrolled 1,176 subjects who were randomized to one of 4 arms: 1 mg estradiol unopposed (n=296), 1 mg E 2 + 0.1 mg NETA (n=294), 1 mg E 2 + 0.25 mg NETA (n=291), and Activella® [1 mg E 2 + 0.5 mg NETA] (n=295). At the end of the study, endometrial biopsy results were available for 988 subjects. The results of the 1 mg estradiol unopposed arm compared to Activella® are shown in TABLE 2.
TABLE 2
INCIDENCE OF ENDOMETRIAL
HYPERPLASIA WITH UNOPPOSED
ESTRADIOL AND ACTIVELLA® IN
A 12-MONTH STUDY1 mg E 2
(n=296)Activella®
(n=295)No. of subjects with histological evaluation at the end of the study247 241 No. (%) of subjects with endometrial hyperplasia at the end of the study36 (14.6%) 1 (0.4%)
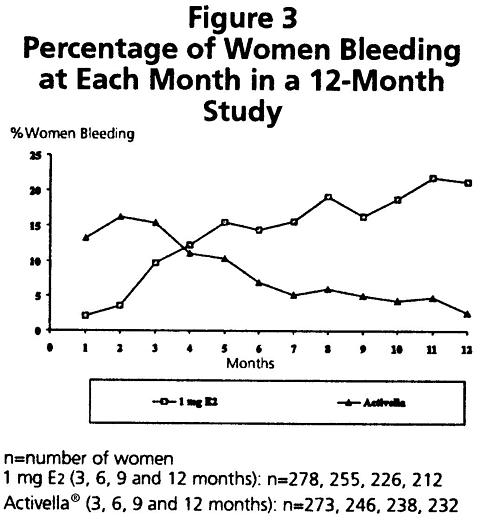
During the initial months of therapy, irregular bleeding or spotting occurred with Activella® treatment. However, bleeding tended to decrease over time, and after 12 months of treatment with Activella®, fewer than 3% of women reported bleeding (see Figure 3).
INFORMATION REGARDING LIPID EFFECTS
A 12-month, placebo-controlled clinical trial in 80 postmenopausal Caucasian women at low risk for cardiovascular disease compared the effects of Activella® to placebo on lipid parameters. These results are shown in TABLE 3.
TABLE 3
PERCENTAGE CHANGE FROM BASE-
LINE IN SELECTED LIPID PARAMETERS
WITH ACTIVELLA® IN A 12-MONTH
PLACEBO-CONTROLLED STUDYLipid Parameter %Activella®
(n=35)Placebo
(n=34)Total Cholesterol-10.5% -0.8% HDL-C 1-12.4% -6.1% LDL-C 2-10.8% 0.8% LDL: HDL Ratio0.1% 9.2% Triglycerides2.2% 4.4% 1 High density lipoprotein-cholesterol2 Low density lipoprotein-cholesterolEFFECT ON BONE MINERAL DENSITY
The results of two randomized, multicenter, calcium-supplemented (500-1000 mg/day), placebo-controlled, 2 year clinical trials have shown that Activella® (estradiol/norethindrone acetate tablets) is effective in preventing bone loss in postmenopausal women. A total of 462 postmenopausal women with intact uteri and baseline BMD values for lumbar spine within 2 standard deviations of the mean in healthy young women were enrolled. In a US trial, 327 postmenopausal women (mean time from menopause 2.5 to 3.1 years) with a mean age of 53 years were randomized to 7 groups (0.25 mg, 0.5 mg, and 1 mg of estradiol alone, 1 mg estradiol with 0.25 mg norethindrone acetate, 1 mg estradiol with 0.5 mg norethindrone acetate, and 2 mg estradiol with 1 mg norethindrone acetate, and placebo. In a European trial, 135 postmenopausal women (mean time from menopause 8.4 to 9.3 years) with a mean age of 58 years were randomized to 1 mg estradiol with 0.25 mg norethindrone acetate, 1 mg estradiol with 0.5 mg norethindrone acetate, and placebo.
Approximately 58% and 67% of the randomized subjects in the two clinical trials, respectively, completed the two clinical trials. BMD was measured using dual-energy x-ray absorptiometry (DEXA).
A summary of the results comparing Activella® and placebo from the two prevention trials is shown in Table 4.
TABLE 4
PERCENTAGE CHANGE
(MEAN±SEM) IN BONE MINERAL DENSITY (BMD)
(Intent to Treat Analysis, Last Observation Carried Forward)US Trial EU Trial Placebo
(n=37)Activella®
(n=37)Placebo
(n=40)Activella®
(n=38)Lumbar spine-2.1±0.5 3.8±0.5 * -0.9±0.6 5.4±0.8 * Femoral neck-2.3±0.6 1.8±0.7 * -1.0±0.7 0.7±0.9 Femoral trochanter-2.0±0.7 3.7±0.7 * 0.8±1.1 6.3±1.2 * Ward's triangle- - -1.6±1.3 2.7±1.7 Distal radius- - -0.7±0.5 2.1±0.5 * Total body- - 0.4±0.4 3.0±0.5 * US = United States, EU = European* Significantly (p<0.001) different from placebo
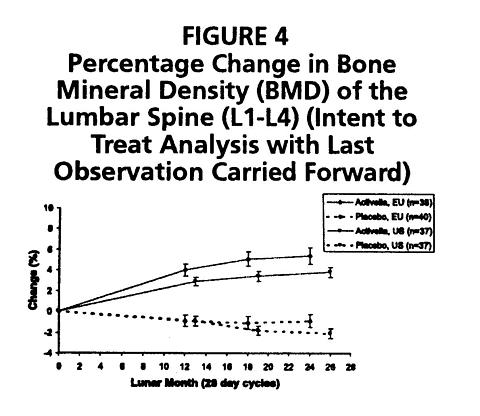
The overall difference in mean percentage change in BMD at the lumbar spine between Activella® and placebo was 5.9% in the US trial (1000 mg/day calcium) and 6.3% in the European trial (500 mg/day calcium). Activella® also increased BMD at the femoral neck and femoral trochanter compared to placebo. The increase in lumbar spine BMD in the US and European clinical trials is displayed in Figure 4.
EFFECT ON BONE TURNOVER
Activella® (estradiol/norethindrone acetate tablets) significantly reduced serum and urine markers of bone turnover with a marked decrease in bone resorption makers (e.g., urinary pyridinoline crosslinks Type 1 collagen C-telopeptide, pyridinoline, deoxypyridinoline) and to a lesser extent in bone formation markers (e.g., serum osteocalcin, bone-specific alkaline phosphatase, C-terminal propetide of Type 1 collagen). The suppression of bone turnover markers was evident by 3 months and persisted throughout the 24-month treatment period.
INDICATIONS AND USAGE
Activella® therapy is indicated in women with an intact uterus for the:
- Treatment of moderate to severe vasomotor symptoms associated with the menopause. There is no adequate evidence that estrogens are effective for nervous symptoms or depression that might occur during menopause and they should not be used to treat these conditions.
- Treatment of vulvar and vaginal atrophy.
- Prevention of postmenopausal osteoporosis.
Most prospective studies of efficacy for the osteoporosis prevention indication have been carried out in white postmenopausal women, without stratification by other risk factors, and tend to show a universally beneficial effect on bone. Since estrogen administration is associated with risk, patient selection must be individualized based on the balance of risks and benefits.
Case-control studies have shown an approximately 60-percent reduction in hip and wrist fractures in women whose estrogen replacement was begun within a few years after menopause. Studies also suggest that estrogen reduces the rate of vertebral fractures. When estrogen therapy is discontinued, bone mass declines at a rate comparable to the immediate postmenopausal period. White and Asian women are at higher risk for osteoporosis than black women, and thin women are at a higher risk than heavier women, who generally have higher endogenous estrogen levels. Early menopause is one of the strongest predictors for the development of osteoporosis. Other factors associated with osteoporosis include genetic factors (small build, family history), lifestyle (cigarette smoking, alcohol abuse, sedentary exercise habits) and nutrition (below average body weight and dietary calcium intake).
The mainstays of prevention and management of osteoporosis are weight-bearing exercise, adequate calcium intake, and, when indicated, estrogen. Postmenopausal women absorb dietary calcium less efficiently than premenopausal women and require an average of 1500 mg/day of elemental calcium to remain in neutral calcium balance. The average calcium intake in the USA is 400-600 mg/day. Therefore, when not contraindicated, calcium supplementation may be helpful for women with suboptimal dietary intake.
CONTRAINDICATIONS
Estrogens/progestins combined should not be used in women under any of the following conditions or circumstances.
- Known or suspected pregnancy, including use for missed abortions or as a diagnostic test for pregnancy. Estrogen or progestin may cause fetal harm when administered to a pregnant woman.
- Known or suspected breast cancer, or past history of breast cancer associated with the use of estrogens.
- Known or suspected estrogen-dependent neoplasia, e.g., endometrial cancer.
- Abnormal genital bleeding unknown etiology.
- Known or suspected active deep venous thrombosis, thromboembolic disorders or stroke or past history of these conditions associated with estrogen use.
- Liver dysfunction or disease.
- Hypersensitivity to any of the components of Activella® (estradiol/norethindrone acetate tablets).
WARNINGS
ALL WARNINGS BELOW PERTAIN TO THE USE OF THIS COMBINATION PRODUCT.
Based on experience with estrogens and/or progestins:
-
Induction of malignant neoplasms
Endometrial cancer. The reported endometrial cancer risk among unopposed estrogen users is about 2- to 12-fold greater than in non-users, and appears dependent on duration of treatment and on estrogen dose. There is no significant increased risk associated with the use of estrogens for less than one year. The greatest risk appears to be associated with prolonged use with increased risks of 15- to 24-fold with five or more years of use. In three studies, persistence of risk was demonstrated for 8 to over 15 years after cessation of estrogen treatment. In one study, a significant decrease in the incidence of endometrial cancer occurred six months after withdrawal. Progestins taken with estrogens have been shown to significantly reduce, but not eliminate, the risk of endometrial cancer associated with estrogen use. In a large clinical trial, the incidence of endometrial hyperplasia with Activella® was 0.4% (one simple hyperplasia without atypia) compared to 14.6% with 1 mg estradiol unopposed (see CLINICAL STUDIES ).
Clinical surveillance of all women taking estrogen/progestin combinations is important. Adequate diagnostic measures, including endometrial sampling when indicated, should be undertaken to rule out malignancy in all cases of undiagnosed persistent or recurring abnormal vaginal bleeding. There is no evidence that "natural" estrogens are more or less hazardous than "synthetic" estrogens at equivalent estrogen doses.
Breast cancer. While the majority of studies have not shown an increased risk of breast cancer in women who have ever used estrogen replacement therapy, some have reported a moderately increased risk (relative risks of 1.3-2.0) in those taking higher doses, or in those taking lower doses for prolonged periods of time, especially in excess of 10 years.
While the effects of added progestins on the risk of breast cancer are also unknown, available epidemiological evidence suggest that progestins do not reduce, and may enhance, the moderately increased breast cancer risk that has been reported with prolonged estrogen replacement therapy.
In a one-year trial among 1,176 women who received either unopposed 1 mg estradiol or a combination of 1 mg estradiol plus one of three different doses of NETA (0.1, 0.25 and 0.5 mg), seven new cases of breast cancer were diagnosed, two of which occurred among the group of 295 Activella® (estradiol/norethindrone acetate tablets) treated women.
Women on hormone replacement therapy should have regular breast examinations and should be instructed in breast self-examination, and women over the age of 40 should have regular mammograms. -
Congenital lesions with malignant potential.
Estrogen therapy during pregnancy is associated with an increased risk of fetal congential reproductive tract disorders, and possible other birth defects.
Studies of women who received diethylstilbestrol (DES) during pregnancy have shown that female offspring have an increased risk of vaginal adenosis, squamous cell dysplasia of the uterine cervix, and clear cell vaginal cancer later in life; male offspring have an increased risk of urogenital abnormalities and possibly testicular cancer later in life.
Although some of these changes are benign, others are precursors of malignancy. -
Cardiovascular disease.
Large doses of estrogens (5 mg conjugated estrogen per day), comparable to those used to treat cancer of the prostate and breast, have been shown in a large prospective clinical trial in men to increase the risk of nonfatal myocardial infarction, pulmonary embolism, and thrombophlebitis.
These risks cannot necessarily be extrapolated from men to women or from unopposed estrogen to combination estrogen/progestin therapy. However, to avoid the theoretical cardiovascular risk to women caused by high estrogen doses, the dose for estrogen replacement therapy should not exceed the lowest effective dose. - Hypercalcemia. Administration of estrogens may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If this occurs, the drugs should be stopped and appropriate measures taken to reduce the serum calcium level.
- Effects during pregnancy. Use in pregnancy is not recommended.
- Gallbladder disease. Two studies have reported a 2- to 4-fold increase in the risk of surgically confirmed gallbladder disease in women receiving postmenopausal estrogens. Among the 1,516 women treated in clinical trials with 1 mg estradiol alone or in combination with several doses of NETA, 3 women had surgically confirmed cholelithiasis, none of them on Activella® (estradiol/norethindrone acetate tablets) treatment.
- Elevated blood pressure. Occasional blood pressure increases during estrogen replacement therapy have been attributed to idiosyncratic reactions to estrogens. More often, blood pressure has remained the same or has dropped. One study showed that postmenopausal estrogen users have higher blood pressure than non-users. Two other studies showed slightly lower blood pressure among estrogen users compared to non-users. Postmenopausal estrogen use does not increase the risk of stroke. Nonetheless, blood pressure should be monitored at regular intervals with estrogen use.
- Thromboembolic disorders. The physician should be alert to the earliest manifestations of thrombotic disorders (thrombophlebitis, cerebrovascular disorders, pulmonary embolism, and retinal thrombosis). Should any of these occur or be suspected, the drugs should be discontinued immediately. In a one-year study where 295 women were exposed to Activella®, there were two cases of deep vein thromboses reported.
- Visual abnormalities. Discontinue medication pending examination if there is a sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examinations reveal papilledema or retinal vascular lesions, medication should be withdrawn.
PRECAUTIONS
GENERAL
Based on experience with estrogens and/or progestins:
-
Cardiovascular risk.
A causal relationship between estrogen replacement therapy and reduction of cardiovascular disease in postmenopausal women has not been proven. Furthermore, the effect of added progestins on this putative benefit is not yet known.
In recent years, many published studies have suggested that there may be a cause-effect relationship between postmenopausal oral estrogen replacement therapy without added progestins and a decrease in cardiovascular disease in women. Although most of the observational studies that assessed this statistical association have reported a 20% to 50% reduction in coronary heart disease risk and associated mortality in estrogen takers, the following should be considered when interpreting these reports. Because only one of these studies was randomized and it was too small to yield statistically significant results, all relevant studies were subject to selection bias. Thus, the apparently reduced risk of coronary artery disease cannot be attributed with certainty to estrogen replacement therapy. It may instead have been caused by life-style and medical characteristics of the women studied with the result that healthier women were selected for estrogen therapy. In general, treated women were of higher socioeconomic and educational status, more slender, more physically active, more likely to have undergone surgical menopause, and less likely to have diabetes than the untreated women. Although some studies attempted to control for these selection factors, it is common for properly designed randomized trials to fail to confirm benefits suggested by less rigorous study designs. Thus, ongoing and future large-scale randomized trials may fail to confirm this apparent benefit.
Current medical practice often includes the use of concomitant progestin therapy in women with intact uterus. While the effects of added progestins on the risk of ischemic heart disease are not known, all available progestins attenuate at least some of the favorable effects of estrogens on HDL levels, although they maintain the favorable effect of estrogens on LDL levels.
The safety data regarding Activella® (estradiol/norethindrone acetate tablets) were obtained primarily from clinical trials and epidemiologic studies of postmenopausal Caucasian women, who were at generally low risk of cardiovascular disease and higher than average risk for osteoporosis. The safety profile of Activella® derived from these study populations cannot necessarily be extrapolated to other populations of diverse racial and/or demographic composition. When considering prescribing Activella®, physicians are advised to weigh the potential benefits and risks of therapy as applicable to each individual patient. -
Use in hysterectomized women.
Existing data do not support the use of the combination of estrogen and progestin in postmenopausal women without a uterus. Risks that may be associated with the inclusion of progestin in estrogen replacement regimens include deterioration in glucose tolerance, and less favorable effects on lipid metabolism compared to the effects of estrogen alone.
The effects of Activella® on glucose tolerance and lipid metabolism have been studied (see CLINICAL PHARMACOLOGY , CLINICAL STUDIES , and PRECAUTIONS, DRUG/LABORATORY TEST INTERACTIONS ). - Physical examination. A complete medical and family history should be taken prior to the initiation of any estrogen/progestin therapy. The pretreatment and periodic physical examinations should include special reference to blood pressure, breasts, abdomen, and pelvic organs, and should include a Papanicolaou smear. As a general rule, estrogen should not be prescribed for longer than one year without another physical examination being performed.
- Fluid retention. Because estrogens/progestins may cause some degree of fluid retention, conditions that might be influenced by this factor, such as asthma, epilepsy, migraine, and cardiac or renal dysfunction, require careful observation.
- Uterine bleeding. Certain patients may develop abnormal uterine bleeding. In cases of undiagnosed abnormal uterine bleeding, adequate diagnostic measures are indicated (see WARNINGS ).
- The pathologist should be advised of estrogen/progestin therapy when relevant specimens are submitted.
Based on experience with estrogens:
- Familial hyperlipoproteinemia. Estrogen therapy may be associated with massive elevations of plasma triglycerides leading to pancreatitis and other complications in patients with familial defects in lipoprotein metabolism.
-
Hypercoagulability.
Some studies have shown that women taking estrogen replacement therapy have hypercoagulability primarily related to decreased antithrombin activity. This effect appears dose- and duration-dependent and is less pronounced than that associated with oral contraceptive use. Also, postmenopausal women tend to have changes in levels of coagulation parameters at baseline compared to premenopausal women. Epidemiological studies have suggested that estrogen use is associated with a higher relative risk of developing venous thromboembolism, i.e., deep vein thrombosis or pulmonary embolism. The studies found a 2- 3- fold higher risk for estrogen users compared to non-users. There is insufficient information on hypercoagulability in women who have had previous thromboembolic disease. The effects of Activella® (estradiol/norethindrone acetate tablets) (n=40) compared to placebo (n=40) on selected clotting factors were evaluated in a 12-month study with postmenopausal women.
Activella® decreased factor VII, plasminogen activator inhibitor-1, and, to a lesser extent, antithrombin III activity, compared to placebo. Fibrinogen remained unchanged during Activella® treatment in comparison with an increase over time in the placebo group. - Mastodynia. Certain patients may develop undesirable manifestations of estrogenic stimulation such as mastodynia. In clinical trials, less than one-fifth of the women treated with Activella® reported breast tenderness or breast pain. The majority of the cases were reported as breast tenderness, primarily during the initial months of the treatment.
Based on experience with progestins:
- Lipoprotein metabolism. (see CLINICAL STUDIES )
-
Impaired glucose tolerance.
Diabetic patients should be carefully observed while receiving estrogen/progestin therapy.
The effects of Activella® on glucose tolerance have been studied (see PRECAUTIONS, DRUG/LABORATORY TEST INTERACTIONS ). - Depression. Patients who have a history of depression should be observed and the drugs discontinued if the depression recurs to a serious degree.
INFORMATION FOR THE PATIENT
See text of Patient Package Insert which appears after the HOW SUPPLIED section.
DRUG/LABORATORY TEST INTERACTIONS
The following interactions have been observed with estrogen therapy, and/or Activella® (estradiol/norethindrone acetate tablets):
- Activella® decreases factor VII, plasminogen activator inhibitor-1, and, to a lesser extent, antithrombin III activity.
- Estrogen therapy increases thyroid-binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T 4 levels (by column or by radioimmunoassay) or T 3 levels by radioimmunoassay. T 3 resin uptake is decreased, reflecting the elevated TBG. Free T 4 and free T 3 concentrations are unaltered.
- Estrogen therapy may elevate other binding proteins in serum i.e., corticosteroid-binding globulin (CBG), sex-hormone-binding globulin (SHBG), leading to increased circulating corticosteroids and sex steroids respectively. Free or biologically active hormone concentrations are unchanged. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin). In a 12-month clinical trial, SHBG was found to increase with Activella®.
- Estrogen therapy increases plasma HDL and HDL-2 subfraction concentrations, reduces LDL cholesterol concentration, and increases triglyceride levels. (For effects during Activella® treatment, see CLINICAL PHARMACOLOGY , CLINICAL STUDIES ).
- Activella® treatment of healthy postmenopausal women does not decrease glucose tolerance when assessed by an oral glucose tolerance test; the insulin response decreases without any increase in the glucose serum levels. Activella® treatment does not deteriorate insulin sensitivity in healthy postmenopausal women when assessed by an hyperinsulinemic euglycemic clamp.
- Estrogen therapy reduces response to metyrapone test.
- Estrogen therapy reduces serum folate concentration.
CARCINOGENESIS, MUTAGENESIS, and IMPAIRMENT OF INFERTILITY
Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver. (See CONTRAINDICATIONS and WARNINGS ).
PREGNANCY CATEGORY X:
Estrogens/progestins should not be used during pregnancy. (See CONTRAINDICATIONS and WARNINGS ).
NURSING MOTHERS:
Detectable amounts of estradiol and norethindrone acetate have been identified in the milk of mothers receiving these products and has been reported to decrease the quantity and the quality of the milk.
As a general principle, the administration of any drug to nursing mothers should be done only when clearly necessary since many drugs are excreted in human milk.
PEDIATRIC USE:
Safety and effectiveness in pediatric patients have not been established.
GERIATRIC USE:
Clinical studies of Activella® (estradiol/norethindrone acetate tablets) did not include sufficient number of subjects aged 65 and over to determine if they responded differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
(See WARNINGS regarding induction of neoplasia, adverse effects on the fetus, increased incidence of gallbladder disease, elevated blood pressure, thromboembolic disorders, cardiovascular disease, visual abnormalities, and hypercalcemia and PRECAUTIONS regarding cardiovascular disease).
Adverse events reported by investigators in the Phase 3 studies regardless of causality assessment are shown in TABLE 5.
TABLE 5
ALL TREATMENT-EMERGENT ADVERSE EVENTS REGARDLESS OF RELATIONSHIP REPORTED AT A FREQUENCY OF >/=5% WITH ACTIVELLA®Endometrial
Hyperplasia Study
(12-Months)Vasomotor
Symptoms Study
(3-Months)Osteoporosis
Study
(2 Years)Activella®
(n=295)1 mg E2
(n=296)Activella®
(n=29)Placebo
(n=34)Activella®
(n=47)Placebo
(n=48)Body as a WholeBack Pain6% 5% 3% 3% 6% 4% Headache16% 16% 17% 18% 11% 6% Digestive SystemNausea3% 5% 10% 0% 11% 0% Gastroenteritis2% 2% 0% 0% 6% 4% Nervous SystemInsomnia6% 4% 3% 3% 0% 8% Emotional Lability1% 1% 0% 0% 6% 0% Respiratory SystemUpper Respiratory Tract Infection18% 15% 10% 6% 15% 19% Sinusitis7% 11% 7% 0% 15% 10% Metabolic and NutritionalWeight increase0% 0% 0% 0% 9% 6% Urogenital SystemBreast Pain24% 10% 21% 0% 17% 8% Post-Menopausal Bleeding5% 15% 10% 3% 11% 0% Uterine Fibroid5% 4% 0% 0% 4% 8% Ovarian Cyst3% 2% 7% 0% 0% 8% Resistance MechanismInfection Viral4% 6% 0% 3% 6% 6% Moniliasis Genital4% 7% 0% 0% 6% 0% Secondary TermsInjury Accidental4% 3% 3% 0% 17% * 4% * Other Events2% 3% 3% 0% 6% 4% * including one upper extremity fracture in each group
The following adverse reactions have been reported with estrogen and/or progestin therapy:
Genitourinary system: changes in vaginal bleeding pattern and abnormal withdrawal bleeding or flow, breakthrough bleeding, spotting, increase in size of uterine leiomyomata, vaginal candidiasis, changes in amount of cervical secretion, premenstrual-like syndrome, cystitis-like syndrome.
Breasts: tenderness, enlargement.
Gastrointestinal: nausea, vomiting, changes in appetite, cholestatic jaundice, abdominal pain, flatulence, bloating, increased incidence of gallbladder disease.
Skin: chloasma or melasma that may persist when drug is discontinued, erythema multiforme, erythema nodosum, hemorrhagic eruption, loss of scalp hair, hirsutism, itching, skin rash and pruritus.
Cardiovascular: changes in blood pressure, cerebrovascular accidents, deep venous thrombosis and pulmonary embolism.
CNS: headache, migraine, dizziness, depression, chorea, insomnia, nervousness.
Eyes: steepening of corneal curvature, intolerance to contact lenses.
Miscellaneous: increase or decrease in weight, aggravation of porphyria, edema, changes in libido, fatigue, allergic reactions, back pain, arthralgia, myalgia.
OVERDOSAGE
Acute Overdose: Serious ill effects have not been reported following acute ingestion of large doses of estrogen/progestin-containing oral contraceptives by young children. Overdosage may cause nausea and vomiting and withdrawal bleeding may occur in females.
DOSAGE AND ADMINISTRATION
Activella® (estradiol/norethindrone acetate tablets) therapy consists of a single tablet to be taken once daily. For the treatment of moderate to severe vasomotor symptoms associated with the menopause, treatment of vulvar and vaginal atrophy, and the prevention of postmenopausal osteoporosis - Activella® 1 mg E 2 / 0.5 mg NETA daily. The doses of 17beta-estradiol and norethindrone acetate in Activella® may not be the lowest effective dose-combination for the prevention of osteoporosis.
Treated patients with an intact uterus should be monitored closely for signs of endometrial cancer, and appropriate diagnostic measures should be taken to rule out malignancy in the event of persistent or recurring abnormal vaginal bleeding.
HOW SUPPLIED
Activella®, 1 mg estradiol and 0.5 mg norethindrone acetate, is a white, film-coated tablet, engraved with NOVO 288 on one side and the APIS bull on the other. It is round, 6 mm in diameter and bi-convex.
Activella® is supplied as:
28 tablets in a calendar dial pack dispenser NDC 0169-5174-02.
Store in a dry place protected from light. Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
[See USP Controlled Room Temperature]
© 2000/2003
Rx only
Activella® is a trademark owned by
Novo Nordisk A/S
Revised July 2003
Novo Nordisk Pharmaceuticals, Inc.
Princeton, NJ 08540
1-866-668-6336
www.novonordisk-us.com
Manufactured by
Novo Nordisk AS
2880 Bagsvaerd, Denmark
Subscribe to the "News" RSS Feed 
TOP ۞
