-
Adriamycin Injection, USP, Adriamycin for Injection, USP (Bedford)
WARNINGS
- Severe local tissue necrosis will occur if there is extravasation during administration (see DOSAGE AND ADMINISTRATION ). Doxorubicin must not be given by the intramuscular or subcutaneous route.
- Myocardial toxicity manifested in its most severe form by potentially fatal congestive heart failure may occur either during therapy or months to years after termination of therapy. The probability of developing impaired myocardial function based on a combined index of signs, symptoms and decline in left ventricular ejection fraction (LVEF) is estimated to be 1 to 2% at a total cumulative dose of 300 mg/m 2 of doxorubicin, 3 to 5% at a dose of 400 mg/m 2 , 5 to 8% at 450 mg/m 2 and 6 to 20% at 500 mg/m 2 . * The risk of developing CHF increases rapidly with increasing total cumulative doses of doxorubicin in excess of 450 mg/m 2 . "Risk factors (active or dormant cardiovascular disease, prior or concomitant radiotherapy to the mediastinal/pericardial area, previous therapy with other anthracyclines or anthracenediones, concomitant use of other cardiotoxic drugs) may increase the risk of cardiac toxicity. Cardiac toxicity with doxorubicin may occur at lower cumulative doses whether or not cardiac risk factors are present." Pediatric patients are at increased risk for developing delayed cardiotoxicity.
- Secondary acute myelogenous leukemia (AML) has been reported in patients treated with anthracyclines, including doxorubicin (see ADVERSE REACTIONS ). The occurrence of refractory secondary leukemia is more common when such drugs are given in combination with DNA-damaging anti-neoplastic agents, when patients have been heavily pretreated with cytotoxic drugs, or when doses of anthracyclines have been escalated. The rate of developing treatment-related leukemia was estimated in an analysis of 1474 breast cancer patients who received adjuvant treatment with doxorubicin-containing regimens (i.e., FAC) in clinical trials. The estimated risk of developing treatment-related leukemia at 10 years was 2.5% for the 810 patients receiving radiotherapy plus chemotherapy and 0.5% for the 664 patients receiving chemotherapy alone. The overall risk was estimated at 1.5% at 10 years for the entire patient population. Pediatric patients are also at risk of developing secondary AML.
- Dosage should be reduced in patients with impaired hepatic function.
- Severe myelosuppression may occur.
- Doxorubicin should be administered only under the supervision of a physician who is experienced in the use of cancer chemotherapeutic agents.
*Data on file at Pharmacia & Upjohn
DESCRIPTION
Doxorubicin is a cytotoxic anthracycline antibiotic isolated from cultures of Streptomyces peucetius var. caesius .
Doxorubicin consists of a naphthacenequinone nucleus linked through a glycosidic bond at ring atom 7 to an amino sugar, daunosamine.
Chemically, doxorubicin hydrochloride is (8 S ,10 S )-10-[(3-Amino-2,3,6-trideoxy-(alpha)-L- lyxo -hexopyranosyl)-oxy]-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione hydrochloride. The structural formula is as follows:
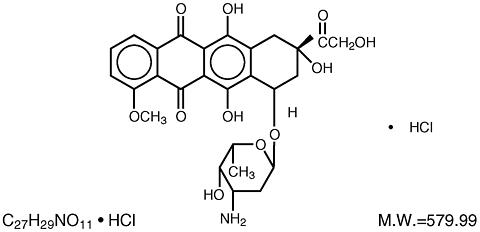
Doxorubicin binds to nucleic acids, presumably by specific intercalation of the planar anthracycline nucleus with the DNA double helix. The anthracycline ring is lipophilic, but the saturated end of the ring system contains abundant hydroxyl groups adjacent to the amino sugar, producing a hydrophilic center. The molecule is amphoteric, containing acidic functions in the ring phenolic groups and a basic function in the sugar amino group. It binds to cell membranes, as well as plasma proteins.
It is supplied in the hydrochloride form as a sterile red-orange lyophilized powder containing lactose and as a sterile parenteral, isotonic solution with sodium chloride for intravenous use only.
Adriamycin (DOXOrubicin HCI) for Injection, USP:
Each 10 mg lyophilized vial contains 10 mg of Doxorubicin Hydrochloride, USP and 50 mg of Lactose Monohydrate, NF.
Each 20 mg lyophilized vial contains 20 mg of Doxorubicin Hydrochloride, USP and 100 mg of Lactose Monohydrate, NF.
Each 50 mg lyophilized vial contains 50 mg of Doxorubicin Hydrochloride, USP and 250 mg of Lactose Monohydrate, NF.
Adriamycin (DOXOrubicin HCI) Injection, USP:
Each 2 mg/mL, 5 mL (10 mg) vial contains 10 mg Doxorubicin Hydrochloride, USP; Sodium Chloride 0.9% (to adjust tonicity) and Water for Injection q.s.; pH adjusted to 3 using Hydrochloric Acid.
Each 2 mg/mL, 10 mL (20 mg) vial contains 20 mg Doxorubicin Hydrochloride, USP; Sodium Chloride 0.9% (to adjust tonicity) and Water for Injection q.s.; pH adjusted to 3 using Hydrochloric Acid.
Each 2 mg/ mL, 25 mL (50 mg) vial contains 50 mg Doxorubicin Hydrochloride, USP; Sodium Chloride 0.9% (to adjust tonicity) and Water for Injection q.s.; pH adjusted to 3 using Hydrochloric Acid.
Each 2 mg/mL, 100 mL (200 mg) multiple dose vial contains 200 mg Doxorubicin Hydrochloride, USP; Sodium Chloride 0.9% (to adjust tonicity) and Water for Injection q.s.; pH adjusted to 3 using Hydrochloric Acid.
CLINICAL PHARMACOLOGY
The cytotoxic effect of doxorubicin on malignant cells and its toxic effects on various organs are thought to be related to nucleotide base intercalation and cell membrane lipid binding activities of doxorubicin. Intercalation inhibits nucleotide replication and action of DNA and RNA polymerases. The interaction of doxorubicin with topoisomerase II to form DNA-cleavable complexes appears to be an important mechanism of doxorubicin cytocidal activity. Doxorubicin cellular membrane binding may effect a variety of cellular functions. Enzymatic electron reduction of doxorubicin by a variety of oxidases, reductases and dehydrogenases generate highly reactive species including the hydroxyl free radical OH·. Free radical formation has been implicated in doxorubicin cardiotoxicity by means of Cu (II) and Fe (III) reduction at the cellular level. Cells treated with doxorubicin have been shown to manifest the characteristic morphologic changes associated with apoptosis or programmed cell death. Doxorubicin-induced apoptosis may be an integral component of the cellular mechanism of action relating to therapeutic effects, toxicities, or both.
Animal studies have shown activity in a spectrum of experimental tumors, immunosuppression, carcinogenic properties in rodents, induction of a variety of toxic effects, including delayed and progressive cardiac toxicity, myelosuppression in all species and atrophy to testes in rats and dogs.
Pharmacokinetic studies, determined in patients with various types of tumors undergoing either single or multi-agent therapy have shown that doxorubicin follows a multiphasic disposition after intravenous injection. The initial distributive half-life of approximately 5 minutes suggests rapid tissue uptake of doxorubicin, while its slow elimination from tissues is reflected by a terminal half-life of 20 to 48 hours. Steady-state distribution volumes exceed 20 to 30 L/kg and are indicative of extensive drug uptake into tissues. Plasma clearance is in the range of 8 to 20 mL/min/kg and is predominately by metabolism and biliary excretion. Approximately 40% of the dose appears in the bile in 5 days, while only 5 to 12% of the drug and its metabolites appear in the urine during the same time period. Binding of doxorubicin and its major metabolite, doxorubicinol to plasma proteins is about 74 to 76% and is independent of plasma concentration of doxorubicin up to 2 µM. Enzymatic reduction at the 7 position and cleavage of the daunosamine sugar yields aglycones which are accompanied by free radical formation, the local production of which may contribute to the cardiotoxic activity of doxorubicin. Disposition of doxorubicinol (DOX-OL) in patients is formation rate limited. The terminal half-life of DOX-OL is similar to doxorubicin. The relative exposure of DOX-OL, compared to doxorubicin ranges between 0.4 to 0.6. In urine, <3% of the dose was recovered as DOX-OL over 7 days.
A published clinical study involving 6 men and 21 women with no prior anthracycline therapy reported a significantly higher median doxorubicin clearance in the men compared to the women (113 versus 45 L/hr). However, the terminal half-life of doxorubicin was longer in men compared to the women (54 versus 35 hrs).
In four patients , dose-independent pharmacokinetics have been shown for doxorubicin in the dose range of 30 to 70 mg/m 2 . Systemic clearance of doxorubicin is significantly reduced in obese women with ideal body weight greater than 130%. There was a significant reduction in clearance without any change in volume of distribution in obese patients when compared with normal patients with less than 115% ideal body weight. The clearance of doxorubicin and doxorubicinol was also reduced in patients with impaired hepatic function. Doxorubicin was excreted in the milk of one lactating patient, with peak milk concentration at 24 hours after treatment being approximately 4.4 -fold greater than the corresponding plasma concentration . Doxorubicin was detectable in the milk up to 72 hours after therapy with 70 mg/m 2 of doxorubicin given as a 15 minute intravenous infusion and 100 mg/m 2 of cisplatin as a 26 hour intravenous infusion. The peak concentration of doxorubicinol in milk at 24 hours was 0.2 µM and AUC up to 24 hours was 16.5 µM.hr while the AUC for doxorubicin was 9.9 µM.hr.
Following administration of 10 to 75-mg/m 2 doses of doxorubicin to 60 children and adolescents ranging from 2 months to 20 years of age, doxorubicin clearance averaged 1443 +/- 114 mL/min/m 2 . Further analysis demonstrated that clearance in 52 children greater than 2 years of age (1540 mL/min/m 2 ) was increased compared with adults. However, clearance in infants younger than 2 years of age (813 mL/min/m 2 ) was decreased compared with older children and approached the range of clearance values determined in adults.
Doxorubicin does not cross the blood brain barrier.
INDICATIONS AND USAGE
Adriamycin (DOXOrubicin HCl) Injection, USP and Adriamycin (DOXOrubicin HCl) for Injection, USP have been used successfully to produce regression in disseminated neoplastic conditions such as acute lymphoblastic leukemia, acute myeloblastic leukemia, Wilms' tumor, neuroblastoma, soft tissue and bone sarcomas, breast carcinoma, ovarian carcinoma, transitional cell bladder carcinoma, thyroid carcinoma, gastric carcinoma, Hodgkin's disease, malignant lymphoma and bronchogenic carcinoma in which the small cell histologic type is the most responsive compared to other cell types.
CONTRAINDICATIONS
Doxorubicin therapy should not be started in patients who have marked myelosuppression induced by previous treatment with other antitumor agents or by radiotherapy. Doxorubicin treatment is contraindicated in patients who received previous treatment with complete cumulative doses of doxorubicin, daunorubicin, idarubicin, and/or other anthracyclines and anthracenes.
WARNINGS
Special attention must be given to the cardiotoxicity induced by doxorubicin. Irreversible myocardial toxicity, manifested in its most severe form by life-threatening or fatal congestive heart failure, may occur either during therapy or months to years after termination of therapy. The probability of developing impaired myocardial function, based on a combined index of signs, symptoms and decline in left ventricular ejection fraction (LVEF) is estimated to be 1 to 2% at a total cumulative dose of 300 mg/m 2 of doxorubicin, 3 to 5% at a dose of 400 mg/m 2 , 5 to 8% at a dose of 450 mg/m 2 and 6 to 20% at a dose of 500 mg/m 2 given in a schedule of a bolus injection once every 3 weeks (data on file at Pharmacia & Upjohn). In a retrospective review by Von Hoff et al, the probability of developing congestive heart failure was reported to be 5/168 (3%) at a cumulative dose of 430 mg/m 2 of doxorubicin, 8/110 (7%) at 575 mg/m 2 and 3/14 (21%) at 728 mg/m 2 . The cumulative incidence of CHF was 2.2%. In a prospective study of doxorubicin in combination with cyclophosphamide, fluorouracil and/or vincristine in patients with breast cancer or small cell lung cancer, the cumulative incidence of congestive heart failure was 5 to 6%. The probability of CHF at various cumulative doses of doxorubicin was 1.5% at 300 mg/m 2 , 4.9% at 400 mg/m 2 , 7.7% at 450 mg/m 2 and 20.5% at 500 mg/m 2 .
Cardiotoxicity may occur at lower doses in patients with prior mediastinal irradiation, concurrent cyclophosphamide therapy, exposure at an early age and advanced age. Data also suggest that pre-existing heart disease is a co-factor for increased risk of doxorubicin cardiotoxicity. In such cases, cardiac toxicity may occur at doses lower than the respective recommended cumulative dose of doxorubicin. Studies have suggested that concomitant administration of doxorubicin and calcium channel entry blockers may increase the risk of doxorubicin cardiotoxicity. The total dose of doxorubicin administered to the individual patient should also take into account previous or concomitant therapy with related compounds such as daunorubicin, idarubicin and mitoxantrone. Cardiomyopathy and/or congestive heart failure may be encountered several months or years after discontinuation of doxorubicin therapy.
The risk of congestive heart failure and other acute manifestations of doxorubicin cardiotoxicity in pediatric patients may be as much or lower than in adults. Pediatric patients appear to be at particular risk for developing delayed cardiac toxicity in that doxorubicin induced cardiomyopathy impairs myocardial growth as pediatric patients mature, subsequently leading to possible development of congestive heart failure during early adulthood. As many as 40% of pediatric patients may have subclinical cardiac dysfunction and 5 to 10% of pediatric patients may develop congestive heart failure on long term follow-up. This late cardiac toxicity may be related to the dose of doxorubicin. The longer the length of follow-up the greater the increase in the detection rate.
Treatment of doxorubicin induced congestive heart failure includes the use of digitalis, diuretics, after load reducers such as angiotensin I converting enzyme (ACE) inhibitors, low salt diet, and bed rest. Such intervention may relieve symptoms and improve the functional status of the patient.
Monitoring Cardiac Function: In adult patients severe cardiac toxicity may occur precipitously without antecedent ECG changes. Cardiomyopathy induced by anthracyclines is usually associated with very characteristic histopathologic changes on an endomyocardial biopsy (EM biopsy), and a decrease of left ventricular ejection fraction (LVEF), as measured by multi-gated radionuclide angiography (MUGA scans) and/or echocardiogram (ECHO), from pretreatment baseline values. However, it has not been demonstrated that monitoring of the ejection fraction will predict when individual patients are approaching their maximally tolerated cumulative dose of doxorubicin. Cardiac function should be carefully monitored during treatment to minimize the risk of cardiac toxicity. A baseline cardiac evaluation with an ECG, LVEF, and/or an echocardiogram (ECHO) is recommended especially in patients with risk factors for increased cardiac toxicity (pre-existing heart disease, mediastinal irradiation, or concurrent cyclophosphamide therapy). Subsequent evaluations should be obtained at a cumulative dose of doxorubicin of at least 400 mg/m 2 and periodically thereafter during the course of therapy. Pediatric patients are at increased risk for developing delayed cardiotoxicity following doxorubicin administration and therefore a follow-up cardiac evaluation is recommended periodically to monitor for this delayed cardiotoxicity.
In adults, a 10% decline in LVEF to below the lower limit of normal or an absolute LVEF of 45%, or a 20% decline in LVEF at any level is indicative of deterioration in cardiac function. In pediatric patients, deterioration in cardiac function during or after the completion of therapy with doxorubicin is indicated by a drop in fractional shortening (FS) by an absolute value of >/=10 percentile units or below 29%, and a decline in LVEF of 10 percentile units or an LVEF below 55%. In general, if test results indicate deterioration in cardiac function associated with doxorubicin, the benefit of continued therapy should be carefully evaluated against the risk of producing irreversible cardiac damage.
Acute life-threatening arrhythmias have been reported to occur during or within a few hours after doxorubicin administration.
There is a high incidence of bone marrow depression, primarily of leukocytes, requiring careful hematologic monitoring. With the recommended dosage schedule, leukopenia is usually transient, reaching its nadir at 10 to 14 days after treatment with recovery usually occurring by the 21st day. White blood counts as low as 1000/mm 3 are to be expected during treatment with appropriate doses of doxorubicin. Red blood and platelet levels should also be monitored since they may also be depressed. Hematologic toxicity may require dose reduction or suspension or delay of doxorubicin therapy. Persistent severe myelosuppression may result in superinfection or hemorrhage.
Doxorubicin may potentiate the toxicity of other anticancer therapies. Exacerbation of cyclophosphamide-induced hemorrhagic cystitis and enhancement of the hepatotoxicity of 6-mercaptopurine have been reported. Radiation-induced toxicity to the myocardium, mucosae, skin, and liver have been reported to be increased by the administration of doxorubicin. Pediatric patients receiving concomitant doxorubicin and actinomycin-D have manifested acute `recall' pneumonitis at variable times after local radiation therapy.
Since metabolism and excretion of doxorubicin occurs predominantly by the hepatobiliary route, toxicity to recommended doses of doxorubicin can be enhanced by hepatic impairment; therefore, prior to the individual dosing, evaluation of hepatic function is recommended using conventional laboratory tests such as SGOT, SGPT, alkaline phosphatase, and bilirubin (see DOSAGE AND ADMINISTRATION ).
Necrotizing colitis manifested by typhlitis (cecal inflammation), bloody stools and severe and sometimes fatal infections, have been associated with a combination of doxorubicin given by IV push daily for 3 days and cytarabine given by continuous infusion daily for 7 or more days.
On intravenous administration of doxorubicin, extravasation may occur with or without an accompanying stinging or burning sensation, even if blood returns well on aspiration of the infusion needle (see DOSAGE AND ADMINISTRATION ). If any signs or symptoms of extravasation have occurred the injection or infusion should be immediately terminated and restarted in another vein.
Pregnancy Category D: Safe use of doxorubicin in pregnancy has not been established. Doxorubicin is embryotoxic and teratogenic in rats and embryotoxic and abortifacient in rabbits. There are no adequate and well-controlled studies in pregnant women. If doxorubicin is to be used during pregnancy, or if the patient becomes pregnant during therapy, the patient should be apprised of the potential hazard to the fetus. Women of childbearing age should be advised to avoid becoming pregnant.
PRECAUTIONS
General: Doxorubicin is not an anti-microbial agent.
Information for Patients: Adriamycin (DOXOrubicin HCl) Injection, USP and Adriamycin (DOXOrubicin HCl) for Injection, USP impart a red coloration to the urine for 1 to 2 days after administration, and patients should be advised to expect this during active therapy.
Drug Interactions: Literature contains the following drug interactions with doxorubicin in humans: cyclosporine (Sandimmune) may induce coma and/or seizures, phenobarbital increases the elimination of doxorubicin, phenytoin levels may be decreased by doxorubicin, streptozocin (Zanosar) may inhibit the hepatic metabolism, and administration of live vaccines to immunosuppressed patients, including those undergoing cytotoxic chemotherapy, may be hazardous.
Paclitaxel: Two published studies report that initial administration of paclitaxel infused over 24 hours followed by doxorubicin administered over 48 hours resulted in a significant decrease in doxorubicin clearance with more profound neutropenic and stomatitis episodes than the reverse sequence of administration.
Progesterone: In a published study, progesterone was given intravenously to patients with advanced malignancies (ECOG PS < 2) at high doses (up to 10 g over 24 hours) concomitantly with a fixed doxorubicin dose (60 mg/m 2 ) via bolus. Enhanced doxorubicin-induced neutropenia and thrombocytopenia were observed.
Verapamil: A study of the effects of verapamil on the acute toxicity of doxorubicin in mice revealed higher intial peak concentrations of doxorubicin in the heart with a higher incidence and severity of degenerative changes in cardiac tissue resulting in a shorter survival.
Cyclosporine: The addition of cyclosporine to doxorubicin may result in increases in AUC for both doxorubicin and doxorubicinol possibly due to a decrease in clearance of parent drug and a decrease in metabolism of doxorubicinol. Literature reports suggest that adding cyclosporine to doxorubicin results in more profound and prolonged hematologic toxicity than doxorubicin alone. Coma and/or seizures have also been described.
Literature reports have also described the following drug interactions: phenobarbital increases the elimination of doxorubicin, phenytoin levels may be decreased by doxorubicin, streptozocin (Zanosar) may inhibit hepatic metabolism of doxorubicin, and administration of live vaccines to immunosuppressed patients including those undergoing cytotoxic chemotherapy may be hazardous.
Laboratory Tests: Initial treatment with doxorubicin requires observation of the patient and periodic monitoring of complete blood counts, hepatic function tests, and radionuclide left ventricular ejection fraction (see WARNINGS ).
Like other cytotoxic drugs, doxorubicin may induce "tumor lysis syndrome" and hyperuricemia in patients with rapidly growing tumors. Appropriate supportive and pharmacologic measures may prevent or alleviate this complication.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Formal long-term carcinogenicity studies have not been conducted with doxorubicin. Doxorubicin and related compounds have been shown to have mutagenic and carcinogenic properties when tested in experimental models (including bacterial systems, mammalian cells in culture, and female Sprague-Dawley rats).
The possible adverse effect on fertility in males and females in humans or experimental animals have not been adequately evaluated. Testicular atrophy was observed in rats and dogs.
Treatment-related acute myelogenous leukemia has been reported in patients treated with doxorubicin-containing adjuvant chemotherapy regimens (see ADVERSE REACTIONS , Hematologic ). The exact role of doxorubicin has not been elucidated. Pediatric patients treated with doxorubicin or other topoisomerase II inhibitors are at risk for developing acute myelogenous leukemia and other neoplasms. The extent of increased risk associated with doxorubicin has not been precisely quantified.
Pregnancy Category D: (See WARNINGS. )
Nursing Mothers: Because of the potential for serious adverse reactions in nursing infants from doxorubicin, mothers should be advised to discontinue nursing during doxorubicin therapy.
Pediatric Use: Pediatric patients are at increased risk for developing delayed cardiotoxicity. Follow-up cardiac evaluations are recommended periodically to monitor for this delayed cardiotoxicity (see WARNINGS ).
Doxorubicin, as a component of intensive chemotherapy regimens administered to pediatric patients, may contribute to prepubertal growth failure. It may also contribute to gonadal impairment, which is usually temporary.
ADVERSE REACTIONS
Dose-limiting toxicities of therapy are myelosuppression and cardiotoxicity (see WARNINGS ). Other reactions reported are:
Cardiotoxicity: (See WARNINGS .)
Cutaneous: Reversible complete alopecia occurs in most cases. Hyperpigmentation of nailbeds and dermal creases, primarily in children, and onycholysis have been reported in a few cases. Recall of skin reaction due to prior radiotherapy has occurred with doxorubicin administration.
Gastrointestinal: Acute nausea and vomiting occurs frequently and may be severe. This may be alleviated by antiemetic therapy. Mucositis (stomatitis and esophagitis) may occur 5 to 10 days after administration. The effect may be severe leading to ulceration and represents a site of origin for severe infections. The dosage regimen consisting of administration of doxorubicin on 3 successive days results in the greater incidence and severity of mucositis. Ulceration and necrosis of the colon, especially the cecum, may occur leading to bleeding or severe infections which can be fatal. This reaction has been reported in patients with acute non-lymphocytic leukemia treated with a 3-day course of doxorubicin combined with cytarabine. Anorexia and diarrhea have been occasionally reported.
Vascular: Phlebosclerosis has been reported especially when small veins are used or a single vein is used for repeated administration. Facial flushing may occur if the injection is given too rapidly.
Local: Severe cellulitis, vesication and tissue necrosis will occur if extravasation of doxorubicin occurs during administration. Erythematous streaking along the vein proximal to the site of the injection has been reported (see DOSAGE AND ADMINISTRATION ).
Hematologic: The occurrence of secondary acute myeloid leukemia with or without a preleukemic phase has been reported in patients concurrently treated with doxorubicin in association with DNA-damaging antineoplastic agents. Such cases could have a short (1-3 years) latency period. An analysis of 1474 breast cancer patients who received adjuvant doxorubicin treatment in clinical trials, showed a 10-year estimated risk of developing treatment-related leukemia at 2.5% (95% confidence interval [CI], 1.0% to 5.1%) for the 810 patients receiving radiotherapy plus chemotherapy and 0.5% (95% CI, 0.1% to 2.4%) for the 664 patients receiving chemotherapy alone. The overall risk was 1.5% (95% CI, 0.7%-2.9%) at 10 years for the entire patient population. Pediatric patients are also at risk of developing secondary acute myeloid leukemia.
Hypersensitivity: Fever, chills, and urticaria have been reported occasionally. Anaphylaxis may occur. A case of apparent cross-sensitivity to lincomycin has been reported.
Other: Conjunctivitis and lacrimation occur rarely.
OVERDOSAGE
Acute overdosage with doxorubicin enhances the toxic effects of mucositis, leukopenia, and thrombocytopenia. Treatment of acute overdosage consists of treatment of the severely myelosuppressed patient with hospitalization, antimicrobials, platelet transfusion and symptomatic treatment of mucositis. Use of hemopoietic growth factor (G-CSF, GM-CSF) may be considered.
The 200 mg Adriamycin (DOXOrubicin HCI) Injection, USP is packaged as a multiple dose vial, and caution should be exercised to prevent inadvertent overdosage.
Cumulative dosage with doxorubicin increases the risk of cardiomyopathy and resultant congestive heart failure (see WARNINGS ). Treatment consists of vigorous management of congestive heart failure with digitalis preparations, diuretics, and afterload reducers such as ACE inhibitors.
DOSAGE AND ADMINISTRATION
Care in the administration of Adriamycin (DOXOrubicin HCl) Injection, USP and Adriamycin (DOXOrubicin HCl) for Injection, USP will reduce the chance of perivenous infiltration (see WARNINGS ). It may also decrease the chance of local reactions such as urticaria and erythematous streaking. On intravenous administration of doxorubicin, extravasation may occur with or without an accompanying burning or stinging sensation, even if blood returns well on aspiration of the infusion needle. If any signs or symptoms of extravasation have occurred, the injection or infusion should be immediately terminated and restarted in another vein. If extravasation is suspected, intermittent application of ice to the site for 15 min. q.i.d. × 3 days may be useful. The benefit of local administration of drugs has not been clearly established. Because of the progressive nature of extravasation reactions, close observation and plastic surgery consultation is recommended. Blistering, ulceration and/or persistent pain are indications for wide excision surgery, followed by split-thickness skin grafting. 1
The most commonly used dose schedule when used as a single agent is 60 to 75 mg/m 2 as a single intravenous injection administered at 21-day intervals. The lower dosage should be given to patients with inadequate marrow reserves due to old age, or prior therapy, or neoplastic marrow infiltration. Adriamycin (DOXOrubicin HCl) Injection, USP and Adriamycin (DOXOrubicin HCl) for Injection, USP have been used concurrently with other approved chemotherapeutic agents. Evidence is available that in some types of neoplastic disease, combination chemotherapy is superior to single agents. The benefits and risks of such therapy continue to be elucidated. When used in combination with other chemotherapy drugs, the most commonly used dosage of doxorubicin is 40 to 60 mg/m 2 given as a single intravenous injection every 21 to 28 days. Doxorubicin dosage must be reduced in case of hyperbilirubinemia as follows:
Plasma bilirubin
concentration (mg/dL)Dosage reduction
(%)1.2-3.0 50 3.1-5.0 75 Reconstitution Directions: Adriamycin (DOXOrubicin HCl) for Injection, USP 10 mg, 20 mg, and 50 mg vials should be reconstituted with 5 mL, 10 mL, and 25 mL respectively of 0.9% Sodium Chloride Injection to give a final concentration of 2 mg/mL of doxorubicin hydrochloride. An appropriate volume of air should be withdrawn from the vial during reconstitution to avoid excessive pressure build up. Bacteriostatic diluents are not recommended.
After adding the diluent, the vial should be shaken and the contents allowed to dissolve. The reconstituted solution is stable for 7 days at room temperature and under normal room light (100 foot-candles) and 15 days under refrigeration, 2° to 8°C (36° to 46°F). It should be protected from exposure to sunlight. Discard any unused solution from the 10 mg, 20 mg, and 50 mg single dose vials. Unused solutions of the multiple dose vial remaining beyond the recommended storage times should be discarded.
It is recommended that Adriamycin (DOXOrubicin HCl) Injection, USP and Adriamycin (DOXOrubicin HCl) for Injection, USP be slowly administered into the tubing of a freely running intravenous infusion of Sodium Chloride Injection or 5% Dextrose Injection. The tubing should be attached to a Butterfly® needle inserted preferably into a large vein. If possible, avoid veins over joints or in extremities with compromised venous or lymphatic drainage. The rate of administration is dependent on the size of the vein and the dosage. However, the dose should be administered in not less than 3 to 5 minutes. Local erythematous streaking along the vein as well as facial flushing may be indicative of too rapid an administration. A burning or stinging sensation may be indicative of perivenous infiltration and the infusion should be immediately terminated and restarted in another vein. Perivenous infiltration may occur painlessly.
Doxorubicin should not be mixed with heparin or fluorouracil since it has been reported that these drugs are incompatible to the extent that a precipitate may form. Until specific compatibility data are available, it is not recommended that doxorubicin be mixed with other drugs.
Note: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Handling and Disposal: Skin reactions associated with doxorubicin have been reported. Skin accidently exposed to doxorubicin should be rinsed copiously with soap and warm water, and if the eyes are involved, standard irrigation techniques should be used immediately. The use of goggles, gloves, and protective gowns is recommended during preparation and administration of the drug.
Procedures for proper handling and disposal of anti-cancer drugs should be considered. Several guidelines on this subject have been published. 1-8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Caregivers of pediatric patients receiving doxorubicin should be counseled to take precautions (such as wearing latex gloves) to prevent contact with the patient's urine and other body fluids for at least 5 days after each treatment.
HOW SUPPLIED
Adriamycin (DOXOrubicin HCl) for Injection, USP is supplied as a sterile red-orange lyophilized powder in single dose flip-top vials in the following package strengths:
NDC 55390-231-10: 10 mg vial; carton of 10.
NDC 55390-232-10: 20 mg vial; carton of 10.
NDC 55390-233-01: 50 mg vial; individually boxed.
Store unreconstituted vial at controlled room temperature, 15° to 30°C (59° to 86°F) [see USP]. Protect from light. Retain in carton until time of use. Discard unused portion.
Reconstituted Solution Stability: After adding the diluent, the vial should be shaken and the contents allowed to dissolve. The reconstituted solution is stable for 7 days at room temperature and under normal room light (100 foot-candles) and 15 days under refrigeration (2° to 8°C). It should be protected from exposure to sunlight. Discard any unused solution from the 10 mg, 20 mg and 50 mg single dose vials. Unused solutions of the multiple dose vial remaining beyond the recommended storage times should be discarded.
Adriamycin (DOXOrubicin HCl) Injection, USP is supplied in single-dose, flip-top vials, as a red-orange solution containing Doxorubicin Hydrochloride, USP 2 mg/mL in the following package strengths:
NDC 55390-235-10: 510 mg in 5 mL; carton of 10.
NDC 55390-236-10: 20 mg in 10 mL; carton of 10.
NDC 55390-237-01: 50 mg in 25 mL; individually boxed.
Store refrigerated, 2° to 8°C (36° to 46°F).
Protect from light. Retain in carton until time of use. Discard unused portion.
Adriamycin (DOXOrubicin HCl) Injection, USP is supplied in a sterile, multiple dose, flip-top vial, as a red-orange solution containing Doxorubicin Hydrochloride, USP 2 mg/mL in the following package strength:
NDC 55390-238-01: 200 mg in 100 mL; individually boxed.
Store refrigerated, 2° to 8°C (36° to 46°F).
Protect from light. Retain in carton until contents are used.
REFERENCES
- Recommendations for the Safe Handling of Parenteral Antineoplastic Drugs. NIH Publication No. 83-2621. For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402.
- AMA Council Report. Guidelines for Handling Parenteral Antineoplastics. JAMA , 1985; 253 (11): 1590-1592.
- National Study Commission on Cytotoxic Exposure -- Recommendation for Handling Cytotoxic Agents. Available from Louis P. Jeffrey, ScD, Chairman, National Study Commission on Cytotoxic Exposure, Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Ave., Boston, Massachusetts 02115.
- Clinical Oncological Society of Australia: Guidelines and Recommendations for Safe Handling of Antineoplastic Agents. Med J Australia 1983; 1 :426-428.
- Jones RB, et al. Safe Handling of Chemotherapeutic Agents: A Report From the Mount Sinai Medical Center. CA-A Cancer Journal for Clinicians 1983; Sept/Oct, 258-263.
- American Society of Hospital Pharmacists Technical Assistance Bulletin on Handling Cytotoxic and Hazardous Drugs. Am J Hosp Pharm 1990; 47 :1033-1049.
- Controlling Occupational Exposure to Hazardous Drugs. (OSHA Work-Practice Guidelines), Am J Healthsyst Pharm , 1996; 53 : 1669-1685.
- ONS Clinical Practice Committee. Cancer Chemotherapy Guidelines and Recommendations for Practice Pittsburgh, Pa: Oncology Nursing Society; 1999:32-41.
Manufactured by: Manufactured for:
Ben Venue Laboratories, Inc. Bedford Laboratories™
Bedford, OH 44146 Bedford, OH 44146
December 2002 ADR-P00
Subscribe to the "News" RSS Feed 
TOP ۞
