-
Alphagan P Ophthalmic Solution (Allergan)
DESCRIPTION
ALPHAGAN® P (brimonidine tartrate ophthalmic solution) 0.15% is a relatively selective alpha-2 adrenergic agonist for ophthalmic use. The chemical name of brimonidine tartrate is 5-bromo-6- (2-imidazolidinylideneamino) quinoxaline L-tartrate. It is an off-white to pale yellow powder. It has a molecular weight of 442.24 as the tartrate salt, and is both soluble in water (1.5 mg/mL) and in the product vehicle (3.0 mg/mL) at pH 7.2. The structural formula is:
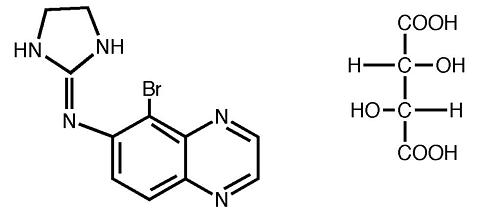
Formula: C 11 H 10 BrN 5 ·C 4 H 6 O 6
CAS Number: 70359-46-5
In solution, ALPHAGAN® P (brimonidine tartrate ophthalmic solution) 0.15% has a clear, greenish-yellow color. It has an osmolality of 250-350 mOsmol/kg and a pH of 6.6-7.4.
Each mL of ALPHAGAN® P contains:
Active Ingredient: brimonidine tartrate 0.15% (1.5 mg/mL)
Preservative: Purite® 0.005% (0.05 mg/mL)
Inactives: boric acid; calcium chloride; magnesium chloride; potassium chloride; purified water; sodium borate; sodium carboxymethylcellulose; sodium chloride; with hydrochloric acid and/or sodium hydroxide to adjust pH.
CLINICAL PHARMACOLOGY
Mechanism of Action: ALPHAGAN® P ophthalmic solution is an alpha adrenergic receptor agonist. It has a peak ocular hypotensive effect occurring at two hours post-dosing. Fluorophotometric studies in animals and humans suggest that brimonidine tartrate has a dual mechanism of action by reducing aqueous humor production and increasing uveoscleral outflow.
Pharmacokinetics: After ocular administration of either a 0.1% or 0.2% solution, plasma concentrations peaked within 0.5 to 2.5 hours and declined with a systemic half-life of approximately 2 hours. In humans, systemic metabolism of brimonidine is extensive. It is metabolized primarily by the liver. Urinary excretion is the major route of elimination of the drug and its metabolites. Approximately 87% of an orally-administered radioactive dose was eliminated within 120 hours, with 74% found in the urine.
Clinical Evaluations: Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss. Brimonidine tartrate has the action of lowering intraocular pressure with minimal effect on cardiovascular and pulmonary parameters.
Two clinical studies were conducted to evaluate the safety, efficacy, and acceptability of ALPHAGAN® P (brimonidine tartrate ophthalmic solution) 0.15% compared with ALPHAGAN®, administered three-times-daily in patients with open-angle glaucoma or ocular hypertension. Those results indicated that ALPHAGAN® P (brimonidine tartrate ophthalmic solution) 0.15% is comparable in IOP lowering effect to ALPHAGAN® (brimonidine tartrate ophthalmic solution) 0.2%, and effectively lowers IOP in patients with open-angle glaucoma or ocular hypertension by approximately 2-5 mmHg.
INDICATIONS AND USAGE
ALPHAGAN® P is indicated for the lowering of intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
CONTRAINDICATIONS
ALPHAGAN® P is contraindicated in patients with hypersensitivity to brimonidine tartrate or any component of this medication. It is also contraindicated in patients receiving monoamine oxidase (MAO) inhibitor therapy.
PRECAUTIONS
General: Although ALPHAGAN® P ophthalmic solution had minimal effect on the blood pressure of patients in clinical studies, caution should be exercised in treating patients with severe cardiovascular disease.
ALPHAGAN® P has not been studied in patients with hepatic or renal impairment; caution should be used in treating such patients. ALPHAGAN® P should be used with caution in patients with depression, cerebral or coronary insufficiency, Raynaud's phenomenon, orthostatic hypotension, or thromboangiitis obliterans. Patients prescribed IOP-lowering medication should be routinely monitored for IOP.
Information for Patients: As with other drugs in this class, ALPHAGAN® P ophthalmic solution may cause fatigue and/or drowsiness in some patients. Patients who engage in hazardous activities should be cautioned of the potential for a decrease in mental alertness.
Drug Interactions: Although specific drug interaction studies have not been conducted with ALPHAGAN® P, the possibility of an additive or potentiating effect with CNS depressants (alcohol, barbiturates, opiates, sedatives, or anesthetics) should be considered. Alpha-agonists, as a class, may reduce pulse and blood pressure. Caution in using concomitant drugs such as beta-blockers (ophthalmic and systemic), anti-hypertensives and/or cardiac glycosides is advised.
Tricyclic antidepressants have been reported to blunt the hypotensive effect of systemic clonidine. It is not known whether the concurrent use of these agents with ALPHAGAN® P ophthalmic solution in humans can lead to resulting interference with the IOP lowering effect. No data on the level of circulating catecholamines after ALPHAGAN® P administration are available. Caution, however, is advised in patients taking tricyclic antidepressants which can affect the metabolism and uptake of circulating amines.
Carcinogenesis, Mutagenesis, and Impairment of Fertility: No compound-related carcinogenic effects were observed in either mice or rats following a 21-month and 24-month study, respectively. In these studies, dietary administration of brimonidine tartrate at doses up to 2.5 mg/kg/day in mice and 1.0 mg/kg/day in rats achieved 86 and 55 times, respectively, the plasma drug concentration estimated in humans treated with one drop of ALPHAGAN® P ophthalmic solution into both eyes 3 times per day.
Brimonidine tartrate was not mutagenic or cytogenic in a series of in vitro and in vivo studies including the Ames test, chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells, a host-mediated assay and cytogenic studies in mice, and dominant lethal assay.
Reproductive studies performed in rats with oral doses of 0.66 mg base/kg revealed no evidence of impaired fertility due to ALPHAGAN® P.
Pregnancy: Teratogenic Effects: Pregnancy Category B. Reproductive studies performed in rats with oral doses of 0.66 mg base/kg revealed no evidence of harm to the fetus due to ALPHAGAN® P ophthalmic solution. Dosing at this level produced an exposure that is 189 times higher than the exposure seen in humans following multiple ophthalmic doses.
There are no adequate and well-controlled studies in pregnant women. In animal studies, brimonidine crossed the placenta and entered into the fetal circulation to a limited extent. ALPHAGAN® P should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
Nursing Mothers: It is not known whether this drugis excreted in human milk; in animal studies brimonidine tartrate was excreted in breast milk. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use: In a well-controlled clinical study conducted in pediatric glaucoma patients (ages 2 to 7 years) the most commonly observed adverse events with brimonidine tartrate ophthalmic solution 0.2% dosed three times daily were somnolence (50%-83% in patients ages 2 to 6 years) and decreased alertness. In pediatric patients 7 years of age or older (>20kg), somnolence appears to occur less frequently (25%). Approximately 16% of patients on brimonidine tartrate ophthalmic solution discontinued from the study due to somnolence.
The safety and effectiveness of brimonidine tartrate ophthalmic solution have not been studied in pediatric patients below the age of 2 years. Brimonidine tartrate ophthalmic solution is not recommended for use in pediatric patients under the age of 2 years. (Also refer to Adverse Reactions section.)
Geriatric Use: No overall differences in safety or effectiveness have been observed between elderly and other adult patients.
ADVERSE REACTIONS
Adverse events occurring in approximately 10-20% of the subjects included: allergic conjunctivitis, conjunctival hyperemia, and eye pruritus.
Adverse events occurring in approximately 5-9% of the subjects included: burning sensation, conjunctival folliculosis, hypertension, oral dryness, and visual disturbance.
Events occurring in approximately 1-4% of subjects included: allergic reaction, asthenia, blepharitis, bronchitis, conjunctival edema, conjunctival hemorrhage, conjunctivitis, cough, dizziness, dyspepsia, dyspnea, epiphora, eye discharge, eye dryness, eye irritation, eye pain, eyelid edema, eyelid erythema, flu syndrome, follicular conjunctivitis, foreign body sensation, headache, pharyngitis, photophobia, rash, rhinitis, sinus infection, sinusitis, stinging, superficial punctate keratopathy, visual field defect, vitreous floaters, and worsened visual acuity.
The following events were reported in less than 1% of subjects: corneal erosion, insomnia, nasal dryness, somnolence, and taste perversion.
The following events have been identified during post-marketing use of ALPHAGAN® ophthalmic solution in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The events, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to ALPHAGAN®, or a combination of these factors, include: bradycardia; hypotension; iritis; miosis; skin reactions (including erythema, eyelid pruritus, rash, and vasodilation) and tachycardia. Apnea, bradycardia, hypotension, hypothermia, hypotonia, and somnolence have been reported in infants receiving ALPHAGAN® ophthalmic solution.
OVERDOSAGE
No information is available on overdosage in humans. Treatment of an oral overdose includes supportive and symptomatic therapy; a patent airway should be maintained.
DOSAGE AND ADMINISTRATION
The recommended dose is one drop of ALPHAGAN® P in the affected eye(s) three times daily, approximately 8 hours apart.
ALPHAGAN® P ophthalmic solution may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic product is being used, the products should be administered at least 5 minutes apart.
HOW SUPPLIED
ALPHAGAN® P (brimonidine tartrate ophthalmic solution) 0.15% is supplied sterile in opaque teal LDPE plastic bottles and tips with purple high impact polystyrene (HIPS) caps as follows:
5 mL in 10 mL bottle NDC 0023-9177-05
10 mL in 10 mL bottle NDC 0023-9177-10
15 mL in 15 mL bottle NDC 0023-9177-15
NOTE: Store at 15°-25°C (59°-77°F).
Rx Only
Revised April 2004
® Marks owned by Allergan, Inc.
U.S. Pat. 5,424,078; 5,736,165; 6,194,415; 6,248,741; 6,465,464; 6,562,873; 6,627,210; 6,641,834; 6,673,337
© 2005 Allergan, Inc
Irvine, CA 92612, U.S.A.9174X
Subscribe to the "News" RSS Feed 
TOP ۞
