-
AndroGel (Unimed)
DESCRIPTION
AndroGel® (testosterone gel) 1% is a clear, colorless hydroalcoholic gel containing 1% testosterone. AndroGel provides continuous transdermal delivery of testosterone, the primary circulating endogenous androgen, for 24 hours following a single application to intact, clean, dry skin of the shoulders, upper arms and/or abdomen.
A daily application of AndroGel 5 g, 7.5 g, or 10 g contains 50 mg, 75 mg, or 100 mg of testosterone, respectively, to be applied daily to the skin's surface. Approximately 10% of the applied testosterone dose is absorbed across skin of average permeability during a 24-hour period.
The active pharmacologic ingredient in AndroGel is testosterone. Testosterone USP is a white to practically white crystalline powder chemically described as 17-beta hydroxyandrost-4-en-3-one.
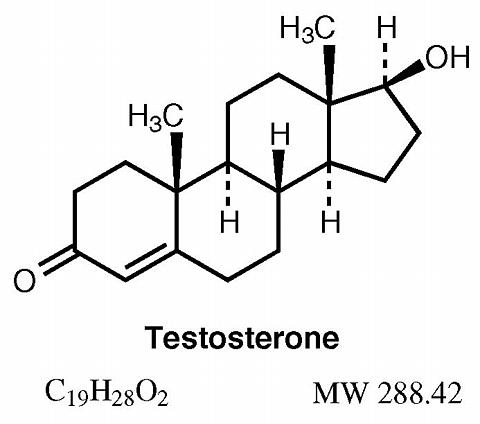
Inactive ingredients in AndroGel are ethanol 67.0%, purified water, sodium hydroxide, carbomer 980 and isopropyl myristate; these ingredients are not pharmacologically active.
CLINICAL PHARMACOLOGY
AndroGel (testosterone gel) delivers physiologic amounts of testosterone, producing circulating testosterone concentrations that approximate normal levels (298 - 1043 ng/dL) seen in healthy men.
Testosterone - General Androgen Effects:
Endogenous androgens, including testosterone and dihydrotestosterone (DHT), are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis, and scrotum; the development of male hair distribution, such as facial, pubic, chest, and axillary hair; laryngeal enlargement, vocal chord thickening, alterations in body musculature, and fat distribution. Testosterone and DHT are necessary for the normal development of secondary sex characteristics. Male hypogonadism results from insufficient secretion of testosterone and is characterized by low serum testosterone concentrations. Symptoms associated with male hypogonadism include impotence and decreased sexual desire, fatigue and loss of energy, mood depression, regression of secondary sexual characteristics and osteoporosis. Hypogonadism is a risk factor for osteoporosis in men.
Drugs in the androgen class also promote retention of nitrogen, sodium, potassium, phosphorus, and decreased urinary excretion of calcium. Androgens have been reported to increase protein anabolism and decrease protein catabolism. Nitrogen balance is improved only when there is sufficient intake of calories and protein.
Androgens are responsible for the growth spurt of adolescence and for the eventual termination of linear growth brought about by fusion of the epiphyseal growth centers. In children, exogenous androgens accelerate linear growth rates but may cause a disproportionate advancement in bone maturation. Use over long periods may result in fusion of the epiphyseal growth centers and termination of the growth process. Androgens have been reported to stimulate the production of red blood cells by enhancing erythropoietin production.
During exogenous administration of androgens, endogenous testosterone release may be inhibited through feedback inhibition of pituitary luteinizing hormone (LH). At large doses of exogenous androgens, spermatogenesis may also be suppressed through feedback inhibition of pituitary follicle-stimulating hormone (FSH).
There is a lack of substantial evidence that androgens are effective in accelerating fracture healing or in shortening postsurgical convalescence.
Pharmacokinetics
Absorption: AndroGel is a hydroalcoholic formulation that dries quickly when applied to the skin surface. The skin serves as a reservoir for the sustained release of testosterone into the systemic circulation. Approximately 10% of the testosterone dose applied on the skin surface from AndroGel is absorbed into systemic circulation. Therefore, 5 g and 10 g of AndroGel systemically delivers approximately 5 mg and 10 mg of testosterone, respectively. In a study with 10 g of AndroGel, all patients showed an increase in serum testosterone within 30 minutes, and eight of nine patients had a serum testosterone concentration within normal range by 4 hours after the initial application. Absorption of testosterone into the blood continues for the entire 24-hour dosing interval. Serum concentrations approximate the steady-state level by the end of the first 24 hours and are at steady state by the second or third day of dosing.
With single daily applications of AndroGel, follow-up measurements 30, 90 and 180 days after starting treatment have confirmed that serum testosterone concentrations are generally maintained within the eugonadal range. Figure 1 summarizes the 24-hour pharmacokinetic profiles of testosterone for hypogonadal men (<300 ng/dL) maintained on 5 g or 10 g of AndroGel for 30 days. The average (± SD) daily testosterone concentration produced by AndroGel 10 g on Day 30 was 792 (± 294) ng/dL and by AndroGel 5 g 566 (± 262) ng/dL.
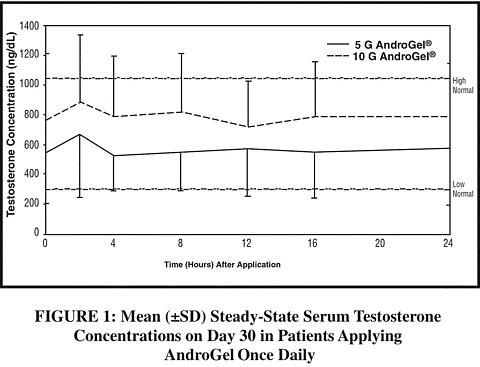
When AndroGel treatment is discontinued after achieving steady state, serum testosterone levels remain in the normal range for 24 to 48 hours but return to their pretreatment levels by the fifth day after the last application.
Distribution: Circulating testosterone is chiefly bound in the serum to sex hormone-binding globulin (SHBG) and albumin. The albumin-bound fraction of testosterone easily dissociates from albumin and is presumed to be bioactive. The portion of testosterone bound to SHBG is not considered biologically active. The amount of SHBG in the serum and the total testosterone level will determine the distribution of bioactive and nonbioactive androgen. SHBG-binding capacity is high in prepubertal children, declines during puberty and adulthood, and increases again during the later decades of life. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free) and the rest is bound to albumin and other proteins.
Metabolism: There is considerable variation in the half-life of testosterone as reported in the literature, ranging from 10 to 100 minutes. Testosterone is metabolized to various 17-keto steroids through two different pathways. The major active metabolites of testosterone are estradiol and DHT. DHT binds with greater affinity to SHBG than does testosterone. In many tissues, the activity of testosterone depends on its reduction to DHT, which binds to cytosol receptor proteins. The steroid-receptor complex is transported to the nucleus where it initiates transcription and cellular changes related to androgen action. In reproductive tissues, DHT is further metabolized to 3-(alpha) and 3-(beta) androstanediol.
DHT concentrations increased in parallel with testosterone concentrations during AndroGel treatment. After 180 days of treatment, mean DHT concentrations were within the normal range with 5 g AndroGel and were about 7% above the normal range after a 10 g dose. The mean steady-state DHT/T ratio during 180 days of AndroGel treatment remained within normal limits (as determined by the analytical laboratory involved with this clinical trial) and ranged from 0.23 to 0.29 (5 g/day) and from 0.27 to 0.33 (10 g/day).
Excretion: About 90% of a dose of testosterone given intramuscularly is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites; about 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver.
Special Populations: In patients treated with AndroGel, there are no observed differences in the average daily serum testosterone concentration at steady state based on age, cause of hypogonadism or body mass index. No formal studies were conducted involving patients with renal or hepatic insufficiencies.
Clinical Studies
AndroGel was evaluated in a multicenter, randomized, parallel-group, active-controlled, 180-day trial in 227 hypogonadal men. The study was conducted in 2 phases. During the Initial Treatment Period (Days 1-90), 73 patients were randomized to AndroGel 5 g daily, 78 patients to AndroGel 10 g daily, and 76 patients to a non-scrotal testosterone transdermal system. The study was double-blind for dose of AndroGel but open-label for active control. Patients who were originally randomized to AndroGel and who had single-sample serum testosterone levels above or below the normal range on Day 60 were titrated to 7.5 g daily on Day 91. During the Extended Treatment Period (Days 91-180), 51 patients continued on AndroGel 5 g daily, 52 patients continued on AndroGel 10 g daily, 41 patients continued on a non-scrotal testosterone transdermal system (5 mg daily), and 40 patients received AndroGel 7.5 g daily. Upon completion of the initial study, 163 enrolled and 162 patients received treatment in an open-label extension study of AndroGel for an additional period of up to 3 years.
Mean peak, trough and average serum testosterone concentrations within the normal range (298-1043 ng/dL) were achieved on the first day of treatment with doses of 5 g and 10 g. In patients continuing on AndroGel 5 g and 10 g, these mean testosterone levels were maintained within the normal range for the 180-day duration of the study. Figure 2 summarizes the 24-hour pharmacokinetic profiles of testosterone administered as AndroGel for 30, 90 and 180 days. Testosterone concentrations were maintained as long as the patient continued to properly apply the prescribed AndroGel treatment.
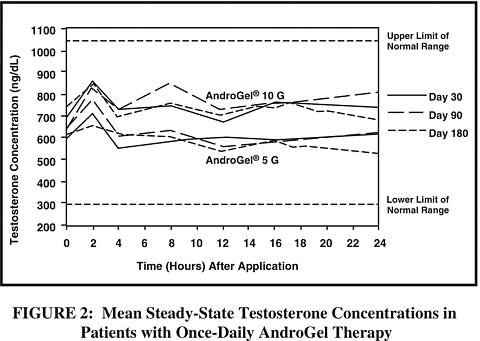
Table 1 summarizes the mean testosterone concentrations on Treatment Day 180 for patients receiving 5 g, 7.5 g, or 10 g of AndroGel. The 7.5 g dose produced mean concentrations intermediate to those produced by 5 g and 10 g of AndroGel.
TABLE 1: Mean (± SD) Steady-State Serum Testosterone
Concentrations During Therapy (Day 180)5 g 7.5 g 10 g N = 44 N = 37 N = 48 Cavg555 ± 225 601 ± 309 713 ± 209 Cmax830 ± 347 901 ± 471 1083 ± 434 Cmin371 ± 165 406 ± 220 485 ± 156
Of 129 hypogonadal men who were appropriately titrated with AndroGel and who had sufficient data for analysis, 87% achieved an average serum testosterone level within the normal range on Treatment Day 180.
AndroGel 5 g/day and 10 g/day resulted in significant increases over time in total body mass and total body lean mass, while total body fat mass and the percent body fat decreased significantly. These changes were maintained for 180 days of treatment during the original study. Changes in the 7.5 g dose group were similar. Bone mineral density in both hip and spine increased significantly from Baseline to Day 180 with 10 g AndroGel.
AndroGel treatment at 5 g/day and 10 g/day for 90 days produced significant improvement in libido (measured by sexual motivation, sexual activity and enjoyment of sexual activity as assessed by patient responses to a questionnaire). The degree of penile erection as subjectively estimated by the patients, increased with AndroGel treatment, as did the subjective score for "satisfactory duration of erection." AndroGel treatment at 5 g/day and 10 g/day produced positive effects on mood and fatigue. Similar changes were seen after 180 days of treatment and in the group treated with the 7.5 g dose. DHT concentrations increased in parallel with testosterone concentrations at AndroGel doses of 5 g/day and 10 g/day, but the DHT/T ratio stayed within the normal range, indicating enhanced availability of the major physiologically active androgen. Serum estradiol (E2) concentrations increased significantly within 30 days of starting treatment with AndroGel 5 or 10 g/day and remained elevated throughout the treatment period but remained within the normal range for eugonadal men. Serum levels of SHBG decreased very slightly (1 to 11%) during AndroGel treatment. In men with hypergonadotropic hypogonadism, serum levels of LH and FSH fell in a dose- and time-dependent manner during treatment with AndroGel.
Potential for Phototoxicity: The phototoxic potential of AndroGel was evaluated in a double-blind, single-dose study in 27 subjects with photosensitive skin types. The Minimal Erythema Dose (MED) of ultraviolet radiation was determined for each subject. A single 24 (+1) hour application of duplicate patches containing test articles (placebo gel, testosterone gel, or saline) was made to naive skin sites on Day 1. On Day 2, each subject received five exposure times of ultraviolet radiation, each exposure being 25% greater than the previous one. Skin evaluations were made on Days 2-5. Exposure of test and control article application sites to ultraviolet light did not produce increased inflammation relative to non-irradiated sites, indicating no phototoxic effect.
Potential for Testosterone Transfer: The potential for dermal testosterone transfer following AndroGel use was evaluated in a clinical study between males dosed with AndroGel and their untreated female partners. Two to 12 hours after AndroGel (10 g) application by the male subjects, the couples (N=38 couples) engaged in daily, 15-minute sessions of vigorous skin-to-skin contact so that the female partners gained maximum exposure to the AndroGel application sites. Under these study conditions, all unprotected female partners had a serum testosterone concentration > 2 times the baseline value at some time during the study. When a shirt covered the application site(s), the transfer of testosterone from the males to the female partners was completely prevented.
INDICATIONS AND USAGE
AndroGel is indicated for replacement therapy in males for conditions associated with a deficiency or absence of endogenous testosterone:
- Primary hypogonadism (congenital or acquired) - testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter's syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone levels and gonadotropins (FSH, LH) above the normal range.
- Hypogonadotropic hypogonadism (congenital or acquired) - idiopathic gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum levels but have gonadotropins in the normal or low range.
AndroGel has not been clinically evaluated in males under 18 years of age.
CONTRAINDICATIONS
Androgens are contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate.
AndroGel is not indicated for use in women, has not been evaluated in women, and must not be used in women.
Pregnant women should avoid skin contact with AndroGel application sites in men. Testosterone may cause fetal harm. In the event that unwashed or unclothed skin to which AndroGel has been applied does come in direct contact with the skin of a pregnant woman, the general area of contact on the woman should be washed with soap and water as soon as possible. In vitro studies show that residual testosterone is removed from the skin surface by washing with soap and water.
AndroGel should not be used in patients with known hypersensitivity to any of its ingredients, including testoste-rone USP that is chemically synthesized from soy.
WARNINGS
- Prolonged use of high doses of orally active 17-alpha-alkyl androgens (e.g., methyltestosterone) has been associated with serious hepatic adverse effects (peliosis hepatis, hepatic neoplasms, cholestatic hepatitis, and jaundice). Peliosis hepatis can be a life-threatening or fatal complication. Long-term therapy with testosterone enanthate, which elevates blood levels for prolonged periods, has produced multiple hepatic adenomas. AndroGel is not known to produce these adverse effects.
- Geriatric patients treated with androgens may be at an increased risk for the development of prostatic hyperplasia and prostatic carcinoma.
- Geriatric patients and other patients with clinical or demographic characteristics that are recognized to be associated with an increased risk of prostate cancer should be evaluated for the presence of prostate cancer prior to initiation of testosterone replacement therapy. In men receiving testosterone replacement therapy, surveillance for prostate cancer should be consistent with current practices for eugonadal men. Increases in serum PSA from baseline values were seen in approximately 18% of individuals in an open label study of 162 hypogonadal men treated with AndroGel for up to 42 months. Most of these increases were seen within the first year of therapy. (see ADVERSE REACTIONS and PRECAUTIONS : Carcinogenesis, Mutagenesis, Impairment of Fertility and Laboratory Tests ).
- Edema with or without congestive heart failure may be a serious complication in patients with preexisting cardiac, renal, or hepatic disease. In addition to discontinuation of the drug, diuretic therapy may be required.
- Gynecomastia frequently develops and occasionally persists in patients being treated for hypogonadism.
- The treatment of hypogonadal men with testosterone esters may potentiate sleep apnea in some patients, especially those with risk factors such as obesity or chronic lung diseases.
- ALCOHOL BASED GELS ARE FLAMMABLE. AVOID FIRE, FLAME OR SMOKING UNTIL THE GEL HAS DRIED.
PRECAUTIONS
Transfer of testosterone to another person can occur when vigorous skin-to-skin contact is made with the application site (see Clinical Studies ). The following precautions are recommended to minimize potential transfer of testosterone from AndroGel-treated skin to another person:
- Patients should wash their hands immediately with soap and water after application of AndroGel.
- Patients should cover the application site(s) with clothing after the gel has dried (e.g. a shirt).
- In the event that unwashed or unclothed skin to which AndroGel has been applied does come in direct contact with the skin of another person, the general area of contact on the other person should be washed with soap and water as soon as possible. In vitro studies show that residual testosterone is removed from the skin surface by washing with soap and water.
Changes in body hair distribution, significant increase in acne, or other signs of virilization of the female partner should be brought to the attention of a physician.
General
The physician should instruct patients to report any of the following:
- Too frequent or persistent erections of the penis.
- Any nausea, vomiting, changes in skin color, or ankle swelling.
- Breathing disturbances, including those associated with sleep.
Information for Patients
Advise patients to carefully read the information brochure that accompanies each carton of 30 AndroGel single-use packets or 75 g AndroGel Pump.
Advise patients of the following:
- AndroGel should not be applied to the scrotum.
- AndroGel should be applied once daily to clean dry skin.
- After application of AndroGel, it is currently unknown for how long showering or swimming should be delayed. For optimal absorption of testosterone, it appears reasonable to wait at least 5-6 hours after application prior to showering or swimming. Nevertheless, showering or swimming after just 1 hour should have a minimal effect on the amount of AndroGel absorbed if done very infrequently.
- SINCE ALCOHOL BASED GELS ARE FLAMMABLE, AVOID FIRE, FLAME OR SMOKING UNTIL THE GEL HAS DRIED.
Laboratory Tests
- Hemoglobin and hematocrit levels should be checked periodically (to detect polycythemia) in patients on long-term androgen therapy.
- Liver function, prostatic specific antigen, cholesterol, and high-density lipoprotein should be checked periodically.
- To ensure proper dosing, serum testosterone concentrations should be measured (see DOSAGE AND ADMINISTRATION ).
Drug Interactions
Oxyphenbutazone: Concurrent administration of oxyphenbutazone and androgens may result in elevated serum levels of oxyphenbutazone.
Insulin: In diabetic patients, the metabolic effects of androgens may decrease blood glucose and, therefore, insulin requirements.
Propranolol: In a published pharmacokinetic study of an injectable testosterone product, administration of testoste-rone cypionate led to an increased clearance of propranolol in the majority of men tested.
Corticosteroids: The concurrent administration of testosterone with ACTH or corticosteroids may enhance edema formation; thus, these drugs should be administered cautiously, particularly in patients with cardiac or hepatic disease.
Drug/Laboratory Test Interactions
Androgens may decrease levels of thyroxin-binding globulin, resulting in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal Data: Testosterone has been tested by subcutaneous injection and implantation in mice and rats. In mice, the implant induced cervical-uterine tumors, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
Human Data: There are rare reports of hepatocellular carcinoma in patients receiving long-term oral therapy with androgens in high doses. Withdrawal of the drugs did not lead to regression of the tumors in all cases.
Geriatric patients treated with androgens may be at an increased risk for the development of prostatic hyperplasia and prostatic carcinoma.
Geriatric patients and other patients with clinical or demographic characteristics that are recognized to be associated with an increased risk of prostate cancer should be evaluated for the presence of prostate cancer prior to initiation of testosterone replacement therapy.
In men receiving testosterone replacement therapy, screening for prostate cancer should be consistent with current practices for eugonadal men. Increases in serum PSA from baseline values were reported in approximately 18% of individual patients treated for up to 42 months in an open-label safety study (see ADVERSE REACTIONS ).
Pregnancy Category X (see CONTRAINDICATIONS ) - Teratogenic Effects: AndroGel is not indicated for women and must not be used in women.
Nursing Mothers: AndroGel is not indicated for women and must not be used in women.
Pediatric Use: Safety and efficacy of AndroGel in pediatric patients have not been established.
ADVERSE REACTIONS
In a controlled clinical study, 154 patients were treated with AndroGel for up to 6 months (see Clinical Studies ). Adverse Events possibly, probably or definitely related to the use of AndroGel and reported by >/=1% of the patients are listed in Table 2.
TABLE 2: Adverse Events Possibly, Probably or
Definitely Related to Use of AndroGel
in the 180-Day Controlled Clinical TrialAdverse EventDose of AndroGel 5 g
n = 777.5 g
n = 4010 g
n = 78Acne1% 3% 8% Alopecia1% 0% 1% Application Site Reaction5% 3% 4% Asthenia0% 3% 1% Depression1% 0% 1% Emotional Lability0% 3% 3% Gynecomastia1% 0% 3% Headache4% 3% 0% Hypertension3% 0% 3% Lab Test Abnormal *6% 5% 3% Libido Decreased0% 3% 1% Nervousness0% 3% 1% Pain Breast1% 3% 1% Prostate Disorder **3% 3% 5% Testis Disorder ***3% 0% 0% * Lab test abnormal occurred in nine patients with one or more of the following events: elevated hemoglobin or hematocrit, hyperlipidemia, elevated triglycerides, hypokalemia, decreased HDL, elevated glucose, elevated creatinine, or elevated total bilirubin.** Prostate disorders included five patients with enlarged prostate, one patient with BPH, and one patient with elevated PSA results.*** Testis disorders were reported from two patients: one patient with left varicocele and one patient with slight sensitivity of left testis.
The following adverse events possibly related to the use of AndroGel occurred in fewer than 1% of patients: amnesia, anxiety, discolored hair, dizziness, dry skin, hirsutism, hostility, impaired urination, paresthesia, penis disorder, peripheral edema, sweating, and vasodilation.
In this clinical trial of AndroGel, skin reactions at the site of application were occasionally reported with AndroGel, but none was severe enough to require treatment or discontinuation of drug.
Six (4%) patients in this trial had adverse events that led to discontinuation of AndroGel. These events included the following: cerebral hemorrhage, convulsion (neither of which were considered related to AndroGel administration), depression, sadness, memory loss, elevated prostate specific antigen and hypertension. No AndroGel patients discontinued due to skin reactions.
In an uncontrolled pharmacokinetic study of 10 patients, two had adverse events associated with AndroGel; these were asthenia and depression in one patient and increased libido and hyperkinesia in the other. Among 17 patients in foreign clinical studies there was one instance each of acne, erythema and benign prostate adenoma associated with a 2.5% testosterone gel formulation applied dermally.
One hundred sixty-two (162) patients received AndroGel for up to 3 years in a long-term follow-up study for patients who completed the controlled clinical trial. Table 3 summarizes those adverse events possibly, probably or definitely related to the use of AndroGel and reported by 2 or more subjects in at least one treatment group.
TABLE 3: Incidence of Treatment-Emergent Adverse Events Possibly, Probably or Definitely Related to the Use of AndroGel
in the 3 Year Open-Label Extension Clinical TrialAdverse Event
Category/ClassificationTreatment Group
% (N = 162)Lab Test Abnormal **/*9.3% (15) Skin dry1.9% (3) Application Site Reaction5.6% (9) Acne3.1% (5) Pruritus1.9% (3) Enlarged Prostate11.7% (19) Carcinoma of Prostate1.2% (2) Urinary Symptoms *3.7% (6) Testis Disorder **1.9% (3) Gynecomastia2.5% (4) Anemia2.5% (4) **/* Lab test abnormal occurred in fifteen patients with one or more of the following events: elevated AST, elevated ALT, elevated testosterone, elevated hemoglobin or hematocrit, elevated cholesterol, elevated cholesterol/LDL ratio, elevated triglycerides, elevated HDL, or elevated serum creatinine.* Urinary symptoms included nocturia, urinary hesitancy, urinary incontinence, urinary retention, urinary urgency and weak urinary stream.** Testis disorder included three patients. There were two patients with a non-palpable testis and one patient with slight right testicular tenderness.
Two patients reported serious adverse events considered possibly related to treatment: deep vein thrombosis (DVT) and prostate disorder requiring a transurethral resection of the prostate (TURP). Nine patients discontinued treatment due to adverse events possibly related to treatment with AndroGel, including two patients with application site reactions, one with kidney failure, and five with prostate disorders (including increase in serum PSA in 4 patients, and increase in PSA with prostate enlargement in a fifth patient). All patients who discontinued due to an increase in serum PSA did so by Day 357.
Increases in Serum PSA
During the initial 6-month study, the mean change in PSA values had a statistically significant increase of 0.26 ng/mL. Serum PSA was measured every 6 months thereafter. While there was no statistically significant increase in mean PSA from 6 months through 36 months of AndroGel treatment for the overall group of 162 patients enrolled in the long-term extension study, there were increases in serum PSA seen in approximately 18% of individual patients. In the long-term extension study, the overall mean change from baseline in serum PSA values for the entire group was 0.11 ng/mL.
Twenty-nine (29) (18%) patients met the per-protocol criterion for increase in serum PSA value, defined as a value >/=2X the baseline value or any single absolute value >/=6 ng/mL. Twenty-five of these patients met this criterion by virtue of a post-baseline value at least twice the baseline value. In most of these cases (22/25), the maximum serum PSA value attained was </=2 ng/mL. The first occurrence of a pre-specified, post-baseline increase in serum PSA was seen at or prior to Month 12 in most of the patients who met this criterion (23 of 29; 79%). Four patients met this criterion by having a serum PSA >/=6 ng/mL and in these, maximum serum PSA values were 6.2 ng/mL, 6.6 ng/mL, 6.7 ng/mL, and 10.7 ng/mL (in AndroGel-treated patients). In two of these AndroGel-treated patients, prostate cancer was detected on biopsy. The first patient's PSA levels were 4.7 ng/mL and 6.2 ng/mL at baseline and at Month 6/Final, respectively. The second patient's PSA levels were 4.2 ng/mL, 5.2 ng/mL, 5.8 ng/mL, and 6.6 ng/mL at baseline, Month 6, Month 12, and Final, respectively.
DRUG ABUSE AND DEPENDENCE
AndroGel contains testosterone, a Schedule III controlled substance as defined by the Anabolic Steroids Control Act.
Oral ingestion of AndroGel will not result in clinically significant serum testosterone concentrations due to extensive first-pass metabolism.
OVERDOSAGE
No reports of AndroGel overdose have been received. However, there is one report of acute overdosage by injection of testosterone enanthate: testosterone levels of up to 11,400 ng/dL were implicated in a cerebrovascular accident.
DOSAGE AND ADMINISTRATION
The recommended starting dose of AndroGel is 5 g delivering 5 mg of testosterone systemically, applied once daily (preferably in the morning) to clean, dry, intact skin of the shoulders and upper arms and/or abdomen. Serum testosterone levels should be measured approximately 14 days after initiation of therapy to ensure proper dosing. If the serum testosterone concentration is below the normal range, or if the desired clinical response is not achieved, the daily AndroGel dose may be increased from 5g to 7.5 g and from 7.5 g to 10 g as instructed by the physician.
AndroGel is available in either unit-dose packets or multiple-dose pumps. The metered-dose pump delivers 1.25 g of product when the pump mechanism is fully depressed once.
AndroGel must not be applied to the genitals.
If using the multi-dose AndroGel Pump, patients should be instructed to prime the pump before using it for the first time by fully depressing the pump mechanism (actuation) 3 times and discard this portion of the product to assure precise dose delivery. After the priming procedure, patients should completely depress the pump one time (actuation) for every 1.25 g of product required to achieve the daily prescribed dosage. The product may be delivered directly into the palm of the hand and then applied to the desired application sites, either one pump actuation at a time or upon completion of all pump actuations required for the daily dose. Alternatively, the product can be applied directly to the application sites. Application directly to the sites may prevent loss of product that may occur during transfer from the palm of the hand onto the application sites. Please refer to the chart below for specific dosing guidelines when the AndroGel Pump is used.
Prescribed
Daily DoseNumber of
Pump Actuations5 g 4 (once daily) 7.5 g 6 (once daily) 10 g 8 (once daily)
If using the packets, the entire contents should be squeezed into the palm of the hand and immediately applied to the application sites. Alternately, patients may squeeze a portion of the gel from the packet into the palm of the hand and apply to application sites. Repeat until entire contents have been applied.
Application sites should be allowed to dry for a few minutes prior to dressing. Hands should be washed with soap and water after AndroGel has been applied.
HOW SUPPLIED
AndroGel contains testosterone, a Schedule III controlled substance as defined by the Anabolic Steroids Control Act.
AndroGel is supplied in non-aerosol, metered-dose pumps. The pump is composed of plastic and stainless steel and an LDPE/aluminum foil inner liner encased in rigid plastic with a polypropylene cap. Each individual packaged AndroGel Pump is capable of dispensing 75 g or 60 metered 1.25 g doses.
AndroGel is also supplied in unit-dose aluminum foil packets in cartons of 30. Each packet of 2.5 g or 5 g gel contains 25 mg or 50 mg testosterone, respectively.
NDC Number Package Size
0051-8488-88 2 × 75 g pumps (each pump dispenses 60 metered 1.25 g doses)
0051-8425-3030 packets (2.5 g per packet)
0051-8450-3030 packets (5 g per packet)
Keep AndroGel out of the reach of children.
Storage
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Disposal
Used AndroGel pumps or used AndroGel packets should be discarded in household trash in a manner that prevents accidental application or ingestion by children or pets. In addition, any discarded gel should be thoroughly rinsed down the sink or discarded in the household trash in a manner that prevents accidental application or ingestion by children or pets.
Manufactured by:
Laboratoires Besins International
Montrouge, France
For:
Unimed Pharmaceuticals, Inc.
A Solvay Pharmaceuticals, Inc. Company
Marietta, GA 30062-2224, USA
500122/500127
Rev Aug 2005
U.S. Patent No. 6,503,894
© 2005 Solvay Pharmaceuticals, Inc.
Patient Information and Instructions for Using
AndroGel® CIII
(testosterone gel) 1%
Read this information carefully before using AndroGel® [AN drow jel]. The following information about AndroGel® should not take the place of your doctor's orders or recommendations. Your doctor will tell you exactly what dose to take, how to safely take it, and when to take it. Make sure you understand the benefits and risks of AndroGel® before you use it. If you have any other questions about your AndroGel® therapy, ask your doctor or pharmacist.
What is AndroGel®?
AndroGel® is a clear, colorless gel medicine that delivers testosterone into your body through your skin. Once AndroGel® is absorbed through your skin, it enters your bloodstream and helps your body reach normal testosterone levels. The type of testosterone delivered by AndroGel® is the same as the testosterone produced in your body.
Your doctor has prescribed this therapy because your body is not making enough testosterone. The medical term for this condition is hypogonadism. Testosterone helps the body produce sperm and the male sexual characteristics. Testosterone is also necessary for normal sexual function and sex drive.
Who should not take AndroGel®?
AndroGel® must not be used by women or by those individuals with known hypersensitivity to any of its components, including individuals who are hypersensitive to testosterone that is chemically synthesized from soy. Pregnant women should avoid skin contact with AndroGel® application sites in men. The active ingredient in AndroGel® is testosterone. (See "Inactive Ingredients" at the end of this leaflet for a list of the other ingredients.) Testosterone may cause fetal harm.
You should not use AndroGel® if you have any of the following conditions:
- prostate cancer (if your doctor knows for sure or suspects it)
- breast cancer (a rare condition for men)
How should I use the AndroGel® Pump?
It is important that you read and follow these directions on how to use the AndroGel® Pump properly.
- Apply AndroGel® at the same time each day (preferably every morning). You should apply your daily dose of gel every morning to clean, dry, intact skin. If you take a bath or shower in the morning, use AndroGel® after your bath or shower. Your doctor will tell you how much AndroGel® to use each day.
- Be sure your skin is completely dry.
- Before using the pump for the first time, you must prime the AndroGel® pump by fully depressing the pump three times and discarding the gel. The unused gel should be discarded by thoroughly rinsing down the sink or discarding in the household trash in a manner to avoid accidental exposure or ingestion by household members or pets.
-
Each full pump depression delivers 1.25 g of AndroGel®. Please refer to the chart below to determine the number of full pump depressions required for the daily dose prescribed by your doctor:
Prescribed
Daily DoseNumber of
Pump Depressions5 g 4 (once daily) 7.5 g 6 (once daily) 10 g 8 (once daily)
-
Fully depress the pump the appropriate number of times to deliver the daily dose prescribed by your doctor. The product may be delivered directly into the palm of your hand and then applied to the desired application sites, either one pump depression at a time or upon completion of all pump depressions required for the daily dose.
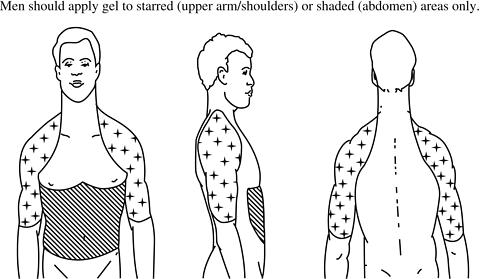
- Apply AndroGel® only to healthy, normal skin on your abdomen (stomach area), shoulders, or upper arms. In this way your body will absorb the right amount of testosterone. Never apply AndroGel® to your genitals (penis or scrotum) or to skin with open sores, wounds, or irritation.
- Wash your hands with soap and water right away after application to reduce the chance that the medicine will spread from your hands to other people.
- Let AndroGel® dry for a few minutes before you dress. This prevents your clothing from wiping the gel off your skin. It ensures that your body will absorb the correct amount of testosterone.
- Allow gel to dry completely before smoking or going near an open flame.
- Wait 5 to 6 hours before showering or swimming. To ensure that the greatest amount of AndroGel® is absorbed into your system, you should wait 5 to 6 hours after application before showering or swimming. Once in a while, you may shower or swim as soon as 1 hour after applying AndroGel®. If done infrequently, this will have little effect on the amount of AndroGel® that is absorbed by your body.
- Maintain normal activities. Once your hands are washed and the application site is covered with clothing, there is little risk of transferring testosterone to someone else's skin due to bodily contact. If, however, you expect direct skin contact with someone else, you should wash your application site(s) with soap and water before that encounter. This will reduce the chance that the medicine will transfer to the other person.
-
The AndroGel® pump contains enough product to allow for priming and a set number of precise doses. Please refer to the chart below to determine the number of days of treatment each pump will provide based on your individual dose. Discard pump afterwards.
Prescribed
Daily DoseNumber of Days of
Treatment per
Pump (after priming)88 g Pump 5 g 15 7.5 g 10 10 g 7.5
How should I use AndroGel® packets?
It is important that you read and follow these directions on how to use AndroGel® properly.
- Apply AndroGel® at the same time each day (preferably every morning). You should apply your daily dose of gel every morning to clean, dry, intact skin. If you take a bath or shower in the morning, use AndroGel® after your bath or shower. Your doctor will tell you how much AndroGel® to use each day.
- Be sure your skin is completely dry.
- Open the packet. Open one AndroGel® aluminum foil packet by folding the top edge at the perforation and tearing completely across the packet along the perforation.
-
Remove the contents from the packet. Squeeze the contents into the palm of your hand.
Squeeze from the bottom of the packet toward the top. If you like, you may squeeze a portion of the gel from the packet into the palm of your hand and apply to application site(s).
Repeat until the entire contents of the packet have been applied.
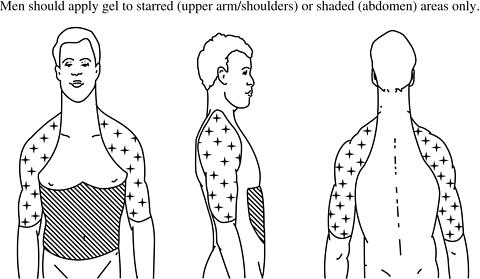
- Apply AndroGel® only to healthy, normal skin on your abdomen (stomach area), shoulders, or upper arms. In this way your body will absorb the right amount of testosterone. Never apply AndroGel® to your genitals (penis or scrotum) or to skin with open sores, wounds, or irritation.
- Wash your hands with soap and water right away after application to reduce the chance that the medicine will spread from your hands to other people.
- Let AndroGel® dry for a few minutes before you dress. This prevents your clothing from wiping the gel off your skin. It ensures that your body will absorb the correct amount of testosterone.
- Allow gel to dry completely before smoking or going near an open flame.
- Wait 5 to 6 hours before showering or swimming. To ensure that the greatest amount of AndroGel® is absorbed into your system, you should wait 5 to 6 hours after application before showering or swimming. Once in a while, you may shower or swim as soon as 1 hour after applying AndroGel®. If done infrequently, this will have little effect on the amount of AndroGel® that is absorbed by your body.
- Maintain normal activities. Once your hands are washed and the application site is covered with clothing, there is little risk of transferring testosterone to someone else's skin due to bodily contact. If, however, you expect direct skin contact with someone else, you should wash your application site(s) with soap and water before that encounter. This will reduce the chance that the medicine will transfer to the other person.
What to do if someone else is exposed to AndroGel®.
If someone else is exposed to AndroGel® either by direct contact with the gel itself or indirectly because of contact with your treated skin, that person should wash the area of contact with soap and water as soon as possible. The longer the gel is in contact with the skin before washing, the greater is the chance that some testosterone will be absorbed by the other person. This is especially important for women (especially pregnant women) and children. They have naturally low levels of testosterone and could be harmed by it.
What to do if you get AndroGel® in your eyes.
If you get AndroGel® in your eyes, rinse your eyes right away with warm clean water to flush out any AndroGel®. Seek medical attention if needed.
What to do if you miss a dose.
If you miss a dose, do not double your next dose the next day to catch up. If your next dose is less than 12 hours away, it is best just to wait. Do not take the skipped dose. If it is more than 12 hours until your next dose, take the dose you missed. Resume your normal dosing the next day.
What should I avoid while using AndroGel®?
It is important that you do not spread the medicine to others, especially women and children. Be sure to wash your hands after applying AndroGel®. Do not allow other persons to contact your skin where you have applied AndroGel®, especially pregnant or nursing women. Testosterone may harm the developing baby. ALCOHOL BASED GELS ARE FLAMMABLE. AVOID FIRE, FLAME OR SMOKING UNTIL THE GEL HAS DRIED.
What are the possible side effects of AndroGel®?
AndroGel® may cause the following side effects:
- breast development and breast discomfort
- extra fluid in the body. This may cause serious problems for patients with heart, kidney, or liver damage.
- sleep disturbance called "sleep apnea." This is more likely in patients who are overweight or who have lung disease.
- prostate enlargement, sometimes accompanied by difficulty urinating
- emotional problems like depression
- changes in blood levels of cholesterol. This may be monitored and prevented by periodic blood tests.
Tell your doctor if you develop any of the following side effects:
- penis erections that are too frequent or continue too long
- nausea, vomiting, yellow or darker skin (jaundice), or ankle swelling
- breathing problems, including problems breathing while sleeping
- difficulty urinating
- any side effect that concerns you
Tell your doctor about other medicines you are taking. AndroGel® may affect how these medicines work, and you may need to have your doses adjusted.
Tell your doctor if your female partner develops changes in hair distribution, increases in acne, or other signs of masculinity.
Older patients may be at increased risk of developing enlarged prostate or prostate cancer. This also may be monitored by periodic blood tests and prostate exams.
Disposal
Used AndroGel® pumps or used AndroGel® packets should be discarded in household trash in a manner that prevents accidental application or ingestion by children or pets. In addition, any discarded gel should be thoroughly rinsed down the sink or discarded in the household trash in a manner that prevents accidental application or ingestion by children or pets.
Other Information
Never share your AndroGel® with anyone. Every patient is different. Your doctor has prescribed AndroGel® specifically for your needs. Use AndroGel® only for the condition for which it was prescribed. Medicines are sometimes prescribed for purposes other than those described in a patient information leaflet. If you have any questions or concerns about your AndroGel® treatment, ask your health care provider or pharmacist. They can answer your questions and give you the printed information about AndroGel® that is written for health professionals.
Keep AndroGel® out of the reach of children.
Inactive Ingredients
Ethanol, purified water, sodium hydroxide, carbomer 980 and isopropyl myristate.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Manufactured by:
Laboratoires Besins International
Montrouge, France
For:
Unimed Pharmaceuticals, Inc.
A Solvay Pharmaceuticals, Inc. Company
Marietta, GA 30062-2224, USA
500100/500121
3E Rev 3/2004
© 2004 Solvay Pharmaceuticals, Inc
Subscribe to the "News" RSS Feed 
TOP ۞
