-
Aptivus Capsules (Boehringer Ingelheim)
WARNING
APTIVUS CO-ADMINISTERED WITH 200 MG RITONAVIR HAS BEEN ASSOCIATED WITH REPORTS OF CLINICAL HEPATITIS AND HEPATIC DECOMPENSATION INCLUDING SOME FATALITIES. EXTRA VIGILANCE IS WARRANTED IN PATIENTS WITH CHRONIC HEPATITIS B OR HEPATITIS C CO-INFECTION, AS THESE PATIENTS HAVE AN INCREASED RISK OF HEPATOTOXICITY. SEE WARNINGS. DESCRIPTION
APTIVUS® (tipranavir) is the brand name for tipranavir (TPV), a non-peptidic protease inhibitor (PI) of HIV belonging to the class of 4-hydroxy-5,6-dihydro-2-pyrone sulfonamides.
APTIVUS soft gelatin capsules are for oral administration. Each capsule contains 250 mg tipranavir. The major inactive ingredients in the capsule are dehydrated alcohol (7% w/w or 0.1 g per capsule), polyoxyl 35 castor oil, propylene glycol, mono/diglycerides of caprylic/capric acid and gelatin.
The chemical name of tipranavir is 2-Pyridinesulfonamide, N-[3-[(1R)-1-[(6R)-5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2H-pyran-3-yl]propyl]phenyl]-5-(trifluoro-methyl). It has a molecular formula of C 31 H 33 F 3 N 2 O 5 S and a molecular weight of 602.7. Tipranavir has the following structural formula and is a single stereoisomer with the 1R, 6R configuration.
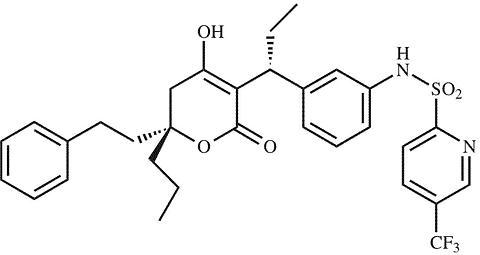
Tipranavir is a white to off-white to slightly yellow solid. It is freely soluble in dehydrated alcohol and propylene glycol, and insoluble in aqueous buffer at pH 7.5.
CLINICAL PHARMACOLOGY
Microbiology
Mechanism of Action
Tipranavir (TPV) is a non-peptidic HIV-1 protease inhibitor that inhibits the virus-specific processing of the viral Gag and Gag-Pol polyproteins in HIV-1 infected cells, thus preventing formation of mature virions.
Antiviral Activity
Tipranavir inhibits the replication of laboratory strains of HIV-1 and clinical isolates in acute models of T-cell infection, with 50% effective concentrations (EC 50 ) ranging from 0.03 to 0.07 µM (18-42 ng/mL). Tipranavir demonstrates antiviral activity in vitro against a broad panel of HIV-1 group M non-clade B isolates (A, C, D, F, G, H, CRF01 AE, CRF02 AG, CRF12 BF). Group O and HIV-2 isolates have reduced susceptibility in vitro to tipranavir with EC 50 values ranging from 0.164 -1 µM and 0.233-0.522 µM, respectively. Protein binding studies have shown that the antiviral activity of tipranavir decreases on average 3.75-fold in conditions where human serum is present. When used with other antiretroviral agents in vitro , the combination of tipranavir was additive to antagonistic with other protease inhibitors (amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, and saquinavir) and generally additive with the NNRTIs (delavirdine, efavirenz, and nevirapine) and the NRTIs (abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, and zidovudine). Tipranavir was synergistic with the HIV fusion inhibitor enfuvirtide. There was no antagonism of the in vitro combinations of tipranavir with either adefovir or ribavirin, used in the treatment of viral hepatitis.
Resistance
In vitro: HIV-1 isolates with a decreased susceptibility to tipranavir have been selected in vitro and obtained from patients treated with APTIVUS/ritonavir (TPV/ritonavir). HIV-1 isolates that were 87-fold resistant to tipranavir were selected in vitro by 9 months and contained 10 protease mutations that developed in the following order: L33F, I84V, K45I, I13V, V32I, V82L, M36I, A71V, L10F, and I54V/T. Changes in the Gag polyprotein CA/P2 cleavage site were also observed following drug selection. Experiments with site-directed mutants of HIV-1 showed that the presence of 6 mutations in the protease coding sequence (I13V, V32I, L33F, K45I, V82L, I84V) conferred > 10-fold reduced susceptibility to tipranavir. Recombinant viruses showing >/= 3-fold reduced susceptibility to tipranavir were growth impaired.
Clinical Studies of Treatment-Experienced Patients: In Phase 3 studies 1182.12 and 1182.48, multiple protease inhibitor-resistant HIV-1 isolates from 59 highly treatment-experienced patients who received APTIVUS/ritonavir and experienced virologic rebound developed amino acid substitutions that were associated with resistance to tipranavir. The most common amino acid substitutions that developed on 500/200 mg APTIVUS/ritonavir in greater than 20% of APTIVUS/ritonavir virologic failure isolates were L33V/I/F, V82T, and I84V. Other substitutions that developed in 10 to 20% of APTIVUS/ritonavir virologic failure isolates included L10V/I/S, I13V, E35D/G/N, I47V, K55R, V82L, and L89V/M. Tipranavir resistance was detected at virologic rebound after an average of 38 weeks of APTIVUS/ritonavir treatment with a median 14-fold decrease in tipranavir susceptibility. The resistance profile in treatment-na[iuml ]ve subjects has not been characterized.
Cross-resistance
Cross-resistance among protease inhibitors has been observed. Tipranavir had < 4-fold decreased susceptibility against 90% (94/105) of HIV-1 isolates resistant to amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, or saquinavir. Tipranavir-resistant viruses which emerged in vitro had decreased susceptibility to the protease inhibitors amprenavir, atazanavir, indinavir, lopinavir, nelfinavir and ritonavir but remained sensitive to saquinavir.
Baseline Genotype and Virologic Outcome Analyses
Genotypic and/or phenotypic analysis of baseline virus may aid in determining tipranavir susceptibility before initiation of APTIVUS/ritonavir therapy. Several analyses were conducted to evaluate the impact of specific mutations and mutational patterns on virologic outcome. Both the type and number of baseline protease inhibitor mutations as well as use of additional active agents (e.g., enfuvirtide) affected APTIVUS/ritonavir response rates in Phase 3 studies 1182.12 and 1182.48 through Week 24 of treatment.
Regression analyses of baseline and/or on-treatment HIV-1 genotypes from 860 highly treatment-experienced patients in Phase 2 and 3 studies demonstrated that mutations at 16 amino acid codons in the HIV protease coding sequence were associated with reduced virologic responses at 24 weeks and/or reduced tipranavir susceptibility: L10V, I13V, K20M/R/V, L33F, E35G, M36I, K43T, M46L, I47V, I54A/M/V, Q58E, H69K, T74P, V82L/T, N83D or I84V.
Analyses were also conducted to assess virologic outcome by the number of primary protease inhibitor mutations present at baseline. Response rates were reduced if five or more protease inhibitor-associated mutations were present at baseline and subjects did not receive concomitant enfuvirtide with APTIVUS/ritonavir. See Table 1.
Table 1 Phase 3 Studies 1182.12 and 1182.48: Proportion of Responders
(confirmed >/= 1 log 10 decrease at Week 24)
by Number of Baseline Primary Protease Inhibitor (PI) MutationsNumber of Baseline
Primary PI Mutations aAPTIVUS/ritonavir
N = 513Comparator PI/ritonavir
N = 502No Enfuvirtide + Enfuvirtide No Enfuvirtide + Enfuvirtide Overall 40%
(147/368)64%
(93/145)19%
(75/390)30%
(34/112)1 - 2 68%
(26/38)75%
(3/4)41%
(17/41)100%
(2/2)3 - 4 44%
(78/176)64%
(39/61)23%
(39/170)40%
(21/52)5+ 28%
(43/151)64%
(51/80)11%
(19/178)19%
(11/57)a Primary PI mutations include any amino acid change at positions 30, 32, 36, 46, 47, 48, 50, 53, 54, 82, 84, 88 and 90
The median change from baseline in HIV-1 RNA at weeks 2, 4, 8, 16 and 24 was evaluated by the number of baseline primary protease inhibitor mutations (1-4 or >/= 5) in subjects who received APTIVUS/ritonavir with or without enfuvirtide. The following observations were made:
- Approximately 1.5 log 10 decrease in HIV-1 RNA at early time points (Week 2) regardless of the number of baseline primary protease inhibitor mutations (1-4 or 5+).
- Subjects with 5 or more primary protease inhibitor mutations in their HIV-1 at baseline who received APTIVUS/ritonavir without enfuvirtide (n=204) began to lose their antiviral response after Week 4.
- Early HIV-1 RNA decreases (1.5-2 log 10 ) were sustained through Week 24 in subjects with 5 or more primary protease inhibitor mutations at baseline who received enfuvirtide with APTIVUS/ritonavir (n=88).
Conclusions regarding the relevance of particular mutations or mutational patterns are subject to change pending additional data.
Baseline Phenotype and Virologic Outcome Analyses
APTIVUS/ritonavir response rates were also assessed by baseline tipranavir phenotype. Relationships between baseline phenotypic susceptibility to tipranavir, mutations at protease amino acid codons 33, 82, 84 and 90, tipranavir resistance-associated mutations, and response to APTIVUS/ritonavir therapy at Week 24 are summarized in Table 2. These baseline phenotype groups are not meant to represent clinical susceptibility breakpoints for APTIVUS/ritonavir because the data are based on the select 1182.12 and 1182.48 patient population. The data are provided to give clinicians information on the likelihood of virologic success based on pre-treatment susceptibility to APTIVUS/ritonavir in highly protease inhibitor-experienced patients.
Table 2 Response by Baseline Tipranavir Phenotype in the 1182.12 and 1182.48 Trials Baseline
Tipranavir Phenotype
(Fold Change) aProportion of
Responders b with
No Enfuvirtide
UseProportion of
Responders b with
Enfuvirtide
Use# of Baseline
Protease
Mutations at
33, 82, 84, 90# of Baseline
Tipranavir Resistance-
Associated
Mutations cTipranavir
Susceptibility0-3 45% (74/163) 77% (46/60) 0-2 0-4 Susceptible> 3-10 21% (10/47) 43% (12/28) 3 5-7 Decreased
Susceptibility> 10 0% (0/8) 57% (4/7) 4 8+ Resistanta Change in tipranavir IC 50 value from wild-type reference b Confirmed >/= 1 log 10 decrease at Week 24 c Number of amino acid substitutions in HIV protease among L10V, I13V, K20M/R/V, L33F, E35G, M36I, K43T, M46L, I47V, I54A/M/V, Q58E, H69K, T74P, V82L/T, N83D or I84V Pharmacodynamics
The median Inhibitory Quotient (IQ) determined from 301 highly treatment-experienced patients was about 75 (inter-quartile range: 29-189), from pivotal clinical trials 1182.12 and 1182.48. The IQ is defined as the tipranavir trough concentration divided by the viral IC 50 value, corrected for protein binding. There was a relationship between the proportion of patients with a >/= 1 log 10 reduction of viral load from baseline at week 24 and their IQ value. Among the 206 patients receiving APTIVUS/ritonavir without enfuvirtide, the response rate was 23% in those with an IQ value < 75 and 55% in those with an IQ value >/= 75. Among the 95 patients receiving APTIVUS/ritonavir with enfuvirtide, the response rates in patients with an IQ value < 75 versus those with an IQ value >/= 75 were 43% and 84%, respectively. These IQ groups are derived from a select population and are not meant to represent clinical breakpoints.
Pharmacokinetics in Adult Patients
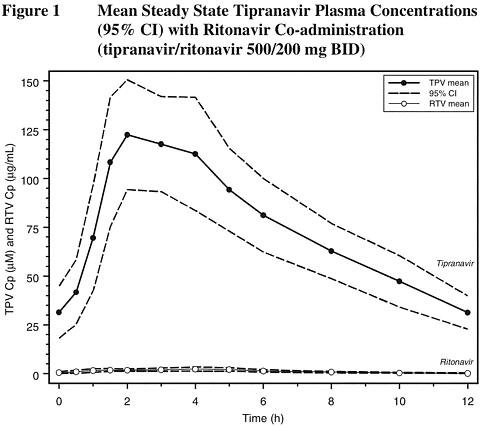
In order to achieve effective tipranavir plasma concentrations and a twice-daily dosing regimen, co-administration of APTIVUS with 200 mg of ritonavir is essential (see PRECAUTIONS and DOSAGE AND ADMINISTRATION ). Ritonavir inhibits hepatic cytochrome P450 3A (CYP 3A), the intestinal P-glycoprotein (P-gp) efflux pump and possibly intestinal CYP 3A. In a dose-ranging evaluation in 113 HIV-negative male and female volunteers, there was a 29-fold increase in the geometric mean morning steady-state trough plasma concentrations of tipranavir following tipranavir co-administered with low-dose ritonavir (500/200 mg twice daily) compared to tipranavir 500 mg twice daily without ritonavir.
Figure 1 displays mean plasma concentrations of tipranavir and ritonavir at steady state for the 500/200 mg tipranavir/ritonavir dose.
Absorption and Bioavailability
Absorption of tipranavir in humans is limited, although no absolute quantification of absorption is available. Tipranavir is a P-gp substrate, a weak P-gp inhibitor, and appears to be a potent P-gp inducer as well. In vivo data suggest that the net effect of tipranavir/ritonavir at the proposed dose regimen (500/200 mg) is P-gp induction at steady-state, although ritonavir is a P-gp inhibitor. Tipranavir trough concentrations at steady-state are about 70% lower than those on Day 1, presumably due to intestinal P-gp induction. Steady state is attained in most subjects after 7-10 days of dosing.
Dosing with APTIVUS 500 mg concomitant with 200 mg ritonavir twice-daily for greater than 2 weeks and without meal restriction produced the following pharmacokinetic parameters for female and male HIV-positive patients. See Table 3.
Table 3 Pharmacokinetic Parameters a of tipranavir/ritonavir 500/200 mg for HIV+ Patients by Gender Females
(n = 14)Males
(n = 106)Cp trough (µM)41.6 ± 24.3 35.6 ± 16.7 C max (µM)94.8 ± 22.8 77.6 ± 16.6 T max (h)2.9 3.0 AUC 0-12h (µM·h)851 ± 309 710 ± 207 CL (L/h)1.15 1.27 V (L)7.7 10.2 t 1/2 (h)5.5 6.0 a Population pharmacokinetic parameters reported as mean ± standard deviationEffects of Food on Oral Absorption
APTIVUS capsules co-administered with ritonavir should be taken with food. Bioavailability is increased with a high fat meal. Tipranavir capsules, administered under high fat meal conditions or with a light snack of toast and skimmed milk, were tested in a multiple dose study. High-fat meals (868 kcal, 53% derived from fat, 31% derived from carbohydrates) enhanced the extent of bioavailability (AUC point estimate 1.31, confidence interval 1.23-1.39), but had minimal effect on peak tipranavir concentrations (C max point estimate 1.16, confidence interval 1.09-1.24).
When APTIVUS, co-administered with low-dose ritonavir, was co-administered with 20 mL of aluminum and magnesium-based liquid antacid, tipranavir AUC 12h , C max and C 12h were reduced by 25-29%. Consideration should be given to separating tipranavir/ritonavir dosing from antacid administration to prevent reduced absorption of tipranavir.
Distribution
Tipranavir is extensively bound to plasma proteins (> 99.9%). It binds to both human serum albumin and (alpha)-1-acid glycoprotein. The mean fraction of APTIVUS (dosed without ritonavir) unbound in plasma was similar in clinical samples from healthy volunteers (0.015% ± 0.006%) and HIV-positive patients (0.019% ± 0.076%). Total plasma tipranavir concentrations for these samples ranged from 9 to 82 µM. The unbound fraction of tipranavir appeared to be independent of total drug concentration over this concentration range.
No studies have been conducted to determine the distribution of tipranavir into human cerebrospinal fluid or semen.
Metabolism
In vitro metabolism studies with human liver microsomes indicated that CYP 3A4 is the predominant CYP enzyme involved in tipranavir metabolism.
The oral clearance of tipranavir decreased after the addition of ritonavir, which may represent diminished first-pass clearance of the drug at the gastrointestinal tract as well as the liver.
The metabolism of tipranavir in the presence of 200 mg ritonavir is minimal. Administration of 14 C-tipranavir to subjects that received tipranavir/ritonavir 500/200 mg dosed to steady-state demonstrated that unchanged tipranavir accounted for 98.4% or greater of the total plasma radioactivity circulating at 3, 8, or 12 hours after dosing. Only a few metabolites were found in plasma, and all were at trace levels (0.2% or less of the plasma radioactivity). In feces, unchanged tipranavir represented the majority of fecal radioactivity (79.9% of fecal radioactivity). The most abundant fecal metabolite, at 4.9% of fecal radioactivity (3.2% of dose), was a hydroxyl metabolite of tipranavir. In urine, unchanged tipranavir was found in trace amounts (0.5% of urine radioactivity). The most abundant urinary metabolite, at 11.0% of urine radioactivity (0.5% of dose) was a glucuronide conjugate of tipranavir.
Elimination
Administration of 14 C-tipranavir to subjects (n=8) that received tipranavir/ritonavir 500/200 mg dosed to steady-state demonstrated that most radioactivity (median 82.3%) was excreted in feces, while only a median of 4.4% of the radioactive dose administered was recovered in urine. In addition, most radioactivity (56%) was excreted between 24 and 96 hours after dosing. The effective mean elimination half-life of tipranavir/ritonavir in healthy volunteers (n=67) and HIV-infected adult patients (n=120) was approximately 4.8 and 6.0 hours, respectively, at steady state following a dose of 500/200 mg twice daily with a light meal.
Pharmacokinetics in Special Populations
Renal Impairment
APTIVUS pharmacokinetics has not been studied in patients with renal dysfunction. However, since the renal clearance of tipranavir is negligible, a decrease in total body clearance is not expected in patients with renal insufficiency.
Hepatic Impairment
In a study comparing 9 patients with mild (Child-Pugh A) hepatic impairment to 9 controls, the single and multiple dose plasma concentrations of tipranavir and ritonavir were increased in patients with hepatic impairment, but were within the range observed in clinical trials. No dosing adjustment is required in patients with mild hepatic impairment.
The influence of moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) on the multiple-dose pharmacokinetics of tipranavir administered with ritonavir has not been evaluated (see DOSAGE AND ADMINISTRATION , CONTRAINDICATIONS , and WARNINGS ).
Gender
Evaluation of steady-state plasma tipranavir trough concentrations at 10-14 h after dosing from the 1182.12 and 1182.48 studies demonstrated that females generally had higher tipranavir concentrations than males. After 4 weeks of tipranavir/ritonavir 500/200 mg BID, the median plasma trough concentration of tipranavir was 43.9 µM for females and 31.1 µM for males. The difference in concentrations does not warrant a dose adjustment.
Race
Evaluation of steady-state plasma tipranavir trough concentrations at 10-14 h after dosing from the 1182.12 and 1182.48 studies demonstrated that white males generally had more variability in tipranavir concentrations than black males, but the median concentration and the range making up the majority of the data are comparable between the races.
Geriatric Patients
Evaluation of steady-state plasma tipranavir trough concentrations at 10-14 h after dosing from the 1182.12 and 1182.48 studies demonstrated that there was no change in median trough tipranavir concentrations as age increased for either gender through 65 years of age. There were an insufficient number of women greater than age 65 years in the two trials to evaluate the elderly, but the trend of consistent trough tipranavir concentrations with increasing age through 80 years for men was supported.
Pediatric Patients
The pharmacokinetic profile of tipranavir in pediatric patients has not been established.
Drug Interactions
See also CONTRAINDICATIONS , WARNINGS and PRECAUTIONS , Drug Interactions .
APTIVUS co-administered with 200 mg of ritonavir can alter plasma exposure of other drugs and other drugs may alter plasma exposure of tipranavir.
Potential for tipranavir/ritonavir to Affect Other Drugs
- APTIVUS co-administered with 200 mg of ritonavir at the recommended dose, is a net inhibitor of CYP 3A and may increase plasma concentrations of agents that are primarily metabolized by CYP 3A. Thus, co-administration of APTIVUS/ritonavir with drugs highly dependent on CYP 3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events should be contraindicated. Co-administration with other CYP 3A substrates may require a dose adjustment or additional monitoring (see CONTRAINDICATIONS and PRECAUTIONS ).
- Studies in human liver microsomes indicated tipranavir is an inhibitor of CYP 1A2, CYP 2C9, CYP 2C19 and CYP 2D6. The potential net effect of tipranavir/ritonavir on CYP 2D6 is inhibition, because ritonavir is a CYP 2D6 inhibitor. The in vivo net effect of tipranavir administered with ritonavir on CYP 1A2, CYP 2C9 and CYP 2C19 is not known. Data are not available to indicate whether tipranavir inhibits or induces glucuronosyl transferases and whether tipranavir induces CYP 1A2, CYP 2C9 and CYP 2C19.
- Tipranavir is a P-gp substrate, a weak P-gp inhibitor, and appears to be a potent P-gp inducer as well. Data suggest that the net effect of tipranavir co-administered with 200 mg of ritonavir is P-gp induction at steady-state, although ritonavir is a P-gp inhibitor.
- It is difficult to predict the net effect of APTIVUS administered with ritonavir on oral bioavailability and plasma concentrations of drugs that are dual substrates of CYP 3A and P-gp. The net effect will vary depending on the relative affinity of the co-administered drugs for CYP 3A and P-gp, and the extent of intestinal first-pass metabolism/efflux.
Potential for Other Drugs to Affect tipranavir
- Tipranavir is a CYP 3A substrate and a P-gp substrate. Co-administration of APTIVUS/ritonavir and drugs that induce CYP 3A and/or P-gp may decrease tipranavir plasma concentrations. Co-administration of APTIVUS/ritonavir and drugs that inhibit P-gp may increase tipranavir plasma concentrations.
- Co-administration of APTIVUS/ritonavir with drugs that inhibit CYP 3A may not further increase tipranavir plasma concentrations, because the level of metabolites is low following steady-state administration of APTIVUS/ritonavir 500/200 mg twice daily.
Drug interaction studies were performed with APTIVUS, co-administered with 200 mg of ritonavir, and other drugs likely to be co-administered and some drugs commonly used as probes for pharmacokinetic interactions. The effects of co-administration of APTIVUS with 200 mg ritonavir, on the AUC, C max and C min , are summarized in Tables 4 and 5. For information regarding clinical recommendations see PRECAUTIONS , Drug Interactions , Tables 8 and 9 .
Table 4 Drug Interactions: Pharmacokinetic Parameters for
Tipranavir in the Presence of Co-administered DrugsCo-administered
DrugCo-administered
Drug Dose
(Schedule)TPV/ritonavir
Drug Dose
(Schedule)n PK Ratio (90% Confidence Interval)
of Tipranavir
Pharmacokinetic Parameters with/without
Co-administered Drug;
No Effect = 1.00C max AUC C min Atorvastatin10 mg
(1 dose)500/200 mg BID
(14 doses)22 <-> 0.96
(0.86, 1.07)1.08
(1.00, 1.15)1.04
(0.89, 1.22)Clarithromycin500 mg BID
(25 doses)500/200 mg BID * 24 (68) up 1.40
(1.24, 1.47)1.66
(1.43, 1.73)2.00
(1.58, 2.47)Didanosine400 mg
(1 dose)500/100 mg BID
(27 doses)5 down 1.32
(1.09, 1.60)1.08
(0.82, 1.42)0.66
(0.31, 1.43)Efavirenz600 mg QD
(8 doses)500/100 mg BID * 21 (89) down 0.79
(0.69, 0.89)0.69
(0.57, 0.83)0.58 (0.36, 0.86) 750/200 mg BID * 25 (100) <-> 0.97
(0.85, 1.09)1.01
(0.85, 1.18)0.97
(0.69, 1.28)Ethinyl estradiol
/Norethindrone0.035/1.0 mg
(1 dose)500/100 mg BID
(21 doses)21 down 1.10
(0.98, 1.24)0.98
(0.88, 1.11)0.73
(0.59, 0.90)750/200 mg BID
(21 doses)13 <-> 1.01
(0.96, 1.06)0.98
(0.90, 1.07)0.91
(0.69, 1.20)Fluconazole100 mg QD
(12 dose)500/200 mg BID * 20 (68) up 1.32
(1.18, 1.47)1.50
(1.29, 1.73)1.69
(1.33, 2.09)Loperamide16 mg
(1 dose)750/200 mg BID
(21 doses)24 down 1.03
(0.92, 1.17)0.98
(0.86, 1.12)0.74
(0.62, 0.88)Rifabutin150 mg
(1 dose)500/200 mg BID
(15 doses)21 <-> 0.99
(0.93, 1.07)1.00 (0.96, 1.04) 1.16
(1.07, 1.27)Tenofovir300 mg
(1 dose)500/100 mg BID 22 down 0.83
(0.74, 0.94)0.82
(0.75, 0.91)0.79
(0.70, 0.90)750/200 mg BID
(23 doses)20 <-> 0.89
(0.84, 0.96)0.91
(0.85, 0.97)0.88
(0.78, 1.00)Zidovudine300 mg
(1 dose)500/100 mg BID 29 down 0.87 (0.80, 0.94) 0.82
(0.76, 0.89)0.77
(0.68, 0.87)750/200 mg BID
(23 doses)25 <-> 1.02 (0.94, 1.10) 1.02
(0.92, 1.13)1.07
(0.86, 134)*steady state comparison to historical data (n)Table 5 Drug Interactions: Pharmacokinetic Parameters for
Co-administered Drug in the Presence of tipranavir/ritonavirCo-administered
DrugCo-administered
Drug Dose
(Schedule)TPV/ritonavir
Drug Dose
(Schedule)n PK Ratio (90% Confidence Interval)
of Co-administered
Drug Pharmacokinetic Parameters
with/without TPV/ritonavir;
No Effect = 1.00C max AUC C min Amprenavir/RTV a600/100 mg BID
(27 doses)500/200 mg BID
(28 doses)16
74down
down0.61
(0.51, 073) d
-0.56
(0.49, 0.64) d
-0.45
(0.38, 0.53) d
0.44
(0.39, 0.49) eAbacavir a300 mg BID 250/200 mg BID 28 down 0.56
(0.48, 0.66)0.56
(0.49, 0.63)- (43 doses) 750/100 mg BID 14 down 0.54
(0.47, 0.63)0.64
(0.55, 0.74)- 1250/100 mg BID
(42 doses)11 down 0.48
(0.42, 0.53)0.65
(0.55, 0.76)- Atorvastatin10 mg
(1 dose)500/200 mg BID
(17 doses)22 up 8.61
(7.25, 10.21)9.36
(8.02, 10.94)5.19
(4.21, 6.40)Orthohydroxy-atorvastatin21,
12,
17down 0.02
(0.02, 0.03)0.11
(0.08, 0.17)0.07
(.06, 0.08)Parahydroxy-atorvastatin13,
22,
1down 1.04
(0.87, 1.25)0.18
(0.14, 0.24)0.33 (NA) Clarithromycin500 mg BID
(25 doses)500/200 mg BID
(15 doses)21 up 0.95
(0.83, 1.09)1.19
(1.04, 1.37)1.68
(1.42, 1.98)14-OH-clarithromycin21 down 0.03
(0.02, 0.04)0.03
(0.02, 0.04)0.05
(0.04, 0.07)Didanosine b200 mg BID, 250/200 mg BID 10 down 0.57
(0.42, 0.79)0.67
(0.51, 0.88)- >/=60 kg 750/100 mg BID 8 <-> 0.76
(0.49, 1.17)0.97
(0.64, 1.47)- 125 mg BID,
< 60 kg
(43 doses)1250/100 mg BID
(42 doses)9 <-> 0.77
(0.47, 1.26)0.87
(0.47, 1.65)- 400 mg
(1 dose)500/100 mg BID
(27 doses)5 <-> 0.80
(0.63, 1.02)0.90
(0.72, 1.11)1.17
(0.62, 2.20)Efavirenz b600 mg QD 500/100 mg BID 24 <-> 1.09
(0.99, 1.19)1.04 (0.97, 1.12) 1.02
(0.92, 1.12)(15 doses) 750/200 mg BID
(15 doses)22 <-> 1.12
(0.98, 1.28)1.00
(0.93, 1.09)0.94
(0.84, 1.04)Ethinyl estradiol0.035 mg 500/100 mg BID 21 down 0.52
(0.47, 0.57)0.52
(0.48, 0.56)- (1 dose) 750/200 mg BID
(21 doses)13 down 0.48
(0.42, 0.57)0.57
(0.54, 0.60)- Fluconazole200 mg (Day 1) 500/200 mg BID 19 <-> 0.97
(0.94, 1.01)0.99
(0.97, 1.02)0.98
(0.94, 1.02)then
100 mg QD
(6 or 12 doses)(2 or 14 doses) 19 <-> 0.94
(0.91, 0.98)0.92
(0.88, 0.95)0.89
(0.85, 0.92)Lopinavir/RTV a400/100 mg BID 500/200 mg BID 21 down 0.53
(0.40, 0.69) d0.45
(0.32, 0.63) d0.30
(0.17, 0.51) d(27 doses) (28 doses) 69 down - - 0.48
(0.40, 0.58) eLoperamide16 mg
(1 dose)750/200 mg BID
(21 doses)24 down 0.39
(0.31, 0.48)0.49 (0.40, 0.61) - N-Demethyl-Loperamide24 down 0.21
(0.17, 0.25)0.23
(0.19, 0.27)Lamivudine a150 mg BID 250/200 mg BID 64 <-> 0.96
(0.89, 1.03)0.95
(0.89, 1.02)- (43 doses) 750/100 mg BID 46 <-> 0.86
(0.78, 0.94)0.96
(0.90, 1.03)- 1250/100 mg BID
(42 doses)35 <-> 0.71
(0.62, 0.81)0.82
(0.66, 1.00)- Nevirapine a200 mg BID 250/200 mg BID 26 <-> 0.97
(0.90, 1.04)0.97
(0.91, 1.04)0.96
(0.87, 1.05)(43 doses) 750/100 mg BID 22 <-> 0.86
(0.76, 0.97)0.89
(0.78, 1.01)0.93
(0.80, 1.08)1250/100 mg BID
(42 doses)17 <-> 0.71
(0.62, 0.82)0.76
(0.63, 0.91)0.77
(0.64, 0.92)Norethindrone1.0 mg 500/100 mg BID 21 <-> 1.03
(0.94, 1.13)1.14
(1.06, 1.22)- (1 dose) 750/200 mg BID
(21 doses)13 <-> 1.08
(0.97, 1.20)1.27
(1.13, 1.43)- Rifabutin150 mg
(1 dose)500/200 mg BID
(15 doses)20 up 1.70
(1.49, 1.94)2.90
(2.59, 3.26)2.14
(1.90, 2.41)25-O-desacetyl-rifabutin20 up 3.20
(2.78, 3.68)20.71
(17.66, 24.28)7.83
(6.70, 9.14)Rifabutin + 25-O-desacetyl-rifabutin c20 up 1.86
(1.63, 2.12)4.33
(3.86, 4.86)2.76
(2.44, 3.12)Saquinavir/RTV a600/100 mg BID 500/200 mg BID 20 down 0.30
(0.23, 0.40) d0.24
(0.19, 0.32) d0.18
(0.13, 0.26) d(27 doses) (28 doses) 68 down - - 0.20
(0.16, 0.25) eStavudine a400 mg BID, 250/200 mg BID 26 <-> 0.90
(0.81, 1.02)1.00
(0.91, 1.11)- >/= 60 kg 750/100 mg BID 22 <-> 0.76
(0.66, 0.89)0.84
(0.74, 0.96)- 30 mg BID,
< 60 kg
(43 doses)1250/100 mg BID
(42 doses)19 <-> 0.74
(0.69, 0.80)0.93
(0.83, 1.05)- Tenofovir300 mg 500/100 mg BID 22 down 0.77
(0.68, 0.87)0.98
(0.91, 1.05)1.07
(0.98, 1.17)(1 dose) 750/200 mg BID
(23 doses)20 down 0.62 (0.54, 0.71) 1.02
(0.94, 1.10)1.14
(1.01, 1.27)Zidovudine b300 mg BID 250/200 mg BID 48 down 0.54
(0.47, 0.62)0.58
(0.51, 0.66)- 300 mg BID 750/100 mg BID 31 down 0.51
(0.44, 0.60)0.64
(0.55, 0.75)- 300 mg BID
(43 doses)1250/100 mg BID
(42 doses)23 down 0.49
(0.40, 0.59)0.69
(0.49, 0.97)- 300 mg 500/100 mg BID 29 down 0.39
(0.33, 0.45)0.57
(0.52, 0.63)0.89
(0.81, 0.99)(1 dose) 750/200 mg BID
(23 doses)25 [varr ] 0.44
(0.36, 0.54)0.67
(0.62, 0.73)1.25
(1.08, 1.44)Zidovudine glucuronide500/100 mg BID 29 up 0.82
(0.74, 0.90)1.02
(0.97, 1.06)1.52
(1.34, 1.71)750/200 mg BID
(23 doses)25 up 0.82
(0.73, 0.92)1.09
(1.05, 1.14)1.94
(1.62 2.31)a HIV+ patients b HIV+ patients (TPV/ritonavir 250 mg/200 mg, 750 mg/200 mg and 1250 mg/100 mg) and healthy volunteers (TPV/ritonavir 500 mg/100 mg and 750 mg/200 mg) c Normalized sum of parent drug (rifabutin) and active metabolite (25-O-desacetyl-rifabutin) d Intensive PK analysis e Drug levels obtained at 8-16 hrs post-dose INDICATIONS AND USAGE
APTIVUS (tipranavir), co-administered with 200 mg of ritonavir, is indicated for combination antiretroviral treatment of HIV-1 infected adult patients with evidence of viral replication, who are highly treatment-experienced or have HIV-1 strains resistant to multiple protease inhibitors.
This indication is based on analyses of plasma HIV-1 RNA levels in two controlled studies of APTIVUS/ritonavir of 24 weeks duration. Both studies were conducted in clinically advanced, 3-class antiretroviral (NRTI, NNRTI, PI) treatment-experienced adults with evidence of HIV-1 replication despite ongoing antiretroviral therapy.
The following points should be considered when initiating therapy with APTIVUS/ritonavir:
- The use of other active agents with APTIVUS/ritonavir is associated with a greater likelihood of treatment response (see CLINICAL PHARMACOLOGY , Microbiology and INDICATIONS AND USAGE , Description of Clinical Studies ).
- Genotypic or phenotypic testing and/or treatment history should guide the use of APTIVUS/ritonavir (see CLINICAL PHARMACOLOGY , Microbiology ). The number of baseline primary protease inhibitor mutations affects the virologic response to APTIVUS/ritonavir (see CLINICAL PHARMACOLOGY , Microbiology ).
- Liver function tests should be performed at initiation of therapy with APTIVUS/ritonavir and monitored frequently throughout the duration of treatment (see WARNINGS ).
- Use caution when prescribing APTIVUS/ritonavir to patients with elevated transaminases, hepatitis B or C co-infection or other underlying hepatic impairment (see WARNINGS ).
- The extensive drug-drug interaction potential of APTIVUS/ritonavir when co-administered with multiple classes of drugs must be considered prior to and during APTIVUS/ritonavir use (see CLINICAL PHARMACOLOGY and CONTRAINDICATIONS ).
- The risk-benefit of APTIVUS/ritonavir has not been established in treatment-na[iuml ]ve adult patients or pediatric patients.
There are no study results demonstrating the effect of APTIVUS/ritonavir on clinical progression of HIV-1.
Description of Clinical Studies
The following clinical data is derived from analyses of 24-week data from ongoing studies measuring effects on plasma HIV-1 RNA levels and CD4+ cell counts. At present there are no results from controlled studies evaluating the effect of APTIVUS/ritonavir on clinical progression of HIV.
Treatment-Experienced Patients
Studies 1182.12 and 1182.48: APTIVUS/ritonavir 500/200 mg BID + optimized background regimen (OBR) vs. Comparator Protease Inhibitor/ritonavir BID + OBR
Studies 1182.12 and 1182.48 are ongoing, randomized, controlled, open-label, multicenter studies in HIV-positive, triple antiretroviral class experienced patients. All patients were required to have previously received at least two protease inhibitor-based antiretroviral regimens and were failing a protease inhibitor-based regimen at the time of study entry with baseline HIV-1 RNA at least 1000 copies/mL and any CD4+ cell count. At least one primary protease gene mutation from among 30N, 46I, 46L, 48V, 50V, 82A, 82F, 82L, 82T, 84V or 90M had to be present at baseline, with not more than two mutations at codons 33, 82, 84 or 90.
These studies evaluated treatment response at 24 weeks in a total of 1159 patients receiving either APTIVUS co-administered with 200 mg of ritonavir plus OBR versus a control group receiving a ritonavir-boosted protease inhibitor (lopinavir, amprenavir, saquinavir or indinavir) plus OBR. Prior to randomization, OBR was individually defined for each patient based on genotypic resistance testing and patient history. The investigator had to declare OBR, comparator protease inhibitor, and use of enfuvirtide prior to randomization. Randomization was stratified by choice of comparator protease inhibitor and use of enfuvirtide.
After Week 8, patients in the control group who met the protocol defined criteria of initial lack of virologic response had the option of discontinuing treatment and switching over to APTIVUS/ritonavir in a separate roll-over study.
Demographics and baseline characteristics were balanced between the APTIVUS/ritonavir arm and control arm. In both studies combined, the 1159 patients had a median age of 43 years (range 17-80), were 88% male, 73% white, 14% black and 1% Asian. The median baseline plasma HIV-1 RNA was 4.82 (range 2 to 6.8) log 10 copies/mL and median baseline CD4+ cell count was 155 (range 1 to 1893) cells/mm 3 . Forty percent (40%) of the patients had baseline HIV-1 RNA of >/= 100,000 copies/mL, 61% had a baseline CD4+ cell count < 200 cells/mm 3 , and 57% had experienced an AIDS defining Class C event at baseline.
Patients had prior exposure to a median of 6 NRTIs, 1 NNRTI, and 4 PIs. A total of 12% of patients had previously used enfuvirtide. In baseline patient samples (n=454), 97% of the isolates were resistant to at least one protease inhibitor, 95% of the isolates were resistant to at least one NRTI, and > 75% of the isolates were resistant to at least one NNRTI.
The individually pre-selected protease inhibitor based on genotypic testing and the patient's medical history was lopinavir in 50%, amprenavir in 26%, saquinavir in 20% and indinavir in 4% of patients. A total of 86% were possibly resistant or resistant to the pre-selected comparator protease inhibitors. Approximately 25% of patients used enfuvirtide during study. There were differences between Studies 1182.12 and 1182.48 in the use of the protease inhibitors and in the use of enfuvirtide.
Treatment response and efficacy outcomes of randomized treatment through Week 24 of Studies 1182.12 and 1182.48 are shown in Table 6.
Table 6 Outcomes of Randomized Treatment Through Week 24
(Pooled Studies 1182.12 and 1182.48)Outcome Tipranavir/ritonavir
(500/200 mg BID) + OBR
(N=582)Comparator Protease
Inhibitor * /ritonavir + OBR
(N=577)Virological Responders a (confirmed at least
1 log 10 HIV-1 RNA below baseline)40% 18% Virological failures54% 79% Initial lack of virologic
response by Week 8 b35% 59% Rebound12% 11% Never suppressed7% 8% Death c or discontinued due to adverse events1% 1% Discontinued due to other reasons d5% 2% *Comparator protease inhibitors were lopinavir, amprenavir, saquinavir or indinavir and 86% of patients were possibly resistant or resistant to the chosen protease inhibitors. a Patients achieved and maintained a confirmed >/= 1 log 10 HIV-1 RNA drop from baseline through Week 24 without prior evidence of treatment failure. b Patients did not achieve a 0.5 log 10 HIV-1 RNA drop from baseline and did not have viral load < 100,000 copies/mL by Week 8. c Patients who died while being virologically suppressed. d Includes patients who were lost to-follow-up, withdrawn consent, non-adherent, protocol violations, added/changed background antiretroviral drugs for reasons other than tolerability or toxicity, or discontinued while suppressed. Through 24 weeks of treatment, the proportion of patients in the APTIVUS/ritonavir arm compared to the comparator PI/ritonavir arm with HIV-1 RNA < 400 copies/mL was 34% and 16% respectively, and with HIV-1 RNA < 50 copies/mL was 23% and 9% respectively. Among all randomized and treated patients, the median change from baseline in HIV-1 RNA at the last measurement up to Week 24 was -0.80 log 10 copies/mL in patients receiving APTIVUS/ritonavir versus -0.25 log 10 copies/mL in the comparator PI/ritonavir arm.
Among all randomized and treated patients, the median change from baseline in CD4+ cell count at the last measurement up to Week 24 was +34 cells/mm 3 in patients receiving tipranavir/ritonavir (N=582) versus +4 cells/mm 3 in the comparator PI/ritonavir (N=577) arm.
Patients in the APTIVUS/ritonavir arm achieved a significantly better virologic outcome when APTIVUS/ritonavir was combined with enfuvirtide (see CLINICAL PHARMACOLOGY , Microbiology ).
CONTRAINDICATIONS
APTIVUS (tipranavir) is contraindicated in patients with known hypersensitivity to any of the ingredients of the product.
APTIVUS is contraindicated in patients with moderate and severe (Child-Pugh Class B and C, respectively) hepatic insufficiency (see WARNINGS ).
Co-administration of APTIVUS with 200 mg of ritonavir, with drugs that are highly dependent on CYP 3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated. These drugs are listed in Table 7 below. For information regarding clinical recommendations see PRECAUTIONS , Drug Interactions , Tables 8 and 9.
Table 7 Drugs that are Contraindicated with Tipranavir,
Co-Administered with 200 mg of RitonavirDrug ClassDrugs within Class that are Contraindicated with APTIVUS, Co-administered with 200 mg of ritonavirAntiarrhythmicsAmiodarone, bepridil, flecainide, propafenone, quinidineAntihistaminesAstemizole, terfenadineErgot derivativesDihydroergotamine, ergonovine, ergotamine, methylergonovineGI motility agentCisaprideNeurolepticPimozideSedatives/hypnoticsMidazolam, triazolam
Due to the need for co-administration of APTIVUS with 200 mg of ritonavir, please refer to ritonavir prescribing information for a description of ritonavir contraindications.
WARNINGS
ALERT: Find out about medicines that should NOT be taken with APTIVUS®. This statement is included on the product's bottle label.
APTIVUS (tipranavir) must be co-administered with 200 mg of ritonavir to exert its therapeutic effect (see DOSAGE AND ADMINISTRATION ). Failure to correctly co-administer APTIVUS with ritonavir will result in reduced plasma levels of tipranavir that will be insufficient to achieve the desired antiviral effect and will alter some drug interactions (effect of tipranavir and ritonavir on other drugs).
Please refer to ritonavir prescribing information for additional information on precautionary measures.
Hepatic Impairment and Toxicity
APTIVUS co-administered with 200 mg of ritonavir, has been associated with reports of clinical hepatitis and hepatic decompensation, including some fatalities. These have generally occurred in patients with advanced HIV disease taking multiple concomitant medications. A causal relationship to APTIVUS/ritonavir could not be established. All patients should be followed closely with clinical and laboratory monitoring, especially those with chronic hepatitis B or C co-infection, as these patients have an increased risk of hepatotoxicity. Liver function tests should be performed prior to initiating therapy with APTIVUS/ritonavir, and frequently throughout the duration of treatment.
Patients with chronic hepatitis B or hepatitis C co-infection or elevations in transaminases are at approximately 2.5-fold risk for developing further transaminase elevations or hepatic decompensation. Additionally, Grade 3 and 4 increases in hepatic transaminases were observed in 6% of healthy volunteers in Phase 1 studies and 6% of subjects receiving APTIVUS/ritonavir in Phase 3 studies.
Tipranavir is principally metabolized by the liver. Therefore caution should be exercised when administering APTIVUS/ritonavir to patients with hepatic impairment because tipranavir concentrations may be increased. APTIVUS/ritonavir is contraindicated in patients with moderate to severe (Child-Pugh Class B and Child-Pugh Class C) hepatic insufficiency.
Physicians and patients should be vigilant for the appearance of signs or symptoms of hepatitis, such as fatigue, malaise, anorexia, nausea, jaundice, bilirubinuria, acholic stools, liver tenderness or hepatomegaly. Patients with signs or symptoms of clinical hepatitis should discontinue APTIVUS/ritonavir treatment and seek medical evaluation.
For information on the multi-dose pharmacokinetics of tipranavir in hepatically impaired patients see CLINICAL PHARMACOLOGY , Pharmacokinetics in Special Populations , Hepatic Impairment .
Diabetes Mellitus/Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus and hyperglycemia have been reported during post-marketing surveillance in HIV-1 infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
PRECAUTIONS
Sulfa Allergy
APTIVUS (tipranavir) should be used with caution in patients with a known sulfonamide allergy. Tipranavir contains a sulfonamide moiety. The potential for cross-sensitivity between drugs in the sulfonamide class and tipranavir is unknown.
Rash
Mild to moderate rashes including urticarial rash, maculopapular rash, and possible photosensitivity have been reported in subjects receiving APTIVUS/ritonavir. In Phase 2 and 3 trials rash was observed in 14% of females and in 8-10% of males receiving APTIVUS/ritonavir. Additionally, in one drug interaction trial in healthy female volunteers administered a single dose of ethinyl estradiol followed by APTIVUS/ritonavir, 33% of subjects developed a rash. Rash accompanied by joint pain or stiffness, throat tightness, or generalized pruritus has been reported in both men and women receiving APTIVUS/ritonavir (see PRECAUTIONS , Drug Interactions and ADVERSE REACTIONS ).
Patients with Hemophilia
There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis in patients with hemophilia type A and B treated with protease inhibitors. In some patients additional Factor VIII was given. In more than half of the reported cases, treatment with protease inhibitors was continued or reintroduced if treatment had been discontinued. A causal relationship between protease inhibitors and these events has not been established.
Lipid Elevations
Treatment with APTIVUS co-administered with 200 mg of ritonavir has resulted in large increases in the concentration of total cholesterol and triglycerides (see ADVERSE REACTIONS , Table 11 ). Triglyceride and cholesterol testing should be performed prior to initiating APTIVUS/ritonavir therapy and at periodic intervals during therapy. Lipid disorders should be managed as clinically appropriate (see PRECAUTIONS , Drug Interactions , Table 9: Established and Other Potentially Significant Drug Interactions for additional information on potential drug interactions with APTIVUS/ritonavir and HMG-CoA reductase inhibitors).
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including tipranavir. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jeroveci pneumonia, tuberculosis, or reactivation of herpes simplex and herpes zoster), which may necessitate further evaluation and treatment.
Information for Patients
Patients should be informed that APTIVUS co-administered with 200 mg of ritonavir, has been associated with severe liver disease, including some deaths. Patients with signs or symptoms of clinical hepatitis should discontinue APTIVUS/ritonavir treatment and seek medical evaluation. Symptoms of hepatitis include fatigue, malaise, anorexia, nausea, jaundice, bilirubinuria, acholic stools, liver tenderness or hepatomegaly. Extra vigilance is needed for patients with chronic hepatitis B or C co-infection, as these patients have an increased risk of hepatotoxicity.
Liver function tests should be performed prior to initiating therapy with tipranavir and 200 mg of ritonavir, and frequently throughout the duration of treatment. Patients with chronic hepatitis B or C co-infection or elevations in liver enzymes prior to treatment are at increased risk (approximately 2.5-fold) for developing further liver enzyme elevations or severe liver disease. Caution should be exercised when administering APTIVUS/ritonavir to patients with liver enzyme abnormalities or history of chronic liver disease. Increased liver function testing is warranted in these patients. APTIVUS should not be given to patients with moderate to severe liver disease.
Mild to moderate rash has been reported in HIV-infected men and women receiving APTIVUS/ritonavir.
Women receiving estrogen-based hormonal contraceptives should be instructed that additional or alternative contraceptive measures should be used during therapy with APTIVUS/ritonavir. There may be an increased risk of rash when APTIVUS is given with hormonal contraceptives.
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
Patients should be informed that APTIVUS must be co-administered with 200 mg ritonavir to ensure its therapeutic effect. Failure to correctly co-administer APTIVUS with ritonavir will result in reduced plasma levels of tipranavir that may be insufficient to achieve the desired antiviral effect.
Patients should be told that sustained decreases in plasma HIV-1 RNA have been associated with a reduced risk of progression to AIDS and death. Patients should remain under the care of a physician while using APTIVUS. Patients should be advised to take APTIVUS and other concomitant antiretroviral therapy every day as prescribed. APTIVUS, co-administered with ritonavir, must be given in combination with other antiretroviral drugs. Patients should not alter the dose or discontinue therapy without consulting with their doctor. If a dose of APTIVUS is missed, patients should take the dose as soon as possible and then return to their normal schedule. However, if a dose is skipped the patient should not double the next dose.
Patients should be informed that APTIVUS is not a cure for HIV-1 infection and that they may continue to develop opportunistic infections and other complications associated with HIV disease. The long-term effects of APTIVUS are unknown at this time. Patients should be told that there are currently no data demonstrating that therapy with APTIVUS can reduce the risk of transmitting HIV to others through sexual contact.
APTIVUS may interact with some drugs; therefore, patients should be advised to report to their health care provider the use of any other prescription, non-prescription medication or herbal products, particularly St. John's wort.
APTIVUS should be taken with food to enhance absorption.
The Patient Package Insert provides written information for the patients, and should be dispensed with each new prescription and refill.
Drug Interactions
Tipranavir administered with ritonavir can alter plasma exposure of other drugs and other drugs can alter plasma exposure of tipranavir and ritonavir.
Tipranavir co-administered with 200 mg of ritonavir at the recommended dosage is a net inhibitor of CYP 3A and may increase plasma concentrations of agents that are primarily metabolized by CYP 3A. Thus, co-administration of tipranavir/ritonavir with drugs highly dependent on CYP 3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events should be contraindicated. Co-administration with other CYP 3A substrates may require a dose adjustment or additional monitoring (see CONTRAINDICATIONS and PRECAUTIONS ).
The mechanisms of the potential interactions are described in the CLINICAL PHARMACOLOGY , Drug Interactions section.
Drugs that are contraindicated or not recommended for co-administration with APTIVUS are included in Table 8 below. These recommendations are based on either drug interaction studies or they are predicted interactions due to the expected magnitude of interaction and potential for serious events or loss of efficacy.
Table 8 Drugs that Should Not be Co-administered with APTIVUS Co-administered with 200 mg of Ritonavir Drug Class/Drug NameClinical CommentAntiarrhythmics
Amiodarone, bepridil, flecainide, propafenone, quinidineCONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias secondary to increases in plasma concentrations of antiarrhythmics.Antihistamines
Astemizole, terfenadineCONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias.Antimycobacterials
RifampinMay lead to loss of virologic response and possible resistance to tipranavir or to the class of protease inhibitors.Ergot derivatives
Dihydroergotamine, ergonovine, ergotamine, methylergonovineCONTRAINDICATED due to potential for serious and/or life-threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues.GI motility agents
CisaprideCONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias.Herbal products
St. John's wortMay lead to loss of virologic response and possible resistance to tipranavir or to the class of protease inhibitors.HMG CoA reductase inhibitors
Lovastatin, simvastatinPotential for serious reactions such as risk of myopathy including rhabdomyolysis.Neuroleptics
PimozideCONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias.Sedatives/hypnotics
Midazolam, triazolamCONTRAINDICATED due to potential for serious and/or life threatening reactions such as prolonged or increased sedation or respiratory depression.
Clinically significant drug-drug interactions of APTIVUS co-administered with 200 mg of ritonavir are summarized in Table 9 below.
Table 9 Established and Other Potentially Significant Drug Interactions:
Alterations in Dose or Regimen May be Recommended Based
on Drug Interaction Studies or Predicted InteractionConcomitant Drug Class:
Drug nameEffect on Concentration of
Tipranavir or Concomitant DrugClinical CommentHIV-Antiviral AgentsNucleoside reverse transcriptase inhbitors:Abacavirdown Abacavir AUC by approximately 40%Clinical relevance of reduction in abacavir levels not established. Dose adjustment of abacavir cannot be recommended at this time.Didanosine (EC)
down DidanosineClinical relevance of reduction in didanosine levels not established. For optimal absorption, didanosine should be separated from TPV/ritonavir dosing by at least 2 hours.Zidovudinedown Zidovudine AUC by approximately 35%. ZDV glucuronide concentrations were unaltered.Clinical relevance of reduction in zidovudine levels not established. Dose adjustment of zidovudine cannot be recommended at this time.Protease inhibitors
(co-administered with 200 mg of ritonavir):Combining amprenavir, lopinavir or saquinavir with APTIVUS/ritonavir is not recommended. No formal drug interaction data are currently available for the concomitant use of APTIVUS, co-administered with 200 mg of ritonavir, with protease inhibitors other than those listed above.Amprenavirdown Amprenavir,Lopinavirdown Lopinavir,Saquinavirdown SaquinavirOther Agents for Opportunistic InfectionsAntifungals:Fluconazole increases TPV concentrations but dose adjustments are not needed. Fluconazole doses > 200 mg/day are not recommended.Fluconazoleup Tipranavir, <-> FluconazoleItraconazoleup Itraconazole (not studied)Ketoconazoleup Ketoconazole (not studied)Voriconazole[varr ] Voriconazole (not studied)Based on theoretical considerations itraconazole and ketoconazole should be used with caution. High doses (200 mg/day) are not recommended.
Due to multiple enzymes involved with voriconazole metabolism, it is difficult to predict the interaction.Antimycobacterials:Clarithromycinup Tipranavir, up Clarithromycin,
down14-hydroxy-clarithromycin metaboliteNo dose adjustment of tipranavir or clarithromycin for patients with normal renal function is necessary.
For patients with renal impairment the following dosage adjustments should be considered:
· For patients with CL CR 30 to 60 mL/min the dose of clarithromycin should be reduced by 50%.
For patients with CL CR < 30 mL/min the dose of clarithromycin should be decreased by 75%.RifabutinTipranavir not changed, up Rifabutin
up Desacetyl-rifabutinSingle dose study. Dosage reductions of rifabutin by 75% are recommended (e.g. 150 mg every other day). Increased monitoring for adverse events in patients receiving the combination is warranted. Further dosage reduction may be necessary.Other Agents Commonly usedCalcium Channel Blockers:
Diltiazem
Felodipine
Nicardipine
Nisoldipine
VerapamilCombination with TPV/ritonavir not studied. Cannot predict effect of TPV/ritonavir on calcium channel blockers that are dual substrates of CYP 3A and P-gp due to conflicting effect of TPV/ritonavir on CYP 3A and P-gp.
[varr ] Diltiazem
upFelodipine (CYP 3A substrate but not P-gp substrate)
[varr ] Nicardipine
[varr ] Nisoldipine (CYP 3A substrate but not clear whether it is a P-gp substrate)
[varr ] VerapamilCaution is warranted and clinical monitoring of patients is recommended.DespiramineCombination with TPV/ritonavir not studied
up DespiramineDosage reduction and concentration monitoring of despiramine is recommended.Disulfiram/MetronidazoleCombination with TPV/ritonavir not studiedAPTIVUS capsules contain alcohol that can produce disulfiram-like reactions when co-administered with disulfiram or other drugs which produce this reaction (e.g. metronidazole).HMG-CoA reductase inhibitors:
Atorvastatinup Tipranavir, upAtorvastatin
downHydroxy-atorvastatin metabolitesStart with the lowest possible dose of atorvastatin with careful monitoring, or consider other HMG-CoA reductase inhibitors. Concomitant use of APTIVUS, co-administered with 200 mg of ritonavir, with lovastatin or simvastatin is not recommended.Hypoglycemics:
Glimepiride
Glipizide
Glyburide
Pioglitazone
Repaglinide
TolbutamideCombination with TPV/ritonavir not studied.
[varr ] Glimepiride (CYP 2C9)
[varr ] Glipizide (CYP 2C9)
[varr ] Glyburide (CYP 2C9)
[varr ] Pioglitazone (CYP 2C8 and CYP 3A4)
[varr ] Repaglinide (CYP 2C8 and CYP 3A4)
[varr ] Tolbutamide (CYP 2C9)
The effect of TPV/ritonavir on CYP 2C8 and CYP 2C9 substrates is not known.Careful glucose monitoring is warranted.Immunosuppressants:
Cyclosporine
Sirolimus
TacrolimusCombination with TPV/ritonavir not studied. Cannot predict effect of TPV/ritonavir on immunosuppressants due to conflicting effect of TPV/ritonavir on CYP 3A and P-gp.
[varr ] Cyclosporine
[varr ] Sirolimus
[varr ]TacrolimusMore frequent concentration monitoring of these medicinal products is recommended until blood levels have been stabilized.Narcotic analgesics:
Meperidine
Combinations with TPV/ritonavir not studied
down Meperidine, up Normeperidine
Dosage increase and long-term use of meperidine are not recommended due to increased concentrations of the metabolite normeperidine which has both analgesic activity and CNS stimulant activity (e.g. seizures).Methadonedown Methadone by 50%Dosage of methadone may need to be increased when co-administered with tipranavir and 200 mg of ritonavir.Oral contraceptives/Estrogens:
Ethinyl estradiol
down Ethinyl estradiol concentrations by 50%Alternative methods of nonhormonal contraception should be used when estrogen based oral contraceptives are co-administered with tipranavir and 200 mg of ritonavir. Patients using estrogens as hormone replacement therapy should be clinically monitored for signs of estrogen deficiency.
Women using estrogens may have an increased risk of non serious rash.PDE5 inhibitors:
Sildenafil
Tadalafil
VardenafilCombinations with TPV/ritonavir not studied.
up Sildenafil
up Tadalafil
up VardenafilConcomitant use of PDE5 inhibitors with tipranavir and ritonavir should be used with caution and in no case should the starting dose of:
· sildenafil exceed 25 mg within 48 hours
·tadalafil exceed 10 mg every 72 hours
· vardenafil exceed 2.5 mg every 72 hoursSelective Serotonin-Reuptake Inhibitors:
Fluoxetine
Paroxetine
SertralineCombination with TPV/ritonavir not studied
up Fluoxetine
up Paroxetine
up SertralineAntidepressants have a wide therapeutic index, but doses may need to be adjusted upon initiation of APTIVUS/ritonavir therapy.WarfarinCombination with TPV/ritonavir not studied.
Cannot predict the effect of TPV/ritonavir on S-Warfarin due to conflicting effect of TPV and RTV on CYP 2C9Frequent INR (international normalized ratio) monitoring upon initiation of tipranavir/ritonavir therapy.Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term animal carcinogenicity bioassays with tipranavir and tipranavir/ritonavir are currently in progress. However, tipranavir showed no evidence of mutagenicity or clastogenicity in a battery of five in vitro and in vivo tests including the Ames bacterial reverse mutation assay using S. typhimurium and E. coli , unscheduled DNA synthesis in rat hepatocytes, induction of gene mutation in Chinese hamster ovary cells, a chromosome aberration assay in human peripheral lymphocytes, and a micronucleus assay in mice.
Tipranavir had no effect on fertility or early embryonic development in rats at dose levels up to 1000 mg/kg/day, equivalent to a C max of 258 µM in females. Based on C max levels in these rats, as well as an exposure (AUC) of 1670 µM·h in pregnant rats from another study, this exposure was approximately equivalent to the anticipated exposure in humans at the recommended dose level of 500/200 mg tipranavir/ritonavir BID.
Pregnancy
Teratogenic Effects, Pregnancy Category C.
Investigation of fertility and early embryonic development with tipranavir disodium was performed in rats, teratogenicity studies were performed in rats and rabbits, and pre- and post-natal development were explored in rats.
No teratogenicity was detected in reproductive studies performed in pregnant rats and rabbits up to dose levels of 1000 mg/kg/day and 150 mg/kg/day tipranavir, respectively, at exposure levels approximately 1.1-fold and 0.1-fold human exposure. At 400 mg/kg/day and above in rats, fetal toxicity (decreased sternebrae ossification and body weights) was observed, corresponding to an AUC of 1310 µM·h or approximately 0.8-fold human exposure at the recommended dose. In rats and rabbits, fetal toxicity was not noted at 40 mg/kg/day and 150 mg/kg/day, respectively, corresponding accordingly to C max /AUC 0-24h levels of 30.4 µM/340 µM·h and 8.4 µM/120 µM·h. These exposure levels (AUC) are approximately 0.2-fold and 0.1-fold the exposure in humans at the recommended dose.
In pre- and post-development studies in rats, tipranavir showed no adverse effects at 40 mg/kg/day (~0.2-fold human exposure), but caused growth inhibition in pups and maternal toxicity at dose levels of 400 mg/kg/day (~0.8-fold human exposure). No post-weaning functions were affected at any dose level.
There are no adequate and well-controlled studies in pregnant women for the treatment of HIV-1 infection. APTIVUS should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Antiretroviral Pregnancy Registry
To monitor maternal-fetal outcomes of pregnant women exposed to APTIVUS, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling (800) 258-4263.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Because of both the potential for HIV transmission and any possible adverse effects of tipranavir, mothers should be instructed not to breastfeed if they are receiving APTIVUS.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of APTIVUS did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, caution should be exercised in the administration and monitoring of APTIVUS in elderly patients reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
APTIVUS (tipranavir), co-administered with 200 mg of ritonavir, has been studied in a total of 1854 HIV-positive adults as combination therapy in clinical studies. Of these, 1397 patients received the dose of 500/200 mg BID. Seven hundred sixty one (761) adults, including 385 in the 1182.12 and 1182.48 Phase 3 pivotal studies, have been treated for at least 24 weeks.
In 1182.12 and 1182.48 in the APTIVUS/ritonavir arm, the most frequent AEs were diarrhea, nausea, fatigue, headache and vomiting. Adverse events leading to discontinuation were reported by 7.8% of the tipranavir-treated patients and 4.9% of the comparator arm patients.
Due to the need for co-administration of APTIVUS with 200 mg of ritonavir, please refer to ritonavir prescribing information for ritonavir-associated adverse reactions.
The most frequent clinical treatment-emergent adverse events reported in Phase 3 clinical studies (1182.12 and 1182.48) in adults are summarized in Table 10 below. Events of moderate to severe intensity (Grades 2-4) reported in at least 2% of highly treatment-experienced subjects in either treatment group are included.
Table 10 Percentage of Patients with Treatment Emergent Adverse Events of at Least Moderate Intensity (Grades 2-4) in >/= 2% of Patients in Either Treatment Group a Phase 3 Studies 1182.12 and 1182.48
(24-weeks)Tipranavir/ritonavir
(500/200 mg BID) + OBR
(n=746)Comparator PI/ritonavir b +
OBR
(n=737)Gastrointestinal
DisordersDiarrhea10.9% 9.4% Nausea6.7% 4.6% Vomiting3.4% 3.0% Abdominal pain c2.8% 3.7% General DisordersPyrexia4.6% 4.3% Fatigue4.0% 3.9% Asthenia1.5% 2.3% Infections and
InfestationsBronchitis2.9% 1.1% Nervous System
DisordersHeadache3.1% 3.1% Psychiatric DisordersDepression2.0% 3.0% Insomnia1.2% 2.6% Respiratory, Thoracic and
Mediastinal DisordersCough0.8% 2.2% Skin and Subcutaneous
Tissue DisordersRash2.0% 2.0% a Excludes laboratory abnormalities that were Adverse Eventsb Comparator PI/RTV: lopinavir/ritonavir 400/100 mg BID, indinavir/ritonavir 800/100 mg BID, saquinavir/ritonavir 1000/100 mg BID, amprenavir/ritonavir 600/100 mg BIDc Abdominal pain includes Preferred Terms "Abdominal pain" and "Abdominal pain upper"
Clinically meaningful adverse reactions in < 2% of adult patients (n=1397) treated with APTIVUS/ritonavir 500/200 mg in Phase 2 and 3 trials listed below by body system:
Blood and Lymphatic System Disorders: anemia, neutropenia, thrombocytopenia
Gastrointestinal Disorders: abdominal distension, dyspepsia, flatulence, gastroesophageal reflux disease, pancreatitis
General Disorders: influenza like illness, malaise, pyrexia
Hepatobiliary Disorders: hepatitis, hepatic failure
Immune System Disorders: hypersensitivity
Infections and infestations: reactivation of herpes simplex and varicella zoster
Investigations: hepatic enzymes increased, liver function test abnormal, lipase increased, weight decreased
Metabolism and Nutrition Disorders: anorexia, decreased appetite, dehydration, diabetes mellitus, facial wasting, hyperamylasemia, hypercholesterolemia, hyperglycemia
Musculoskeletal and Connective Tissue Disorders: muscle cramp, myalgia
Nervous System Disorders: dizziness, neuropathy peripheral, somnolence
Psychiatric Disorders: insomnia, sleep disorder
Renal and Urinary Disorders: renal insufficiency
Respiratory, Thoracic and Mediastinal Disorders: dyspnea
Skin and Subcutaneous System Disorders: exanthem, lipoatrophy, lipodystrophy acquired, lipohypertrophy, pruritus
Laboratory Abnormalities
Treatment emergent clinical laboratory abnormalities reported at 24 weeks in Phase 3 clinical studies (1182.12 and 1182.48) in adults are summarized in Table 11 below.
Table 11 Treatment Emergent Laboratory Abnormalities Reported in >/= 2% of Adult Patients Studies 1182.12 and 1182.48 (24-weeks) Limit APTIVUS/ritonavir
(500/200 mg BID) +
OBR
(n=732)Comparator PI/ritonavir
+ OBR *
(n=726)HematologyWBC count
decrease
Grade 3-4< 2.0 × 10 3 /µL 3.6% 5.4% ChemistryAmylase
Grade 3-4> 2 × ULN 2.9% 4.8% ALT
Grade 2> 2.5-5 × ULN 10.7% 5.4% Grade 3> 5-10 × ULN 3.1% 1.4% Grade 4> 10 × ULN 2.7% 0.4% AST
Grade 2> 2.5-5 × ULN 6.0% 5.8% Grade 3> 5-10 × ULN 3.3% 1.0% Grade 4> 10 × ULN 0.7% 0.4% ALT and/or AST
Grade 2-4> 2.5 × ULN 17.5% 9.9% Cholesterol
Grade 2> 300 - 400 mg/dL 11.3% 4.3% Grade 3> 400 - 500 mg/dL 2.5% 0.3% Grade 4> 500 mg/dL 0.8% 0% Triglycerides
Grade 2400 - 750 mg/dL 26.2% 14.7% Grade 3> 750 - 1200 mg/dL 12.8% 5.6% Grade 4> 1200 mg/dL 6.1% 3.4% *Comparator PI/RTV: lopinavir/ritonavir 400/100 mg BID, indinavir/ritonavir 800/100 mg BID, saquinavir/ritonavir 1000/100 mg BID, amprenavir/ritonavir 600/100 mg BIDIn clinical trials extending up to 48 weeks, the proportion of patients who developed Grade 2-4 ALT and/or AST elevations increased to 24.4% with APTIVUS/ritonavir and to 12.8% with CPI/ritonavir.
OVERDOSAGE
There is no known antidote for tipranavir overdose. Treatment of overdose should consist of general supportive measures, including monitoring of vital signs and observation of the patient's clinical status. If indicated, elimination of unabsorbed tipranavir should be achieved by emesis or gastric lavage. Administration of activated charcoal may also be used to aid in removal of unabsorbed drug. Since tipranavir is highly protein bound, dialysis is unlikely to be beneficial in significant removal of this medicine.
DOSAGE AND ADMINISTRATION
General
The recommended dose of APTIVUS (tipranavir) Capsules is 500 mg (two 250 mg capsules), co-administered with 200 mg of ritonavir, twice daily.
APTIVUS Capsules, co-administered with 200 mg of ritonavir should be taken with food. Bioavailability is increased with a high fat meal.
HOW SUPPLIED
APTIVUS (tipranavir) Capsules 250 mg are pink, oblong soft gelatin capsules imprinted in black with "TPV 250". They are packaged in HDPE unit-of-use bottles with a child resistant closure and 120 capsules. (NDC 0597-0003-02)
APTIVUS capsules should be stored in a refrigerator 2°-8°C (36°-46°F) prior to opening the bottle. After opening the bottle, the capsules may be stored at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) and must be used within 60 days.
Store in a safe place out of the reach of children.
Address medical inquiries to:
http://us.boehringer-ingelheim.com , (800) 542-6257 or
(800) 459-9906 TTY.
Rx only
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
APTIVUS® is a registered trademark used under license from Boehringer Ingelheim International GmbH
©Copyright Boehringer Ingelheim International GmbH, 2005 ALL RIGHTS RESERVED
APTIVUS Capsules are covered by U.S. Patents 5,852,195; 6,147,095; 6,169,181 and 6,231,887
OT2000
10003515/US/1 10003515/01
Issued: June 23, 2005
Patient Information
Aptivus® (ap' · ti · vas)
(tipranavir)
Capsules, 250 mg
ALERT: Find out about medicines that should not be taken with Aptivus®. Please also read the section " Who Should Not Take APTIVUS? " .
Read the Patient Information that comes with APTIVUS before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or treatment. You should stay under a doctor's care while taking APTIVUS.
What is the most important information I should know about APTIVUS?
Patients taking APTIVUS, together with 200 mg NORVIR® (ritonavir), may develop severe liver disease that can cause death. If you develop any of the following symptoms of liver problems, you should stop taking APTIVUS/ritonavir treatment and call your doctor right away: tiredness, general ill feeling or " flu-like " symptoms, loss of appetite, nausea (feeling sick to your stomach), yellowing of your skin or whites of your eyes, dark (tea-colored) urine, pale stools (bowel movements), or pain, ache, or sensitivity on your right side below your ribs. If you have chronic hepatitis B or C infection, your doctor should check your blood tests more often because you have an increased chance of developing liver problems.
What is APTIVUS?
APTIVUS is a medicine called a "protease inhibitor" that is used to treat adults with Human Immunodeficiency Virus (HIV). APTIVUS blocks HIV protease, an enzyme which is needed for HIV to make more virus. When used with other anti-HIV medicines, APTIVUS may reduce the amount of HIV in your blood and increase the number of CD4+ cells. Reducing the amount of HIV in the blood may keep your immune system healthy, so it can help fight infection.
APTIVUS is always taken with NORVIR® (ritonavir) and at the same time as NORVIR. When you take APTIVUS with NORVIR, you must always use at least 2 other anti-HIV medicines.
Does APTIVUS cure HIV or AIDS?
APTIVUS does not cure HIV infection or AIDS. The long-term effects of APTIVUS are not known at this time. People taking APTIVUS may still get infections or other conditions common in people with HIV (opportunistic infections). It is very important that you stay under the care of your doctor during treatment with APTIVUS.
Does APTIVUS lower the chance of passing HIV to other people?
APTIVUS does not reduce the chance of passing HIV to others through sexual contact, sharing needles, or being exposed to your blood. Continue to practice safer sex. Use a latex or polyurethane condom or other barrier method to lower the chance of sexual contact with any body fluids such as semen, vaginal secretions or blood. Never use or share dirty needles.
Ask your doctor if you have any questions about safer sex or how to prevent passing HIV to other people.
Who should not take APTIVUS?
Do not take APTIVUS if you:
- are allergic to tipranavir or any of the other ingredients in APTIVUS. See the end of this leaflet for a list of major ingredients.
- are allergic to ritonavir (NORVIR®)
- have moderate to severe liver problems
-
take any of the following types of medicines because
you could have serious side effects
:
-- Migraine headache medicines called "ergot alkaloids". If you take migraine headache medicines, ask your doctor or pharmacist if any of them are "ergot alkaloids".
-- Halcion® (triazolam)
-- Hismanal® (astemizole)
-- Orap® (pimozide)
-- Propulsid® (cisapride)
-- Seldane® (terfenadine)
-- Versed® (midazolam)
-- Pacenone® (amiodarone)
-- Vascor® (bepridil)
-- Tambocor® (flecainide)
-- Rythmol® (propafenone)
-- Quinaglute dura® (quinidine)
What should I tell my doctor before I take APTIVUS?
Tell your doctor about all of your medical conditions, including if you:
- have liver problems or are infected with hepatitis B or hepatitis C. These patients may have worsening of their liver disease.
- are allergic to sulfa medicines.
- have hemophilia. APTIVUS may cause increased bleeding.
- have diabetes. APTIVUS may worsen your diabetes or high blood sugar levels.
- are pregnant or planning to become pregnant. It is not known if APTIVUS can harm your unborn baby. You and your doctor will need to decide if APTIVUS is right for you. If you take APTIVUS while you are pregnant, talk to your doctor about how you can be in the Antiretroviral Pregnancy Registry.
- are breast-feeding. Do not breast-feed if you are taking APTIVUS. You should not breast-feed if you have HIV because of the chance of passing the HIV virus to your baby. Talk with your doctor about the best way to feed your baby.
- are using estrogens for birth control or hormone replacement. Women who use estrogens for birth control or hormone replacement have an increased chance of developing a skin rash while taking APTIVUS. If a rash occurs, it is usually mild to moderate, but you should talk to your doctor as you may need to temporarily stop taking either APTIVUS or the other medicine that contains estrogen or female hormones.
Tell your doctor about all the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. APTIVUS and many other medicines can interact. Sometimes serious side effects will happen if APTIVUS is taken with certain other medicines (see " Who should not take APTIVUS? " ).
- Some medicines cannot be taken at all with APTIVUS.
- Some medicines will require a change in dosage if taken with APTIVUS.
- Some medicines will require close monitoring if taken with APTIVUS.
Women taking birth control pills need to use another birth control method. APTIVUS makes birth control pills work less well.
Know all the medicines you take and keep a list of them with you. Show this list to all your doctors and pharmacists anytime you get a new medicine you take. They will tell you if you can take these other medicines with APTIVUS. Do not start any new medicines while you are taking APTIVUS without first talking with your doctor or pharmacist. You can ask your doctor or pharmacist for a list of medicines that can interact with APTIVUS.
How should I take APTIVUS?
- Take APTIVUS exactly as your doctor has prescribed. You should check with your doctor or pharmacist if you are not sure. You must take APTIVUS at the same time as NORVIR® (ritonavir). The usual dose is 500 mg (two 250 mg capsules) of APTIVUS, together with 200 mg (two 100 mg capsules or 2.5 mL of solution) of NORVIR, twice per day. APTIVUS with NORVIR must be used together with other anti-HIV medicines.
APTIVUS comes in a capsule form and you should swallow APTIVUS capsules whole. Do not chew the capsules.
- Always take APTIVUS with food.
- Do not change your dose or stop taking APTIVUS without first talking with your doctor.
- If you take too much APTIVUS, call your doctor or poison control center right away.
- If you forget to take APTIVUS, take the next dose of APTIVUS, together with NORVIR® (ritonavir), as soon as possible. Do not take a double dose to make up for a missed dose.
- It is very important to take all your anti-HIV medicines as prescribed and at the right times of day. This can help your medicines work better. It also lowers the chance that your medicines will stop working to fight HIV (drug resistance).
- When your APTIVUS® supply starts to run low, get more from your doctor or pharmacy. This is very important because the amount of virus in your blood may increase if the medicine is stopped for even a short period of time. The HIV virus may develop resistance to APTIVUS and become harder to treat. You should NEVER stop taking APTIVUS or your other HIV medicines without talking with your doctor.
What are the possible side effects of APTIVUS?
APTIVUS may cause serious side effects, including:
- liver problems, including liver failure and death. Your doctor should do blood tests to monitor your liver function during treatment with APTIVUS. Patients with liver diseases such as hepatitis B and hepatitis C may have worsening of their liver disease with APTIVUS and should have more frequent monitoring blood tests.
- rash. Mild to moderate rash, including flat or raised rashes or sensitivity to the sun, have been reported in approximately 10% of subjects receiving APTIVUS. Some patients who developed rash also had joint pain or stiffness, throat tightness, or generalized itching.
- increased bleeding in patients with hemophilia. This can happen in patients taking APTIVUS or other protease inhibitor medicines.
- diabetes and high blood sugar (hyperglycemia). This can happen in patients taking APTIVUS or other protease inhibitor medicines. Some patients have diabetes before starting treatment with APTIVUS which gets worse. Some patients get diabetes during treatment with APTIVUS. Some patients will need changes in their diabetes medicine. Some patients will need new diabetes medicine.
- increased blood fat (lipid) levels. Your doctor should do blood tests to monitor your blood fat (triglycerides and cholesterol) during treatment with APTIVUS. Some patients taking APTIVUS have large increases in triglycerides and cholesterol. The long-term chance of having a heart attack or stroke due to increases in blood fats caused by APTIVUS is not known at this time.
- changes in body fat. These changes have happened in patients taking APTIVUS and other anti-HIV medicines. The changes may include an increased amount of fat in the upper back and neck ("buffalo hump"), breast, and around the back, chest, and stomach area. Loss of fat from the legs, arms, and face may also happen. The cause and long-term health effects of these conditions are not known.
The most common side effects include diarrhea, nausea, vomiting, stomach pain, tiredness and headache. Women taking birth control pills may get a skin rash.
It may be hard to tell the difference between side effects caused by APTIVUS, by the other medicines you are also taking, or by the complications of HIV infection. For this reason it is very important that you tell your doctor about any changes in your health. You should report any new or continuing symptoms to your doctor right away. Your doctor may be able to help you manage these side effects.
The list of side effects is not complete. Ask your doctor or pharmacist for more information.
How should I store APTIVUS?
- Store APTIVUS capsules in a refrigerator at approximately 36°F to 46°F (2°C to 8°C). Once the bottle is opened, the contents must be used within 60 days. Patients may take the bottle with them for use away from home so long as the bottle remains at a temperature of approximately 59°F to 86°F (15°C to 30°C). You can write the date of opening the bottle on the label. Do not use after the expiration date written on the bottle.
- Keep APTIVUS and all medicines out of the reach of children.
General advice about APTIVUS
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use APTIVUS for a condition for which it was not prescribed. Do not give APTIVUS to other people, even if they have the same condition you have. It may harm them.
This leaflet summarizes the most important information about APTIVUS. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about APTIVUS that is written for health professionals.
For additional information, you may also call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257, or (TTY) 1-800-459-9906. You may also request information through the company website at
http://us.boehringer-ingelheim.com .
What are the ingredients in APTIVUS?
Active Ingredient: tipranavir
Major Inactive Ingredients: dehydrated alcohol, polyoxyl 35 castor oil, propylene glycol, mono/diglycerides of caprylic/capric acid and gelatin.
Rx only
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
APTIVUS is a registered trademark used under license from Boehringer Ingelheim International GmbH
©Copyright Boehringer Ingelheim International GmbH, 2005 ALL RIGHTS RESERVED
APTIVUS Capsules are covered by U.S. Patents 5,852,195; 6,147,095; 6,169,181 and 6,231,887
OT2000
10003515/US/1 10003515/01
Issued: June 23, 2005
Subscribe to the "News" RSS Feed 
TOP ۞
