-
Aricept ODT Tablets, Aricept Tablets (Eisai)
DESCRIPTION
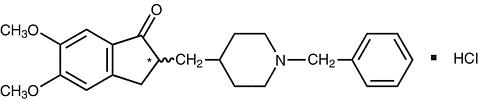
ARICEPT® (donepezil hydrochloride) is a reversible inhibitor of the enzyme acetylcholinesterase, known chemically as (±)-2,3-dihydro-5,6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1 H -inden-1-one hydrochloride. Donepezil hydrochloride is commonly referred to in the pharmacological literature as E2020. It has an empirical formula of C 24 H 29 NO 3 HCl and a molecular weight of 415.96. Donepezil hydrochloride is a white crystalline powder and is freely soluble in chloroform, soluble in water and in glacial acetic acid, slightly soluble in ethanol and in acetonitrile and practically insoluble in ethyl acetate and in n-hexane.
ARICEPT® is available for oral administration in film-coated tablets containing 5 or 10 mg of donepezil hydrochloride. Inactive ingredients are lactose monohydrate, corn starch, microcrystalline cellulose, hydroxypropyl cellulose, and magnesium stearate. The film coating contains talc, polyethylene glycol, hypromellose and titanium dioxide. Additionally, the 10 mg tablet contains yellow iron oxide (synthetic) as a coloring agent.
ARICEPT® ODT tablets are available for oral administration. Each ARICEPT® ODT tablet contains 5 or 10 mg of donepezil hydrochloride. Inactive ingredients are carrageenan, mannitol, colloidal silicon dioxide and polyvinyl alcohol. Additionally, the 10 mg tablet contains ferric oxide (yellow) as a coloring agent.
CLINICAL PHARMACOLOGY
Current theories on the pathogenesis of the cognitive signs and symptoms of Alzheimer's Disease attribute some of them to a deficiency of cholinergic neurotransmission.
Donepezil hydrochloride is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by acetylcholinesterase. If this proposed mechanism of action is correct, donepezil's effect may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. There is no evidence that donepezil alters the course of the underlying dementing process.
Clinical Trial Data
The effectiveness of ARICEPT® as a treatment for Alzheimer's Disease is demonstrated by the results of two randomized, double-blind, placebo-controlled clinical investigations in patients with Alzheimer's Disease (diagnosed by NINCDS and DSM III-R criteria, Mini-Mental State Examination >/= 10 and </= 26 and Clinical Dementia Rating of 1 or 2). The mean age of patients participating in ARICEPT® trials was 73 years with a range of 50 to 94. Approximately 62% of patients were women and 38% were men. The racial distribution was white 95%, black 3% and other races 2%.
Study Outcome Measures: In each study, the effectiveness of treatment with ARICEPT® was evaluated using a dual outcome assessment strategy.
The ability of ARICEPT® to improve cognitive performance was assessed with the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS-cog), a multi-item instrument that has been extensively validated in longitudinal cohorts of Alzheimer's Disease patients. The ADAS-cog examines selected aspects of cognitive performance including elements of memory, orientation, attention, reasoning, language and praxis. The ADAS-cog scoring range is from 0 to 70, with higher scores indicating greater cognitive impairment. Elderly normal adults may score as low as 0 or 1, but it is not unusual for non-demented adults to score slightly higher.
The patients recruited as participants in each study had mean scores on the Alzheimer's Disease Assessment Scale (ADAS-cog) of approximately 26 units, with a range from 4 to 61. Experience gained in longitudinal studies of ambulatory patients with mild to moderate Alzheimer's Disease suggest that they gain 6 to 12 units a year on the ADAS-cog. However, lesser degrees of change are seen in patients with very mild or very advanced disease because the ADAS-cog is not uniformly sensitive to change over the course of the disease. The annualized rate of decline in the placebo patients participating in ARICEPT® trials was approximately 2 to 4 units per year.
The ability of ARICEPT® to produce an overall clinical effect was assessed using a Clinician's Interview Based Impression of Change that required the use of caregiver information, the CIBIC plus. The CIBIC plus is not a single instrument and is not a standardized instrument like the ADAS-cog. Clinical trials for investigational drugs have used a variety of CIBIC formats, each different in terms of depth and structure.
As such, results from a CIBIC plus reflect clinical experience from the trial or trials in which it was used and cannot be compared directly with the results of CIBIC plus evaluations from other clinical trials. The CIBIC plus used in ARICEPT® trials was a semi-structured instrument that was intended to examine four major areas of patient function: General, Cognitive, Behavioral and Activities of Daily Living. It represents the assessment of a skilled clinician based upon his/her observations at an interview with the patient, in combination with information supplied by a caregiver familiar with the behavior of the patient over the interval rated. The CIBIC plus is scored as a seven point categorical rating, ranging from a score of 1, indicating "markedly improved," to a score of 4, indicating "no change" to a score of 7, indicating "markedly worse." The CIBIC plus has not been systematically compared directly to assessments not using information from caregivers (CIBIC) or other global methods.
Thirty-Week Study
In a study of 30 weeks duration, 473 patients were randomized to receive single daily doses of placebo, 5 mg/day or 10 mg/day of ARICEPT®. The 30-week study was divided into a 24-week double-blind active treatment phase followed by a 6-week single-blind placebo washout period. The study was designed to compare 5 mg/day or 10 mg/day fixed doses of ARICEPT® to placebo. However, to reduce the likelihood of cholinergic effects, the 10 mg/day treatment was started following an initial 7-day treatment with 5 mg/day doses.
Effects on the ADAS-cog: Figure 1 illustrates the time course for the change from baseline in ADAS-cog scores for all three dose groups over the 30 weeks of the study. After 24 weeks of treatment, the mean differences in the ADAS-cog change scores for ARICEPT® treated patients compared to the patients on placebo were 2.8 and 3.1 units for the 5 mg/day and 10 mg/day treatments, respectively. These differences were statistically significant. While the treatment effect size may appear to be slightly greater for the 10 mg/day treatment, there was no statistically significant difference between the two active treatments.
Following 6 weeks of placebo washout, scores on the ADAS-cog for both the ARICEPT® treatment groups were indistinguishable from those patients who had received only placebo for 30 weeks. This suggests that the beneficial effects of ARICEPT® abate over 6 weeks following discontinuation of treatment and do not represent a change in the underlying disease. There was no evidence of a rebound effect 6 weeks after abrupt discontinuation of therapy.
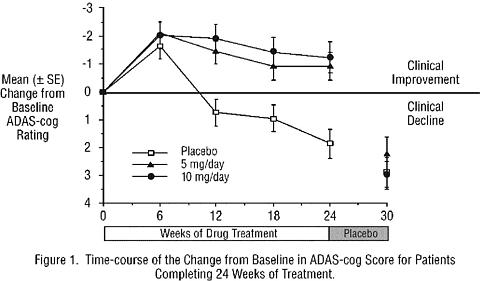
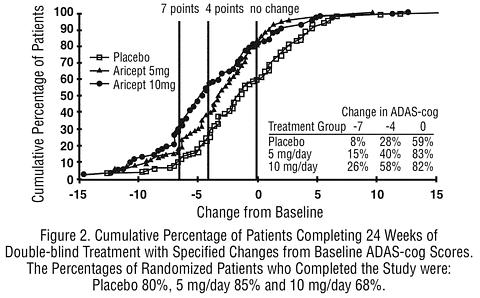
Figure 2 illustrates the cumulative percentages of patients from each of the three treatment groups who had attained the measure of improvement in ADAS-cog score shown on the X axis. Three change scores, (7-point and 4-point reductions from baseline or no change in score) have been identified for illustrative purposes and the percent of patients in each group achieving that result is shown in the inset table.
The curves demonstrate that both patients assigned to placebo and ARICEPT® have a wide range of responses, but that the active treatment groups are more likely to show the greater improvements. A curve for an effective treatment would be shifted to the left of the curve for placebo, while an ineffective or deleterious treatment would be superimposed upon or shifted to the right of the curve for placebo, respectively.
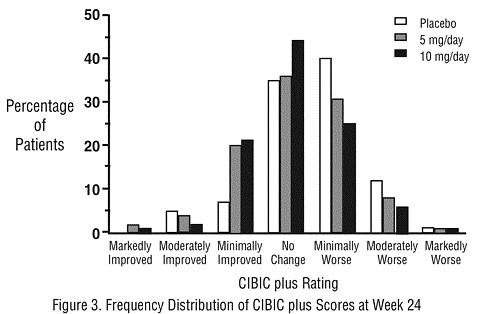
Effects on the CIBIC plus: Figure 3 is a histogram of the frequency distribution of CIBIC plus scores attained by patients assigned to each of the three treatment groups who completed 24 weeks of treatment. The mean drug-placebo differences for these groups of patients were 0.35 units and 0.39 units for 5 mg/day and 10 mg/day of ARICEPT®, respectively. These differences were statistically significant. There was no statistically significant difference between the two active treatments.
Fifteen-Week Study
In a study of 15 weeks duration, patients were randomized to receive single daily doses of placebo or either 5 mg/day or 10 mg/day of ARICEPT® for 12 weeks, followed by a 3-week placebo washout period. As in the 30-week study, to avoid acute cholinergic effects, the 10 mg/day treatment followed an initial 7-day treatment with 5 mg/day doses.
Effects on the ADAS-Cog: Figure 4 illustrates the time course of the change from baseline in ADAS-cog scores for all three dose groups over the 15 weeks of the study. After 12 weeks of treatment, the differences in mean ADAS-cog change scores for the ARICEPT® treated patients compared to the patients on placebo were 2.7 and 3.0 units each, for the 5 and 10 mg/day ARICEPT® treatment groups respectively. These differences were statistically significant. The effect size for the 10 mg/day group may appear to be slightly larger than that for 5 mg/day. However, the differences between active treatments were not statistically significant.
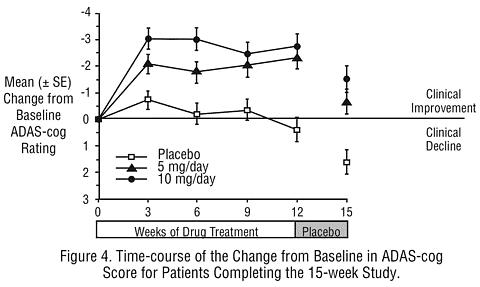
Following 3 weeks of placebo washout, scores on the ADAS-cog for both the ARICEPT® treatment groups increased, indicating that discontinuation of ARICEPT® resulted in a loss of its treatment effect. The duration of this placebo washout period was not sufficient to characterize the rate of loss of the treatment effect, but, the 30-week study (see above) demonstrated that treatment effects associated with the use of ARICEPT® abate within 6 weeks of treatment discontinuation.
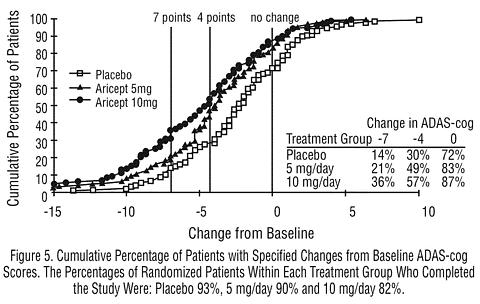
Figure 5 illustrates the cumulative percentages of patients from each of the three treatment groups who attained the measure of improvement in ADAS-cog score shown on the X axis. The same three change scores, (7-point and 4-point reductions from baseline or no change in score) as selected for the 30-week study have been used for this illustration. The percentages of patients achieving those results are shown in the inset table.
As observed in the 30-week study, the curves demonstrate that patients assigned to either placebo or to ARICEPT® have a wide range of responses, but that the ARICEPT® treated patients are more likely to show the greater improvements in cognitive performance.
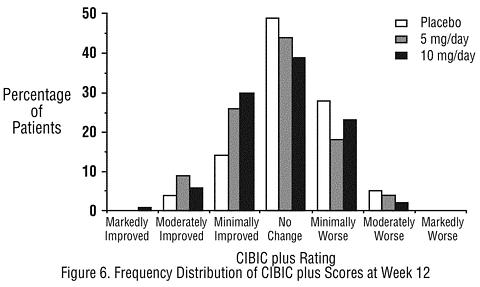
Effects on the CIBIC plus: Figure 6 is a histogram of the frequency distribution of CIBIC plus scores attained by patients assigned to each of the three treatment groups who completed 12 weeks of treatment. The differences in mean scores for ARICEPT® treated patients compared to the patients on placebo at Week 12 were 0.36 and 0.38 units for the 5 mg/day and 10 mg/day treatment groups, respectively. These differences were statistically significant.
In both studies, patient age, sex and race were not found to predict the clinical outcome of ARICEPT® treatment.
Clinical Pharmacokinetics
ARICEPT® ODT is bioequivalent to ARICEPT® Tablets. Donepezil is well absorbed with a relative oral bioavailability of 100% and reaches peak plasma concentrations in 3 to 4 hours. Pharmacokinetics are linear over a dose range of 1-10 mg given once daily. Neither food nor time of administration (morning vs. evening dose) influences the rate or extent of absorption of ARICEPT® Tablets. A food effect study has not been conducted with ARICEPT® ODT; however, the effect of food with ARICEPT® ODT is expected to be minimal. ARICEPT® ODT can be taken without regard to meals.
The elimination half life of donepezil is about 70 hours and the mean apparent plasma clearance (Cl/F) is 0.13 L/hr/kg. Following multiple dose administration, donepezil accumulates in plasma by 4-7 fold and steady state is reached within 15 days. The steady state volume of distribution is 12 L/kg. Donepezil is approximately 96% bound to human plasma proteins, mainly to albumins (about 75%) and alpha 1 -acid glycoprotein (about 21%) over the concentration range of 2-1000 ng/mL.
Donepezil is both excreted in the urine intact and extensively metabolized to four major metabolites, two of which are known to be active, and a number of minor metabolites, not all of which have been identified. Donepezil is metabolized by CYP 450 isoenzymes 2D6 and 3A4 and undergoes glucuronidation. Following administration of 14 C-labeled donepezil, plasma radioactivity, expressed as a percent of the administered dose, was present primarily as intact donepezil (53%) and as 6-O-desmethyl donepezil (11%), which has been reported to inhibit AChE to the same extent as donepezil in vitro and was found in plasma at concentrations equal to about 20% of donepezil. Approximately 57% and 15% of the total radioactivity was recovered in urine and feces, respectively, over a period of 10 days, while 28% remained unrecovered, with about 17% of the donepezil dose recovered in the urine as unchanged drug.
Special Populations:
Hepatic Disease: In a study of 10 patients with stable alcoholic cirrhosis, the clearance of ARICEPT® was decreased by 20% relative to 10 healthy age and sex matched subjects.
Renal Disease: In a study of 11 patients with moderate to severe renal impairment (Cl Cr < 18 mL/min/1.73 m 2 ) the clearance of ARICEPT® did not differ from 11 age and sex matched healthy subjects.
Age: No formal pharmacokinetic study was conducted to examine age related differences in the pharmacokinetics of ARICEPT®. However, mean plasma ARICEPT® concentrations measured during therapeutic drug monitoring of elderly patients with Alzheimer's Disease are comparable to those observed in young healthy volunteers.
Gender and Race: No specific pharmacokinetic study was conducted to investigate the effects of gender and race on the disposition of ARICEPT®. However, retrospective pharmacokinetic analysis indicates that gender and race (Japanese and Caucasians) did not affect the clearance of ARICEPT®.
Drug-Drug Interactions
Drugs Highly Bound to Plasma Proteins: Drug displacement studies have been performed in vitro between this highly bound drug (96%) and other drugs such as furosemide, digoxin, and warfarin. ARICEPT® at concentrations of 0.3-10 µg/mL did not affect the binding of furosemide (5 µg/mL), digoxin (2 ng/mL), and warfarin (3 µg/mL) to human albumin. Similarly, the binding of ARICEPT® to human albumin was not affected by furosemide, digoxin and warfarin.
Effect of ARICEPT® on the Metabolism of Other Drugs: No in vivo clinical trials have investigated the effect of ARICEPT® on the clearance of drugs metabolized by CYP 3A4 (e.g. cisapride, terfenadine) or by CYP 2D6 (e.g. imipramine). However, in vitro studies show a low rate of binding to these enzymes (mean K i about 50-130 µM), that, given the therapeutic plasma concentrations of donepezil (164 nM), indicates little likelihood of interference.
Whether ARICEPT® has any potential for enzyme induction is not known.
Formal pharmacokinetic studies evaluated the potential of ARICEPT® for interaction with theophylline, cimetidine, warfarin, digoxin and ketoconazole. No effects of ARICEPT® on the pharmacokinetics of these drugs were observed.
Effect of Other Drugs on the Metabolism of ARICEPT®: Ketoconazole and quinidine, inhibitors of CYP450, 3A4 and 2D6, respectively, inhibit donepezil metabolism in vitro . Whether there is a clinical effect of quinidine is not known. In a 7-day crossover study in 18 healthy volunteers, ketoconazole (200mg q.d.) increased mean donepezil (5mg q.d.) concentrations (AUC 0-24 and C max ) by 36%. The clinical relevance of this increase in concentration is unknown.
Inducers of CYP 2D6 and CYP 3A4 (e.g., phenytoin, carbamazepine, dexamethasone, rifampin, and phenobarbital) could increase the rate of elimination of ARICEPT®.
Formal pharmacokinetic studies demonstrated that the metabolism of ARICEPT® is not significantly affected by concurrent administration of digoxin or cimetidine.
INDICATIONS AND USAGE
ARICEPT® is indicated for the treatment of mild to moderate dementia of the Alzheimer's type.
CONTRAINDICATIONS
ARICEPT® is contraindicated in patients with known hypersensitivity to donepezil hydrochloride or to piperidine derivatives.
WARNINGS
Anesthesia: ARICEPT®, as a cholinesterase inhibitor, is likely to exaggerate succinylcholine-type muscle relaxation during anesthesia.
Cardiovascular Conditions: Because of their pharmacological action, cholinesterase inhibitors may have vagotonic effects on the sinoatrial and atrioventricular nodes. This effect may manifest as bradycardia or heart block in patients both with and without known underlying cardiac conduction abnormalities. Syncopal episodes have been reported in association with the use of ARICEPT®.
Gastrointestinal Conditions: Through their primary action, cholinesterase inhibitors may be expected to increase gastric acid secretion due to increased cholinergic activity. Therefore, patients should be monitored closely for symptoms of active or occult gastrointestinal bleeding, especially those at increased risk for developing ulcers, e.g., those with a history of ulcer disease or those receiving concurrent nonsteroidal anti-inflammatory drugs (NSAIDS). Clinical studies of ARICEPT® have shown no increase, relative to placebo, in the incidence of either peptic ulcer disease or gastrointestinal bleeding.
ARICEPT®, as a predictable consequence of its pharmacological properties, has been shown to produce diarrhea, nausea and vomiting. These effects, when they occur, appear more frequently with the 10 mg/day dose than with the 5 mg/day dose. In most cases, these effects have been mild and transient, sometimes lasting one to three weeks, and have resolved during continued use of ARICEPT®.
Genitourinary: Although not observed in clinical trials of ARICEPT®, cholinomimetics may cause bladder outflow obstruction.
Neurological Conditions: Seizures: Cholinomimetics are believed to have some potential to cause generalized convulsions. However, seizure activity also may be a manifestation of Alzheimer's Disease.
Pulmonary Conditions: Because of their cholinomimetic actions, cholinesterase inhibitors should be prescribed with care to patients with a history of asthma or obstructive pulmonary disease.
PRECAUTIONS
Drug-Drug Interactions (see Clinical Pharmacology : Clinical Pharmacokinetics : Drug-drug Interactions )
Effect of ARICEPT® on the Metabolism of Other Drugs: No in vivo clinical trials have investigated the effect of ARICEPT® on the clearance of drugs metabolized by CYP 3A4 (e.g. cisapride, terfenadine) or by CYP 2D6 (e.g. imipramine). However, in vitro studies show a low rate of binding to these enzymes (mean K i about 50-130 µM), that, given the therapeutic plasma concentrations of donepezil (164 nM), indicates little likelihood of interference.
Whether ARICEPT® has any potential for enzyme induction is not known.
Formal pharmacokinetic studies evaluated the potential of ARICEPT® for interaction with theophylline, cimetidine, warfarin, digoxin and ketoconazole. No effects of ARICEPT® on the pharmacokinetics of these drugs were observed.
Effect of Other Drugs on the Metabolism of ARICEPT®: Ketoconazole and quinidine, inhibitors of CYP450, 3A4 and 2D6, respectively, inhibit donepezil metabolism in vitro . Whether there is a clinical effect of quinidine is not known. In a 7-day crossover study in 18 healthy volunteers, ketoconazole (200mg q.d.) increased mean donepezil (5mg q.d.) concentrations (AUC 0-24 and C max ) by 36%. The clinical relevance of this increase in concentration is unknown.
Inducers of CYP 2D6 and CYP 3A4 (e.g., phenytoin, carbamazepine, dexamethasone, rifampin, and phenobarbital) could increase the rate of elimination of ARICEPT®.
Formal pharmacokinetic studies demonstrated that the metabolism of ARICEPT® is not significantly affected by concurrent administration of digoxin or cimetidine.
Use with Anticholinergics: Because of their mechanism of action, cholinesterase inhibitors have the potential to interfere with the activity of anticholinergic medications.
Use with Cholinomimetics and Other Cholinesterase Inhibitors: A synergistic effect may be expected when cholinesterase inhibitors are given concurrently with succinylcholine, similar neuromuscular blocking agents or cholinergic agonists such as bethanechol.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of a carcinogenic potential was obtained in an 88-week carcinogenicity study of donepezil hydrochloride conducted in CD-1 mice at doses up to 180 mg/kg/day (approximately 90 times the maximum recommended human dose on a mg/m 2 basis), or in a 104-week carcinogenicity study in Sprague-Dawley rats at doses up to 30 mg/kg/day (approximately 30 times the maximum recommended human dose on a mg/m 2 basis).
Donepezil was not mutagenic in the Ames reverse mutation assay in bacteria, or in a mouse lymphoma forward mutation assay in vitro . In the chromosome aberration test in cultures of Chinese hamster lung (CHL) cells, some clastogenic effects were observed. Donepezil was not clastogenic in the in vivo mouse micronucleus test and was not genotoxic in an in vivo unscheduled DNA synthesis assay in rats.
Donepezil had no effect on fertility in rats at doses up to 10 mg/kg/day (approximately 8 times the maximum recommended human dose on a mg/m 2 basis).
Pregnancy
Pregnancy Category C: Teratology studies conducted in pregnant rats at doses up to 16 mg/kg/day (approximately 13 times the maximum recommended human dose on a mg/m 2 basis) and in pregnant rabbits at doses up to 10 mg/kg/day (approximately 16 times the maximum recommended human dose on a mg/m 2 basis) did not disclose any evidence for a teratogenic potential of donepezil. However, in a study in which pregnant rats were given up to 10 mg/kg/day (approximately 8 times the maximum recommended human dose on a mg/m 2 basis) from day 17 of gestation through day 20 postpartum, there was a slight increase in still births and a slight decrease in pup survival through day 4 postpartum at this dose; the next lower dose tested was 3 mg/kg/day. There are no adequate or well-controlled studies in pregnant women. ARICEPT® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether donepezil is excreted in human breast milk. ARICEPT® has no indication for use in nursing mothers.
Pediatric Use
There are no adequate and well-controlled trials to document the safety and efficacy of ARICEPT® in any illness occurring in children.
Geriatric Use
Alzheimer's disease is a disorder occurring primarily in individuals over 55 years of age. The mean age of patients enrolled in the clinical studies with ARICEPT® was 73 years; 80% of these patients were between 65 and 84 years old and 49% of patients were at or above the age of 75. The efficacy and safety data presented in the clinical trials section were obtained from these patients. There were no clinically significant differences in most adverse events reported by patient groups >/= 65 years old and < 65 years old.
ADVERSE REACTIONS
Adverse Events Leading to Discontinuation
The rates of discontinuation from controlled clinical trials of ARICEPT® due to adverse events for the ARICEPT® 5 mg/day treatment groups were comparable to those of placebo-treatment groups at approximately 5%. The rate of discontinuation of patients who received 7-day escalations from 5 mg/day to 10 mg/day, was higher at 13%.
The most common adverse events leading to discontinuation, defined as those occurring in at least 2% of patients and at twice the incidence seen in placebo patients, are shown in Table 1.
Table 1. Most Frequent Adverse Events Leading
to Withdrawal from Controlled Clinical Trials
by Dose GroupDose GroupPlacebo 5 mg/day
ARICEPT®10 mg/day
ARICEPT®Patients Randomized355 350 315 Event/% DiscontinuingNausea1% 1% 3% Diarrhea0% <1% 3% Vomiting<1% <1% 2% Most Frequent Adverse Clinical Events Seen in Association with the Use of ARICEPT®
The most common adverse events, defined as those occurring at a frequency of at least 5% in patients receiving 10 mg/day and twice the placebo rate, are largely predicted by ARICEPT®'s cholinomimetic effects. These include nausea, diarrhea, insomnia, vomiting, muscle cramp, fatigue and anorexia. These adverse events were often of mild intensity and transient, resolving during contin-ued ARICEPT® treatment without the need for dose modification.
There is evidence to suggest that the frequency of these common adverse events may be affected by the rate of titration. An open-label study was conducted with 269 patients who received placebo in the 15 and 30-week studies. These patients were titrated to a dose of 10 mg/day over a 6-week period. The rates of common adverse events were lower than those seen in patients titrated to 10 mg/day over one week in the controlled clinical trials and were comparable to those seen in patients on 5 mg/day.
See Table 2 for a comparison of the most common adverse events following one and six week titration regimens.
Table 2. Comparison of rates of adverse events in patients
titrated to 10 mg/day over 1 and 6 weeksNo titration One week titration Six week titration Adverse EventPlacebo (n=315) 5 mg/day (n=311) 10 mg/day (n=315) 10 mg/day (n=269) Nausea6% 5% 19% 6% Diarrhea5% 8% 15% 9% Insomnia6% 6% 14% 6% Fatigue3% 4% 8% 3% Vomiting3% 3% 8% 5% Muscle cramps2% 6% 8% 3% Anorexia2% 3% 7% 3% Adverse Events Reported in Controlled Trials
The events cited reflect experience gained under closely monitored conditions of clinical trials in a highly selected patient population. In actual clinical practice or in other clinical trials, these frequency estimates may not apply, as the conditions of use, reporting behavior, and the kinds of patients treated may differ. Table 3 lists treatment emergent signs and symptoms that were reported in at least 2% of patients in placebo-controlled trials who received ARICEPT® and for which the rate of occurrence was greater for ARICEPT® assigned than placebo assigned patients. In general, adverse events occurred more frequently in female patients and with advancing age.
Table 3. Adverse Events Reported in Controlled Clinical
Trials in at Least 2% of Patients Receiving ARICEPT®
and at a Higher Frequency than Placebo-treated PatientsBody System/
Adverse EventPlacebo
(n=355)ARICEPT®
(n=747)Percent of Patients
with any Adverse Event72 74 Body as a WholeHeadache9 10 Pain, various locations8 9 Accident6 7 Fatigue3 5 Cardiovascular SystemSyncope1 2 Digestive SystemNausea6 11 Diarrhea5 10 Vomiting3 5 Anorexia2 4 Hemic and Lymphatic SystemEcchymosis3 4 Metabolic and Nutritional SystemsWeight Decrease1 3 Musculoskeletal SystemMuscle Cramps2 6 Arthritis1 2 Nervous SystemInsomnia6 9 Dizziness6 8 Depression<1 3 Abnormal Dreams0 3 Somnolence<1 2 Urogenital SystemFrequent Urination1 2 Other Adverse Events Observed During Clinical Trials
ARICEPT® has been administered to over 1700 individuals during clinical trials worldwide. Approximately 1200 of these patients have been treated for at least 3 months and more than 1000 patients have been treated for at least 6 months. Controlled and uncontrolled trials in the United States included approximately 900 patients. In regards to the highest dose of 10 mg/day, this population includes 650 patients treated for 3 months, 475 patients treated for 6 months and 116 patients treated for over 1 year. The range of patient exposure is from 1 to 1214 days.
Treatment emergent signs and symptoms that occurred during 3 controlled clinical trials and two open-label trials in the United States were recorded as adverse events by the clinical investigators using terminology of their own choosing. To provide an overall estimate of the proportion of individuals having similar types of events, the events were grouped into a smaller number of standardized categories using a modified COSTART dictionary and event frequencies were calculated across all studies. These categories are used in the listing below. The frequencies represent the proportion of 900 patients from these trials who experienced that event while receiving ARICEPT®. All adverse events occurring at least twice are included, except for those already listed in Tables 2 or 3, COSTART terms too general to be informative, or events less likely to be drug caused. Events are classified by body system and listed using the following definitions: frequent adverse events - those occurring in at least 1/100 patients; infrequent adverse events - those occurring in 1/100 to 1/1000 patients. These adverse events are not necessarily related to ARICEPT® treatment and in most cases were observed at a similar frequency in placebo-treated patients in the controlled studies. No important additional adverse events were seen in studies conducted outside the United States.
Body as a Whole: Frequent: influenza, chest pain, toothache; Infrequent: fever, edema face, periorbital edema, hernia hiatal, abscess, cellulitis, chills, generalized coldness, head fullness, listlessness.
Cardiovascular System: Frequent: hypertension, vasodilation, atrial fibrillation, hot flashes, hypotension; Infrequent: angina pectoris, postural hypotension, myocardial infarction, AV block (first degree), congestive heart failure, arteritis, bradycardia, peripheral vascular disease, supraventricular tachycardia, deep vein thrombosis.
Digestive System: Frequent: fecal incontinence, gastrointestinal bleeding, bloating, epigastric pain; Infrequent: eructation, gingivitis, increased appetite, flatulence, periodontal abscess, cholelithiasis, diverticulitis, drooling, dry mouth, fever sore, gastritis, irritable colon, tongue edema, epigastric distress, gastroenteritis, increased transaminases, hemorrhoids, ileus, increased thirst, jaundice, melena, polydipsia, duodenal ulcer, stomach ulcer.
Endocrine System: Infrequent: diabetes mellitus, goiter.
Hemic and Lymphatic System: Infrequent: anemia, thrombocythemia, thrombocytopenia, eosinophilia, erythrocytopenia.
Metabolic and Nutritional Disorders: Frequent: dehydration; Infrequent: gout, hypokalemia, increased creatine kinase, hyperglycemia, weight increase, increased lactate dehydrogenase.
Musculoskeletal System: Frequent: bone fracture; Infrequent: muscle weakness, muscle fasciculation.
Nervous System: Frequent: delusions, tremor, irritability, paresthesia, aggression, vertigo, ataxia, increased libido, restlessness, abnormal crying, nervousness, aphasia; Infrequent: cerebrovascular accident, intracranial hemorrhage, transient ischemic attack, emotional lability, neuralgia, coldness (localized), muscle spasm, dysphoria, gait abnormality, hypertonia, hypokinesia, neurodermatitis, numbness (localized), paranoia, dysarthria, dysphasia, hostility, decreased libido, melancholia, emotional withdrawal, nystagmus, pacing.
Respiratory System: Frequent: dyspnea, sore throat, bronchitis; Infrequent: epistaxis, post nasal drip, pneumonia, hyperventilation, pulmonary congestion, wheezing, hypoxia, pharyngitis, pleurisy, pulmonary collapse, sleep apnea, snoring.
Skin and Appendages: Frequent: pruritus, diaphoresis, urticaria; Infrequent: dermatitis, erythema, skin discoloration, hyperkeratosis, alopecia, fungal dermatitis, herpes zoster, hirsutism, skin striae, night sweats, skin ulcer.
Special Senses: Frequent: cataract, eye irritation, vision blurred; Infrequent: dry eyes, glaucoma, earache, tinnitus, blepharitis, decreased hearing, retinal hemorrhage, otitis externa, otitis media, bad taste, conjunctival hemorrhage, ear buzzing, motion sickness, spots before eyes.
Urogenital System: Frequent: urinary incontinence, nocturia; Infrequent: dysuria, hematuria, urinary urgency, metrorrhagia, cystitis, enuresis, prostate hypertrophy, pyelonephritis, inability to empty bladder, breast fibroadenosis, fibrocystic breast, mastitis, pyuria, renal failure, vaginitis.
Postintroduction Reports
Voluntary reports of adverse events temporally associated with ARICEPT® that have been received since market introduction that are not listed above, and that there is inadequate data to determine the causal relationship with the drug include the following: abdominal pain, agitation, cholecystitis, confusion, convulsions, hallucinations, heart block (all types), hemolytic anemia, hepatitis, hyponatremia, neuroleptic malignant syndrome, pancreatitis, and rash.
OVERDOSAGE
Because strategies for the management of overdose are continually evolving, it is advisable to contact a Poison Control Center to determine the latest recommendations for the management of an overdose of any drug.
As in any case of overdose, general supportive measures should be utilized. Overdosage with cholinesterase inhibitors can result in cholinergic crisis characterized by severe nausea, vomiting, salivation, sweating, bradycardia, hypotension, respiratory depression, collapse and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Tertiary anticholinergics such as atropine may be used as an antidote for ARICEPT® overdosage. Intravenous atropine sulfate titrated to effect is recommended: an initial dose of 1.0 to 2.0 mg IV with subsequent doses based upon clinical response. Atypical responses in blood pressure and heart rate have been reported with other cholinomimetics when co-administered with quaternary anticholinergics such as glycopyrrolate. It is not known whether ARICEPT® and/or its metabolites can be removed by dialysis (hemodialysis, peritoneal dialysis, or hemofiltration).
Dose-related signs of toxicity in animals included reduced spontaneous movement, prone position, staggering gait, lacrimation, clonic convulsions, depressed respiration, salivation, miosis, tremors, fasciculation and lower body surface temperature.
DOSAGE AND ADMINISTRATION
The dosages of ARICEPT® shown to be effective in controlled clinical trials are 5 mg and 10 mg administered once per day.
The higher dose of 10 mg did not provide a statistically significantly greater clinical benefit than 5 mg. There is a suggestion, however, based upon order of group mean scores and dose trend analyses of data from these clinical trials, that a daily dose of 10 mg of ARICEPT® might provide additional benefit for some patients. Accordingly, whether or not to employ a dose of 10 mg is a matter of prescriber and patient preference.
Evidence from the controlled trials indicates that the 10 mg dose, with a one week titration, is likely to be associated with a higher incidence of cholinergic adverse events than the 5 mg dose. In open label trials using a 6 week titration, the frequency of these same adverse events was similar between the 5 mg and 10 mg dose groups. Therefore, because steady state is not achieved for 15 days and because the incidence of untoward effects may be influenced by the rate of dose escalation, treatment with a dose of 10 mg should not be contemplated until patients have been on a daily dose of 5 mg for 4 to 6 weeks.
ARICEPT® should be taken in the evening, just prior to retiring. ARICEPT® can be taken with or without food.
Allow ARICEPT® ODT tablet to dissolve on the tongue and follow with water.
HOW SUPPLIED
ARICEPT® is supplied as film-coated, round tablets containing either 5 mg or 10 mg of donepezil hydrochloride.
The 5 mg tablets are white. The strength, in mg (5), is debossed on one side and ARICEPT is debossed on the other side.
The 10 mg tablets are yellow. The strength, in mg (10), is debossed on one side and ARICEPT is debossed on the other side.
5 mg (White) Bottles of 30 (NDC# 62856-245-30)
Bottles of 90 (NDC# 62856-245-90)
Unit Dose Blister Package 100 (10 × 10)
(NDC# 62856-245-41)
10 mg (Yellow) Bottles of 30 (NDC# 62856-246-30)
Bottles of 90 (NDC# 62856-246-90)
Unit Dose Blister Package 100 (10 × 10)
(NDC# 62856-246-41)
ARICEPT® ODT is supplied as tablets containing either 5 mg or 10 mg of donepezil hydrochloride.
The 5 mg orally disintegrating tablets are white. The strength, in mg (5), is embossed on one side and ARICEPT is embossed on the other side.
The 10 mg orally disintegrating tablets are yellow. The strength, in mg (10), is embossed on one side and ARICEPT is embossed on the other side.
5 mg (White) Unit Dose Blister Package 30 (10 × 3)
(NDC# 62856-831-30)
10 mg (Yellow)Unit Dose Blister Package 30 (10 × 3)
(NDC# 62856-832-30)
Storage: Store at controlled room temperature, 15°C to 30°C (59°F to 86°F).
Rx only
ARICEPT® is a registered trademark of Eisai Co., Ltd.
Manufactured and Marketed by Eisai Inc., Teaneck, NJ 07666
Marketed by
Pfizer Inc, New York, NY 10017
© 2005 Eisai Inc.
Printed in U.S.A.
200430 Revised March 2005
Subscribe to the "News" RSS Feed 
TOP ۞
