-
Aromasin Tablets (Pharmacia & Upjohn)
DESCRIPTION
AROMASIN® Tablets for oral administration contain 25 mg of exemestane, an irreversible, steroidal aromatase inactivator. Exemestane is chemically described as 6-methyl-enandrosta-1,4-diene-3,17-dione. Its molecular formula is C 20 H 24 0 2 and its structural formula is as follows:
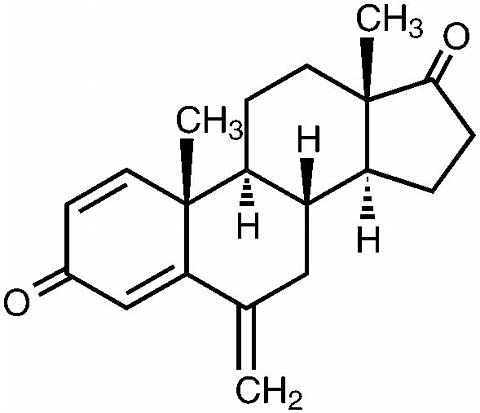
The active ingredient is a white to slightly yellow crystalline powder with a molecular weight of 296.41. Exemestane is freely soluble in N, N-dimethylformamide, soluble in methanol, and practically insoluble in water.
Each AROMASIN Tablet contains the following inactive ingredients: mannitol, crospovidone, polysorbate 80, hypromellose, colloidal silicon dioxide, microcrystalline cellulose, sodium starch glycolate, magnesium stearate, simethicone, polyethylene glycol 6000, sucrose, magnesium carbonate, titanium dioxide, methylparaben, and polyvinyl alcohol.
CLINICAL PHARMACOLOGY
Mechanism of Action
Breast cancer cell growth may be estrogen-dependent. Aromatase is the principal enzyme that converts androgens to estrogens both in pre- and postmenopausal women. While the main source of estrogen (primarily estradiol) is the ovary in premenopausal women, the principal source of circulating estrogens in postmenopausal women is from conversion of adrenal and ovarian androgens (androstenedione and testosterone) to estrogens (estrone and estradiol) by the aromatase enzyme in peripheral tissues. Estrogen deprivation through aromatase inhibition is an effective and selective treatment for some postmenopausal patients with hormone-dependent breast cancer.
Exemestane is an irreversible, steroidal aromatase inactivator, structurally related to the natural substrate androstenedione. It acts as a false substrate for the aromatase enzyme, and is processed to an intermediate that binds irreversibly to the active site of the enzyme causing its inactivation, an effect also known as "suicide inhibition." Exemestane significantly lowers circulating estrogen concentrations in postmenopausal women, but has no detectable effect on adrenal biosynthesis of corticosteroids or aldosterone. Exemestane has no effect on other enzymes involved in the steroidogenic pathway up to a concentration at least 600 times higher than that inhibiting the aromatase enzyme.
Pharmacokinetics
Following oral administration to healthy postmenopausal women, exemestane is rapidly absorbed. After maximum plasma concentration is reached, levels decline polyexponentially with a mean terminal half-life of about 24 hours. Exemestane is extensively distributed and is cleared from the systemic circulation primarily by metabolism. The pharmacokinetics of exemestane are dose proportional after single (10 to 200 mg) or repeated oral doses (0.5 to 50 mg). Following repeated daily doses of exemestane 25 mg, plasma concentrations of unchanged drug are similar to levels measured after a single dose.
Pharmacokinetic parameters in postmenopausal women with advanced breast cancer following single or repeated doses have been compared with those in healthy, postmenopausal women. Exemestane appeared to be more rapidly absorbed in the women with breast cancer than in the healthy women, with a mean t max of 1.2 hours in the women with breast cancer and 2.9 hours in the healthy women. After repeated dosing, the average oral clearance in women with advanced breast cancer was 45% lower than the oral clearance in healthy postmenopausal women, with corresponding higher systemic exposure. Mean AUC values following repeated doses in women with breast cancer (75.4 ng·h/mL) were about twice those in healthy women (41.4 ng·h/mL).
Absorption: Following oral administration of radiolabeled exemestane, at least 42% of radioactivity was absorbed from the gastrointestinal tract. Exemestane plasma levels increased by approximately 40% after a high-fat breakfast.
Distribution : Exemestane is distributed extensively into tissues. Exemestane is 90% bound to plasma proteins and the fraction bound is independent of the total concentration. Albumin and (alpha) 1 -acid glycoprotein both contribute to the binding. The distribution of exemestane and its metabolites into blood cells is negligible.
Metabolism and Excretion: Following administration of radiolabeled exemestane to healthy postmenopausal women, the cumulative amounts of radioactivity excreted in urine and feces were similar (42 ± 3% in urine and 42 ± 6% in feces over a 1-week collection period). The amount of drug excreted unchanged in urine was less than 1% of the dose. Exemestane is extensively metabolized, with levels of the unchanged drug in plasma accounting for less than 10% of the total radioactivity. The initial steps in the metabolism of exemestane are oxidation of the methylene group in position 6 and reduction of the 17-keto group with subsequent formation of many secondary metabolites. Each metabolite accounts only for a limited amount of drug-related material. The metabolites are inactive or inhibit aromatase with decreased potency compared with the parent drug. One metabolite may have androgenic activity (see Pharmacodynamics , Other Endocrine Effects ). Studies using human liver preparations indicate that cytochrome P-450 3A4 (CYP 3A4) is the principal isoenzyme involved in the oxidation of exemestane.
Special Populations
Geriatric: Healthy postmenopausal women aged 43 to 68 years were studied in the pharmacokinetic trials. Age-related alterations in exemestane pharmacokinetics were not seen over this age range.
Gender: The pharmacokinetics of exemestane following administration of a single, 25-mg tablet to fasted healthy males (mean age 32 years) were similar to the pharmacokinetics of exemestane in fasted healthy postmenopausal women (mean age 55 years).
Race: The influence of race on exemestane pharmacokinetics has not been evaluated.
Hepatic Insufficiency: The pharmacokinetics of exemestane have been investigated in subjects with moderate or severe hepatic insufficiency (Childs-Pugh B or C). Following a single 25-mg oral dose, the AUC of exemestane was approximately 3 times higher than that observed in healthy volunteers (see PRECAUTIONS ).
Renal Insufficiency: The AUC of exemestane after a single 25-mg dose was approximately 3 times higher in subjects with moderate or severe renal insufficiency (creatinine clearance <35 mL/min/1.73 m 2 ) compared with the AUC in healthy volunteers (see PRECAUTIONS ).
Pediatric: The pharmacokinetics of exemestane have not been studied in pediatric patients.
Drug-Drug Interactions
Exemestane is metabolized by cytochrome P-450 3A4 (CYP 3A4) and aldoketoreductases. It does not inhibit any of the major CYP isoenzymes, including CYP 1A2, 2C9, 2D6, 2E1, and 3A4. In a clinical pharmacokinetic study, ketoconazole showed no significant influence on the pharmacokinetics of exemestane. Although no other formal drug-drug interaction studies have been conducted, significant effects on exemestane clearance by CYP isoenzymes inhibitors appear unlikely. In a pharmacokinetic interaction study of 10 healthy postmenopausal volunteers pretreated with potent CYP 3A4 inducer rifampicin 600 mg daily for 14 days followed by a single dose of exemestane 25 mg, the mean plasma C max and AUC 0-(infinity) of exemestane were decreased by 41% and 54%, respectively (see PRECAUTIONS and DOSAGE AND ADMINISTRATION ).
Pharmacodynamics
Effect on Estrogens: Multiple doses of exemestane ranging from 0.5 to 600 mg/day were administered to postmenopausal women with advanced breast cancer. Plasma estrogen (estradiol, estrone, and estrone sulfate) suppression was seen starting at a 5-mg daily dose of exemestane, with a maximum suppression of at least 85% to 95% achieved at a 25-mg dose. Exemestane 25 mg daily reduced whole body aromatization (as measured by injecting radiolabeled androstenedione) by 98% in postmenopausal women with breast cancer. After a single dose of exemestane 25 mg, the maximal suppression of circulating estrogens occurred 2 to 3 days after dosing and persisted for 4 to 5 days.
Effect on Corticosteroids: In multiple-dose trials of doses up to 200 mg daily, exemestane selectivity was assessed by examining its effect on adrenal steroids. Exemestane did not affect cortisol or aldosterone secretion at baseline or in response to ACTH at any dose. Thus, no glucocorticoid or mineralocorticoid replacement therapy is necessary with exemestane treatment.
Other Endocrine Effects: Exemestane does not bind significantly to steroidal receptors, except for a slight affinity for the androgen receptor (0.28% relative to dihydrotestosterone). The binding affinity of its 17-dihydrometabolite for the androgen receptor, however, is 100-times that of the parent compound. Daily doses of exemestane up to 25 mg had no significant effect on circulating levels of testosterone, androstenedione, dehydroepiandrosterone sulfate, or 17-hydroxyprogesterone. Increases in testosterone and androstenedione levels have been observed at daily doses of 200 mg or more. A dose-dependent decrease in sex hormone binding globulin (SHBG) has been observed with daily exemestane doses of 2.5 mg or higher. Slight, nondose-dependent increases in serum lutenizing hormone (LH) and follicle-stimulating hormone (FSH) levels have been observed even at low doses as a consequence of feedback at the pituitary level.
CLINICAL STUDIES
Exemestane 25 mg administered once daily was evaluated in a randomized double-blind, multicenter, multinational comparative study and in two multicenter single-arm studies of postmenopausal women with advanced breast cancer who had disease progression after treatment with tamoxifen for metastatic disease or as adjuvant therapy. Some patients also have received prior cytotoxic therapy, either as adjuvant treatment or for metastatic disease.
The primary purpose of the three studies was evaluation of objective response rate (complete response [CR] and partial response [PR]). Time to tumor progression and overall survival were also assessed in the comparative trial. Response rates were assessed based on World Health Organization (WHO) criteria, and in the comparative study, were submitted to an external review committee that was blinded to patient treatment. In the comparative study, 769 patients were randomized to receive AROMASIN (exemestane tablets) 25 mg once daily (N = 366) or megestrol acetate 40 mg four times daily (N = 403). Demographics and baseline characteristics are presented in Table 1.
Table 1. Demographics and Baseline Characteristics from the Comparative Study of Postmenopausal
Women with Advanced Breast Cancer Whose Disease Had Progressed after Tamoxifen TherapyParameterAROMASIN
(N = 366)Megestrol Acetate
(N = 403)Median Age (range)65 (35-89) 65 (30-91) ECOG Performance Status0167 (46%) 187 (46%) 1162 (44%) 172 (43%) 234 (9%) 42 (10%) Receptor StatusER and/or PgR +246 (67%) 274 (68%) ER and PgR unknown116 (32%) 128 (32%) Responders to prior tamoxifen68 (19%) 85 (21%) NE for response to prior tamoxifen46 (13%) 41 (10%) Site of MetastasisVisceral ± other sites207 (57%) 239 (59%) Bone only61 (17%) 73 (18%) Soft tissue only54 (15%) 51 (13%) Bone & soft tissue43 (12%) 38 (9%) Measurable Disease287 (78%) 314 (78%) Prior Tamoxifen TherapyAdjuvant or Neoadjuvant145 (40%) 152 (38%) Advanced Disease, OutcomeCR, PR or SD>/= 6 months179 (49%) 210 (52%) SD< 6 months, PD or NE42 (12%) 41 (10%) Prior ChemotherapyFor advanced disease ± adjuvant58 (16%) 67 (17%) Adjuvant only104 (28%) 108 (27%) No chemotherapy203 (56%) 226 (56%)
The efficacy results from the comparative study are shown in Table 2. The objective response rates observed in the two treatment arms showed that AROMASIN was not different from megestrol acetate. Response rates for exemestane from the two single-arm trials were 23.4% and 28.1%.
Table 2. Efficacy Results from the Comparative Study of Postmenopausal Women with Advanced Breast Cancer Whose Disease Had Progressed after Tamoxifen Therapy Response CharacteristicsAROMASIN
(N=366)Megestrol
Acetate (N=403)Objective Response Rate = CR + PR (%)15.0 12.4 Difference in Response Rate ( AR - MA )2.6 95% C. I.7.5, -2.3 CR (%)2.2 1.2 PR (%)12.8 11.2 SD >/= 24 Weeks (%)21.3 21.1 Median Duration of Response (weeks)76.1 71.0 Median TTP (weeks)20.3 16.6 Hazard Ratio ( AR - MA )0.84 Abbreviations:CR = complete responsePR = partial responseSD = stable disease (no change)TTP = time to tumor progressionC. I. = confidence intervalMA = megestrol acetateAR = AROMASINThere were too few deaths occurring across treatment groups to draw conclusions on overall survival differences. The Kaplan-Meier curve for time to tumor progression in the comparative study is shown in Figure 1.
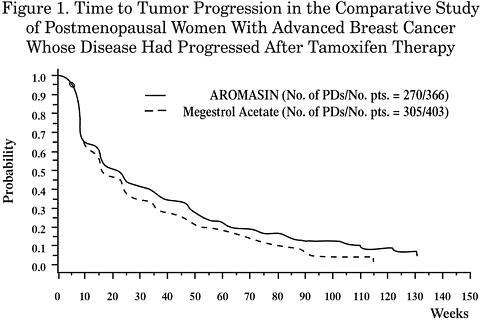
INDICATIONS AND USAGE
AROMASIN Tablets are indicated for the treatment of advanced breast cancer in postmenopausal women whose disease has progressed following tamoxifen therapy.
CONTRAINDICATIONS
AROMASIN Tablets are contraindicated in patients with a known hypersensitivity to the drug or to any of the excipients.
WARNINGS
AROMASIN Tablets may cause fetal harm when administered to a pregnant woman. Radioactivity related to 14 C-exemestane crossed the placenta of rats following oral administration of 1 mg/kg exemestane. The concentration of exemestane and its metabolites was approximately equivalent in maternal and fetal blood. When rats were administered exemestane from 14 days prior to mating until either days 15 or 20 of gestation, and resuming for the 21 days of lactation, an increase in placental weight was seen at 4 mg/kg/day (approximately 1.5 times the recommended human daily dose on a mg/m 2 basis). Prolonged gestation and abnormal or difficult labor was observed at doses equal to or greater than 20 mg/kg/day. Increased resorption, reduced number of live fetuses, decreased fetal weight, and retarded ossification were also observed at these doses. No malformations were noted when exemestane was administered to pregnant rats during the organogenesis period at doses up to 810 mg/kg/day (approximately 320 times the recommended human dose on a mg/m 2 basis). Daily doses of exemestane, given to rabbits during organogenesis caused a decrease in placental weight at 90 mg/kg/day (approximately 70 times the recommended human daily dose on a mg/m 2 basis). Abortions, an increase in resorptions, and a reduction in fetal body weight were seen at 270 mg/kg/day. There was no increase in the incidence of malformations in rabbits at doses up to 270 mg/kg/day (approximately 210 times the recommended human dose on a mg/m 2 basis).
There are no studies in pregnant women using AROMASIN. AROMASIN is indicated for postmenopausal women. If there is exposure to AROMASIN during pregnancy, the patient should be apprised of the potential hazard to the fetus and potential risk for loss of the pregnancy.
PRECAUTIONS
General. AROMASIN Tablets should not be administered to premenopausal women. AROMASIN should not be coadministered with estrogen-containing agents as these could interfere with its pharmacologic action.
Hepatic Insufficiency. The pharmacokinetics of exemestane have been investigated in subjects with moderate or severe hepatic insufficiency (Childs-Pugh B or C). Following a single 25-mg oral dose, the AUC of exemestane was approximately 3 times higher than that observed in healthy volunteers. The safety of chronic dosing in patients with moderate or severe hepatic impairment has not been studied. Based on experience with exemestane at repeated doses up to 200 mg daily that demonstrated a moderate increase in non-life threatening adverse events, dosage adjustment does not appear to be necessary.
Renal Insufficiency. The AUC of exemestane after a single 25-mg dose was approximately 3 times higher in subjects with moderate or severe renal insufficiency (creatinine clearance <35 mL/min/1.73 m 2 ) compared with the AUC in healthy volunteers. The safety of chronic dosing in patients with moderate or severe renal impairment has not been studied. Based on experience with exemestane at repeated doses up to 200 mg daily that demonstrated a moderate increase in non-life threatening adverse events, dosage adjustment does not appear to be necessary.
Laboratory Tests. Approximately 20% of patients receiving exemestane in clinical studies, experienced Common Toxicity Criteria (CTC) grade 3 or 4 lymphocytopenia. Of these patients, 89% had a pre-existing lower grade lymphopenia. Forty percent of patients either recovered or improved to a lesser severity while on treatment. Patients did not have a significant increase in viral infections, and no opportunistic infections were observed. Elevations of serum levels of AST, ALT, alkaline phosphatase and gamma glutamyl transferase > 5 times the upper value of the normal range (i.e., >/= CTC grade 3) have been rarely reported but appear mostly attributable to the underlying presence of liver and/or bone metastases. In the comparative study, CTC grade 3 or 4 elevation of gamma glutamyl transferase without documented evidence of liver metastasis was reported in 2.7% of patients treated with AROMASIN and in 1.8% of patients treated with megestrol acetate.
Drug Interactions. Exemestane is extensively metabolized by CYP 3A4, but coadministration of ketoconazole, a potent inhibitor of CYP 3A4, has no significant effect on exemestane pharmacokinetics. Significant pharmacokinetic interactions mediated by inhibition of CYP isoenzymes therefore appear unlikely. Co-medications that induce CYP 3A4 (e.g., rifampicin, phenytoin, carbamazepine, phenobarbital, or St. John's wort) may significantly decrease exposure to exemestane. Dose modification is recommended for patients who are also receiving a potent CYP 3A4 inducer (see DOSAGE AND ADMINISTRATION and CLINICAL PHARMACOLOGY ).
Drug/Laboratory Tests Interactions. No clinically relevant changes in the results of clinical laboratory tests have been observed.
Carcinogenesis, Mutagenesis, Impairment of Fertility.
A 2-year carcinogenicity study in mice at doses of 50, 150 and 450 mg/kg/day exemestane (gavage), resulted in an increased incidence of hepatocellular adenomas and/or carcinomas in both genders at the high dose level. Plasma AUCs (0-24hr) at the high dose were 2575 ± 386 and 5667 ± 1833 ng·hr/mL in males and females (approx. 34 and 75 fold the AUC in postmenopausal patients at the recommended clinical dose). An increased incidence of renal tubular adenomas was observed in male mice at the high dose of 450 mg/kg/day. Since the doses tested in mice did not achieve an MTD, neoplastic findings in organs other than liver and kidneys remain unknown.
A separate carcinogenicity study was conducted in rats at the doses of 30, 100 and 315 mg/kg/day exemestane (gavage) for 92 weeks in males and 2 years in females. No evidence of carcinogenic activity up to the highest dose tested of 315 mg/kg/day was observed in females. The male rat study was inconclusive since it was terminated prematurely at Week 92. At the highest dose, plasma AUC (0-24hr) levels in male (1418 ± 287 ng·hr/mL) and female (2318 ± 1067 ng·hr/mL) rats were 19 and 31 fold higher than those measured in postmenopausal cancer patients, receiving the recommended clinical dose.
Exemestane was not mutagenic in vitro in bacteria (Ames test) or mammalian cells (V79 Chinese hamster lung cells). Exemestane was clastogenic in human lymphocytes in vitro without metabolic activation but was not clastogenic in vivo (micronucleus assay in mouse bone marrow). Exemestane did not increase unscheduled DNA synthesis in rat hepatocytes when tested in vitro.
In a pilot reproductive study in rats, male rats were treated with doses of 125-1000 mg/kg/day exemestane, beginning 63 days prior to and during cohabitation. Untreated female rats showed reduced fertility when mated to males treated with >/=500 mg/kg/day exemestane (>/= 200 times the recommended human dose on a mg/m 2 basis). In a separate study, exemestane was given to female rats at 4-100 mg/kg/day beginning 14 days prior to mating and through day 15 or 20 of gestation. Exemestane increased the placental weights at >/=4 mg/kg/day (>/= 1.5 times the human dose on a mg/m 2 basis). Exemestane showed no effects on ovarian function, mating behavior, and conception rate in rats given doses up to 20 mg/kg/day (approximately 8 times the recommended human dose on a mg/m 2 basis), however, decreases in mean litter size and fetal body weight, along with delayed ossification were evidenced at >/= 20 mg/kg/day. In general toxicology studies, changes in the ovary, including hyperplasia, an increase in the incidence of ovarian cysts and a decrease in corpora lutea were observed with variable frequency in mice, rats and dogs at doses that ranged from 3-20 times the human dose on a mg/m 2 basis.
Pregnancy. Pregnancy Category D. See WARNINGS .
Nursing Mothers. AROMASIN is only indicated in postmenopausal women. However, radioactivity related to exemestane appeared in rat milk within 15 minutes of oral administration of radiolabeled exemestane. Concentrations of exemestane and its metabolites were approximately equivalent in the milk and plasma of rats for 24 hours after a single oral dose of 1 mg/kg 14 C-exemestane. It is not known whether exemestane is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if a nursing woman is inadvertently exposed to AROMASIN (see WARNINGS ).
Pediatric Use. The safety and effectiveness of AROMASIN in pediatric patients have not been established.
Geriatric Use. The use of AROMASIN in geriatric patients does not require special precautions.
ADVERSE REACTIONS
A total of 1058 patients were treated with exemestane 25 mg once daily in the clinical trials program. Exemestane was generally well tolerated, and adverse events were usually mild to moderate. Only one death was considered possibly related to treatment with exemestane; an 80-year-old woman with known coronary artery disease had a myocardial infarction with multiple organ failure after 9 weeks on study treatment. In the clinical trials program, only 3% of the patients discontinued treatment with exemestane because of adverse events, mainly within the first 10 weeks of treatment; late discontinuations because of adverse events were uncommon (0.3%).
In the comparative study, adverse reactions were assessed for 358 patients treated with AROMASIN and 400 patients treated with megestrol acetate. Fewer patients receiving AROMASIN discontinued treatment because of adverse events than those treated with megestrol acetate (2% vs. 5%). Adverse events that were considered drug related or of indeterminate cause included hot flashes (13% vs. 5%), nausea (9% vs. 5%), fatigue (8% vs. 10%), increased sweating (4% vs. 8%), and increased appetite (3% vs. 6%). The proportion of patients experiencing an excessive weight gain (>10% of their baseline weight) was significantly higher with megestrol acetate than with AROMASIN (17% vs. 8%). Table 3 shows the adverse events of all CTC grades, regardless of causality, reported in 5% or greater of patients in the study treated either with AROMASIN or megestrol acetate.
Table 3. Incidence (%) of Adverse Events of all Grades * and
Causes Occurring in >/=5% of Patients
In Each Treatment Arm in the Comparative StudyEventAROMASIN
25 mg
once daily
(N=358)Megestrol
Acetate 40 mg
QID
(N=400)Autonomic NervousIncreased sweating6 9 Body as a WholeFatigue22 29 Hot flashes13 6 Pain13 13 Influenza-like symptoms6 5 Edema (includes edema,
peripheral edema,
leg edema)7 6 CardiovascularHypertension5 6 NervousDepression13 9 Insomnia11 9 Anxiety10 11 Dizziness8 6 Headache8 7 GastrointestinalNausea18 12 Vomiting7 4 Abdominal pain6 11 Anorexia6 5 Constipation5 8 Diarrhea4 5 Increased appetite3 6 RespiratoryDyspnea10 15 Coughing6 7 * Graded according to Common Toxicity Criteria
Less frequent adverse events of any cause (from 2% to 5%) reported in the comparative study for patients receiving AROMASIN 25 mg once daily were fever, generalized weakness, paresthesia, pathological fracture, bronchitis, sinusitis, rash, itching, urinary tract infection, and lymphedema.
Additional adverse events of any cause observed in the overall clinical trials program (N = 1058) in 5% or greater of patients treated with exemestane 25 mg once daily but not in the comparative study included pain at tumor sites (8%), asthenia (6%) and fever (5%). Adverse events of any cause reported in 2% to 5% of all patients treated with exemestane 25 mg in the overall clinical trials program but not in the comparative study included chest pain, hypoesthesia, confusion, dyspepsia, arthralgia, back pain, skeletal pain, infection, upper respiratory tract infection, pharyngitis, rhinitis, and alopecia.
OVERDOSAGE
Clinical trials have been conducted with exemestane given as a single dose to healthy female volunteers at doses as high as 800 mg and daily for 12 weeks to postmenopausal women with advanced breast cancer at doses as high as 600 mg. These dosages were well tolerated. There is no specific antidote to overdosage and treatment must be symptomatic. General supportive care, including frequent monitoring of vital signs and close observation of the patient, is indicated.
A male child (age unknown) accidentally ingested a 25-mg tablet of exemestane. The initial physical examination was normal, but blood tests performed 1 hour after ingestion indicated leucocytosis (WBC 25000/mm 3 with 90% neutrophils). Blood tests were repeated 4 days after the incident and were normal. No treatment was given.
In mice, mortality was observed after a single oral dose of exemestane of 3200 mg/kg, the lowest dose tested (about 640 times the recommended human dose on a mg/m 2 basis). In rats and dogs, mortality was observed after single oral doses of exemestane of 5000 mg/kg (about 2000 times the recommended human dose on a mg/m 2 basis) and of 3000 mg/kg (about 4000 times the recommended human dose on a mg/m 2 basis), respectively.
Convulsions were observed after single doses of exemestane of 400 mg/kg and 3000 mg/kg in mice and dogs (approximately 80 and 4000 times the recommended human dose on a mg/m 2 basis), respectively.
DOSAGE AND ADMINISTRATION
The recommended dose of AROMASIN Tablets is 25 mg once daily after a meal. Treatment with AROMASIN should continue until tumor progression is evident.
For patients receiving AROMASIN with a potent CYP 3A4 inducer such as rifampicin or phenytoin, the recommended dose of AROMASIN is 50 mg once daily after a meal.
The safety of chronic dosing in patients with moderate or severe hepatic or renal impairment has not been studied. Based on experience with exemestane at repeated doses up to 200 mg daily that demonstrated a moderate increase in non-life threatening adverse events, dosage adjustment does not appear to be necessary (see CLINICAL PHARMACOLOGY , Special Populations and PRECAUTIONS ).
HOW SUPPLIED
AROMASIN Tablets are round, biconvex, and off-white to slightly gray. Each tablet contains 25 mg of exemestane. The tablets are printed on one side with the number "7663" in black. AROMASIN is packaged in either HDPE bottles with a child-resistant screw cap, supplied in packs of 30 tablets.
30-tablet HDPE bottle NDC 0009-7663-04
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].Rx only
MADE IN ITALY Distributed by Pharmacia & Upjohn Co Division of Pfizer Inc, NY, NY 10017 LAB-0098-4.0 Revised September 2004
Subscribe to the "News" RSS Feed 
TOP ۞
