-
Humatrope Vials and Cartridges (Lilly)
DESCRIPTION
Humatrope® (Somatropin, rDNA Origin, for Injection) is a polypeptide hormone of recombinant DNA origin. Humatrope has 191 amino acid residues and a molecular weight of about 22,125 daltons. The amino acid sequence of the product is identical to that of human growth hormone of pituitary origin. Humatrope is synthesized in a strain of Escherichia coli that has been modified by the addition of the gene for human growth hormone.
Humatrope is a sterile, white, lyophilized powder intended for subcutaneous or intramuscular administration after reconstitution. Humatrope is a highly purified preparation. Phosphoric acid and/or sodium hydroxide may have been added to adjust the pH. Reconstituted solutions have a pH of approximately 7.5. This product is oxygen sensitive.
VIAL -- Each vial of Humatrope contains 5 mg somatropin (15 IU or 225 nanomoles); 25 mg mannitol; 5 mg glycine; and 1.13 mg dibasic sodium phosphate. Each vial is supplied in a combination package with an accompanying 5-mL vial of diluting solution. The diluent contains Water for Injection with 0.3% Metacresol as a preservative and 1.7% glycerin.
CARTRIDGE -- The cartridges of somatropin contain either 6 mg (18 IU), 12 mg (36 IU), or 24 mg (72 IU) of somatropin. The 6, 12, and 24 mg cartridges contain respectively: mannitol 18, 36, and 72 mg; glycine 6, 12, and 24 mg; dibasic sodium phosphate 1.36, 2.72, and 5.43 mg. Each cartridge is supplied in a combination package with an accompanying syringe containing approximately 3 mL of diluting solution. The diluent contains Water for Injection; 0.3% Metacresol as a preservative; and 1.7%, 0.29%, and 0.29% glycerin in the 6, 12, and 24 mg cartridges, respectively.
CLINICAL PHARMACOLOGY
General
Linear Growth -- Humatrope stimulates linear growth in pediatric patients who lack adequate normal endogenous growth hormone. In vitro, preclinical, and clinical testing have demonstrated that Humatrope is therapeutically equivalent to human growth hormone of pituitary origin and achieves equivalent pharmacokinetic profiles in normal adults. Treatment of growth hormone-deficient pediatric patients and patients with Turner syndrome with Humatrope produces increased growth rate and IGF-I (Insulin-like Growth Factor-I/Somatomedin-C) concentrations similar to those seen after therapy with human growth hormone of pituitary origin.
In addition, the following actions have been demonstrated for Humatrope and/or human growth hormone of pituitary origin.
- Tissue Growth -- 1. Skeletal Growth: Humatrope stimulates skeletal growth in pediatric patients with growth hormone deficiency. The measurable increase in body length after administration of either Humatrope or human growth hormone of pituitary origin results from an effect on the growth plates of long bones. Concentrations of IGF-I, which may play a role in skeletal growth, are low in the serum of growth hormone-deficient pediatric patients but increase during treatment with Humatrope. Elevations in mean serum alkaline phosphatase concentrations are also seen. 2. Cell Growth: It has been shown that there are fewer skeletal muscle cells in short-statured pediatric patients who lack endogenous growth hormone as compared with normal pediatric populations. Treatment with human growth hormone of pituitary origin results in an increase in both the number and size of muscle cells.
- Protein Metabolism -- Linear growth is facilitated in part by increased cellular protein synthesis. Nitrogen retention, as demonstrated by decreased urinary nitrogen excretion and serum urea nitrogen, follows the initiation of therapy with human growth hormone of pituitary origin. Treatment with Humatrope results in a similar decrease in serum urea nitrogen.
- Carbohydrate Metabolism -- Pediatric patients with hypopituitarism sometimes experience fasting hypoglycemia that is improved by treatment with Humatrope. Large doses of human growth hormone may impair glucose tolerance. Untreated patients with Turner syndrome have an increased incidence of glucose intolerance. Administration of human growth hormone to normal adults or patients with Turner syndrome resulted in increases in mean serum fasting and postprandial insulin levels although mean values remained in the normal range. In addition, mean fasting and postprandial glucose and hemoglobin A 1c levels remained in the normal range.
- Lipid Metabolism -- In growth hormone-deficient patients, administration of human growth hormone of pituitary origin has resulted in lipid mobilization, reduction in body fat stores, and increased plasma fatty acids.
- Mineral Metabolism -- Retention of sodium, potassium, and phosphorus is induced by human growth hormone of pituitary origin. Serum concentrations of inorganic phosphate increased in patients with growth hormone deficiency after therapy with Humatrope or human growth hormone of pituitary origin. Serum calcium is not significantly altered in patients treated with either human growth hormone of pituitary origin or Humatrope.
Pharmacokinetics
Absorption -- Humatrope has been studied following intramuscular, subcutaneous, and intravenous administration in adult volunteers. The absolute bioavailability of somatropin is 75% and 63% after subcutaneous and intramuscular administration, respectively.
Distribution -- The volume of distribution of somatropin after intravenous injection is about 0.07 L/kg.
Metabolism -- Extensive metabolism studies have not been conducted. The metabolic fate of somatropin involves classical protein catabolism in both the liver and kidneys. In renal cells, at least a portion of the breakdown products of growth hormone is returned to the systemic circulation. In normal volunteers, mean clearance is 0.14 L/hr/kg. The mean half-life of intravenous somatropin is 0.36 hours, whereas subcutaneously and intramuscularly administered somatropin have mean half-lives of 3.8 and 4.9 hours, respectively. The longer half-life observed after subcutaneous or intramuscular administration is due to slow absorption from the injection site.
Excretion -- Urinary excretion of intact Humatrope has not been measured. Small amounts of somatropin have been detected in the urine of pediatric patients following replacement therapy.
Special Populations
Geriatric -- The pharmacokinetics of Humatrope has not been studied in patients greater than 65 years of age.
Pediatric -- The pharmacokinetics of Humatrope in pediatric patients is similar to adults.
Gender -- No studies have been performed with Humatrope. The available literature indicates that the pharmacokinetics of growth hormone is similar in both men and women.
Race -- No data are available.
Renal, Hepatic insufficiency -- No studies have been performed with Humatrope.
Table 1
Summary of Somatropin Parameters in the Normal PopulationC max
(ng/mL)t ½
(hr)AUC 0-(infinity)
(ng·hr/mL)Cls
(L/kg·hr)V(beta)
(L/kg)0.02 mg (0.05 IU * )/kg iv MEAN 415 0.363 156 0.135 0.0703 SD 75 0.053 33 0.029 0.0173 0.1 mg (0.27 IU * )/kg im MEAN 53.2 4.93 495 0.215 1.55 SD 25.9 2.66 106 0.047 0.91 0.1 mg (0.27 IU * )/kg sc MEAN 63.3 3.81 585 0.179 0.957 SD 18.2 1.40 90 0.028 0.301 Abbreviations: C max =maximum concentration; t ½ =half-life; AUC 0-(infinity) =area under the curve; Cls=systemic clearance; V(beta)=volume distribution; iv=intravenous; SD=standard deviation; im=intramuscular; sc=subcutaneous. *Based on previous International Standard of 2.7 IU=1 mg.
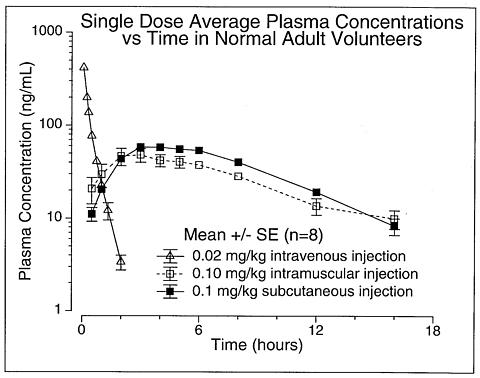
Figure 1
CLINICAL TRIALS
Effects of Humatrope Treatment in Adults with Growth Hormone Deficiency
Two multicenter trials in adult-onset growth hormone deficiency (n=98) and two studies in childhood-onset growth hormone deficiency (n=67) were designed to assess the effects of replacement therapy with Humatrope. The primary efficacy measures were body composition (lean body mass and fat mass), lipid parameters, and the Nottingham Health Profile. The Nottingham Health Profile is a general health-related quality of life questionnaire. These four studies each included a 6-month randomized, blinded, placebo-controlled phase followed by 12 months of open-label therapy for all patients. The Humatrope dosages for all studies were identical: 1 month of therapy at 0.00625 mg/kg/day followed by the proposed maintenance dose of 0.0125 mg/kg/day. Adult-onset patients and childhood-onset patients differed by diagnosis (organic vs. idiopathic pituitary disease), body size (normal vs. small for mean height and weight), and age (mean=44 vs. 29 years). Lean body mass was determined by bioelectrical impedance analysis (BIA), validated with potassium 40. Body fat was assessed by BIA and sum of skinfold thickness. Lipid subfractions were analyzed by standard assay methods in a central laboratory.
Humatrope-treated adult-onset patients, as compared to placebo, experienced an increase in lean body mass (2.59 vs. -0.22 kg, p<0.001) and a decrease in body fat (-3.27 vs. 0.56 kg, p<0.001). Similar changes were seen in childhood-onset growth hormone-deficient patients. These significant changes in lean body mass persisted throughout the 18-month period as compared to baseline for both groups, and for fat mass in the childhood-onset group. Total cholesterol decreased short-term (first 3 months) although the changes did not persist. However, the low HDL cholesterol levels observed at baseline (mean=30.1 mg/mL and 33.9 mg/mL in adult-onset and childhood-onset patients) normalized by the end of 18 months of therapy (a change of 13.7 and 11.1 mg/dL for the adult-onset and childhood-onset groups, p<0.001). Adult-onset patients reported significant improvements as compared to placebo in the following two of six possible health-related domains: physical mobility and social isolation (Table 2). Patients with childhood-onset disease failed to demonstrate improvements in Nottingham Health Profile outcomes.
Two additional studies on the effect of Humatrope on exercise capacity were also conducted. Improved physical function was documented by increased exercise capacity (VO 2 max, p<0.005) and work performance (Watts, p<0.01) (J Clin Endocrinol Metab 1995; 80:552-557).
Table 2
Changes a in Nottingham Health Profile Scores b in
Adult-Onset Growth Hormone-Deficient PatientsOutcome
MeasurePlacebo
(6 Months)Humatrope
Therapy
(6 Months)Significance Energy
level-11.4 -15.5 NS Physical
mobility-3.1 -10.5 p<0.01 Social
isolation0.5 -4.7 p<0.01 Emotional
reactions-4.5 -5.4 NS Sleep -6.4 -3.7 NS Pain -2.8 -2.9 NS a An improvement in score is indicated by a more negative change in the score. b To account for multiple analyses, appropriate statistical methods were applied and the required level of significance is 0.01. NS=not significant. Effects of Growth Hormone Treatment in Patients with Turner Syndrome
One long-term, randomized, open-label multicenter concurrently controlled study, two long-term, open-label multicenter, historically controlled studies and one long-term, randomized, dose-response study were conducted to evaluate the efficacy of growth hormone for the treatment of patients with short stature due to Turner syndrome.
In the randomized study, GDCT, comparing growth hormone-treated patients to a concurrent control group who received no growth hormone, the growth hormone-treated patients who received a dose of 0.3 mg/kg/wk given 6 times per week from a mean age of 11.7 years for a mean duration of 4.7 years attained a mean near final height of 146.0 ± 6.2 cm (n=27, mean ± SD) as compared to the control group who attained a near final height of 142.1 ± 4.8 cm (n=19). By analysis of covariance * , the effect of growth hormone therapy was a mean height increase of 5.4 cm (p=0.001).
In two of the studies (85-023 and 85-044), the effect of long-term growth hormone treatment (0.375 mg/kg/wk given either 3 times per week or daily) on adult height was determined by comparing adult heights in the treated patients with those of age-matched historical controls with Turner syndrome who never received any growth-promoting therapy. The greatest improvement in adult height was observed in patients who received early growth hormone treatment and estrogen after age 14 years. In Study 85-023, this resulted in a mean adult height gain of 7.4 cm (mean duration of GH therapy of 7.6 years) vs. matched historical controls by analysis of covariance.
In Study 85-044, patients treated with early growth hormone therapy were randomized to receive estrogen replacement therapy (conjugated estrogens, 0.3 mg escalating to 0.625 mg daily) at either age 12 or 15 years. Compared with matched historical controls, early GH therapy (mean duration of GH therapy 5.6 years) combined with estrogen replacement at age 12 years resulted in an adult height gain of 5.9 cm (n=26), whereas patients who initiated estrogen at age 15 years (mean duration of GH therapy 6.1 years) had a mean adult height gain of 8.3 cm (n=29). Patients who initiated GH therapy after age 11 (mean age 12.7 years; mean duration of GH therapy 3.8 years) had a mean adult height gain of 5.0 cm (n=51).
In a randomized blinded dose-response study, GDCI, patients were treated from a mean age of 11.1 years for a mean duration of 5.3 years with a weekly dose of either 0.27 mg/kg or 0.36 mg/kg administered 3 or 6 times weekly. The mean near final height of patients receiving growth hormone was 148.7 ± 6.5 cm (n=31). When compared to historical control data, the mean gain in adult height was approximately 5 cm.
In some studies, Turner syndrome patients (n=181) treated to final adult height achieved statistically significant average height gains ranging from 5.0 to 8.3 cm.
Table 3
Summary Table of Efficacy ResultsStudy/
GroupStudy
Design aN at Adult
HeightGH
Age (yr)Estrogen
Age (yr)GH
Duration (yr)Adult Height
Gain (cm) bGDCTRCT 27 11.7 13 4.7 5.4 85-023MHT 17 9.1 15.2 7.6 7.4 85-044: A *MHT 29 9.4 15 6.1 8.3 B *26 9.6 12.3 5.6 5.9 C *51 12.7 13.7 3.8 5 GDCIRDT 31 11.1 8-13.5 5.3 ~5 c a RCT: randomized controlled trial; MHT: matched historical controlled trial; RDT: randomized dose-response trial. b Analysis of covariance vs. controls. c Compared with historical data. *A: GH age <11 yr, estrogen age 15 yr.
B: GH age <11 yr, estrogen age 12 yr.
C: GH age >11 yr, estrogen at month 12.
* Analysis of covariance includes adjustments for baseline height relative to age and for mid-parental height.
Effect of Humatrope Treatment in Pediatric Patients with Idiopathic Short Stature
Two randomized, multicenter trials, 1 placebo-controlled and 1 dose-response, were conducted in pediatric patients with idiopathic short stature, also called non-growth hormone-deficient short stature. The diagnosis of idiopathic short stature was made after excluding other known causes of short stature, as well as growth hormone deficiency. Limited safety and efficacy data are available below the age of 7 years. No specific studies have been conducted in pediatric patients with familial short stature or who were born small for gestational age (SGA).
The placebo-controlled study enrolled 71 pediatric patients (55 males, 16 females) 9 to 15 years old (mean age 12.38 ± 1.51 years), with short stature, 68 of whom received study drug. Patients were predominately Tanner I (45.1%) and Tanner II (46.5%) at baseline.
In this double-blind trial, patients received subcutaneous injections of either Humatrope 0.222 mg/kg/wk or placebo. Study drug was given in divided doses 3 times per week until height velocity decreased to </=1.5 cm/year ("final height"). Thirty-three subjects (22 Humatrope, 11 placebo) had final height measurements after a mean treatment duration of 4.4 years (range 0.11-9.08 years).
The Humatrope group achieved a mean final height Standard Deviation Score (SDS) of -1.8 (Table 4). Placebo-treated patients had a mean final height SDS of -2.3 (mean treatment difference = 0.51, p=0.017). Height gain across the duration of the study and final height SDS minus baseline predicted height SDS were also significantly greater in Humatrope-treated patients than in placebo-treated patients (Table 4 and 5). In addition, the number of patients who achieved a final height above the 5th percentile of the general population for age and sex was significantly greater in the Humatrope group than the placebo group (41% vs. 0%, p<0.05), as was the number of patients who gained at least 1 SDS unit in height across the duration of the study (50% vs. 0%, p<0.05).
Table 4
Baseline Height Characteristics and Effect of Humatrope on Final Height aHumatrope
(n=22)
Mean (SD)Placebo
(n=11)
Mean (SD)Treatment Effect
Mean
(95% CI)p-value Baseline height SDS-2.7 (0.6) -2.75 (0.6) 0.77 BPH SDS-2.1 (0.7) -2.3 (0.8) 0.53 Final height SDS b-1.8 (0.8) -2.3 (0.6) 0.51 (0.10, 0.92) 0.017 FH SDS - baseline height SDS0.9 (0.7) 0.4 (0.2) 0.51 (0.04, 0.97) 0.034 FH SDS - BPH SDS0.3 (0.6) -0.1 (0.6) 0.46 (0.02, 0.89) 0.043 a For final height population. b Between-group comparison was performed using analysis of covariance with baseline predicted height SDS as the covariant. Treatment effect is expressed as least squares mean (95% CI).
Abbreviations: FH=final height; SDS=standard deviation score; BPH=baseline predicted height; CI=confidence interval.
The dose-response study included 239 pediatric patients (158 males, 81 females), 5 to 15 years old, (mean age 9.8 ± 2.3 years). Mean baseline characteristics included: a height SDS of -3.21 (±0.70), a predicted adult height SDS of -2.63 (±1.08), and a height velocity SDS of -1.09 (±1.15). All but 3 patients were Tanner I. Patients were randomized to one of three Humatrope treatment groups: 0.24 mg/kg/wk; 0.24 mg/kg/wk for 1 year, followed by 0.37 mg/kg/wk; and 0.37 mg/kg/wk.
The primary hypothesis of this study was that treatment with Humatrope would increase height velocity during the first 2 years of therapy in a dose-dependent manner. Additionally, after completing the initial 2-year dose-response phase of the study, 50 patients were followed to final height.
Patients receiving 0.37 mg/kg/wk had a significantly greater increase in mean height velocity after 2 years of treatment than patients receiving 0.24 mg/kg/wk (4.04 vs. 3.27 cm/year, p=0.003). The mean difference between final height and baseline predicted height was 7.2 cm for patients receiving 0.37 mg/kg/wk and 5.4 cm for patients receiving 0.24 mg/kg/wk (Table 5). While no patient had height above the 5th percentile in any dose group at baseline, 82% of the patients receiving 0.37 mg/kg/wk and 47% of the patients receiving 0.24 mg/kg/wk achieved a final height above the 5th percentile of the general population height standards (p=NS).
Table 5
Final Height Minus Baseline Predicted Height: Idiopathic Short Stature TrialsPlacebo-controlled Trial
3 × per week dosingDose Response Trial
6 × per week dosingPlacebo
(n=10)Humatrope
0.22 mg/kg
(n=22)Humatrope
0.24 mg/kg
(n=13)Humatrope
0.24/0.37 mg/kg
(n=13)Humatrope
0.37 mg/kg
(n=13)FH -Baseline PH
Mean cm
(95% CI)
-0.7
(-3.6, 2.3)
+ 2.2
(0.4, 3.9)
+ 5.4
(2.8, 7.9)
+ 6.7
(4.1, 9.2)
+ 7.2
(4.6, 9.8)Mean inches
(95% CI)-0.3
(-1.4, 0.9)+ 0.8
(0.2, 1.5)+ 2.1
(1.1, 3.1)+ 2.6
(1.6, 3.6)+ 2.8
(1.8, 3.9)Abbreviations: PH=predicted height; FH=final height; CI=confidence interval.INDICATIONS AND USAGE
Pediatric Patients -- Humatrope is indicated for the long-term treatment of pediatric patients who have growth failure due to an inadequate secretion of normal endogenous growth hormone.
Humatrope is indicated for the treatment of short stature associated with Turner syndrome in patients whose epiphyses are not closed.
Humatrope is indicated for the long-term treatment of idiopathic short stature, also called non-growth hormone-deficient short stature, defined by height SDS </=-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range, in pediatric patients whose epiphyses are not closed and for whom diagnostic evaluation excludes other causes associated with short stature that should be observed or treated by other means.
Adult Patients -- Humatrope is indicated for replacement of endogenous growth hormone in adults with growth hormone deficiency who meet either of the following two criteria:
-
Adult Onset:
Patients who have growth hormone deficiency either alone, or with multiple hormone deficiencies (hypopituitarism), as a result of pituitary disease, hypothalamic disease, surgery, radiation therapy, or trauma;
or - Childhood Onset: Patients who were growth hormone-deficient during childhood who have growth hormone deficiency confirmed as an adult before replacement therapy with Humatrope is started.
CONTRAINDICATIONS
Humatrope should not be used for growth promotion in pediatric patients with closed epiphyses.
Humatrope should not be used or should be discontinued when there is any evidence of active malignancy. Anti-malignancy treatment must be complete with evidence of remission prior to the institution of therapy.
Humatrope should not be reconstituted with the supplied Diluent for Humatrope for use by patients with a known sensitivity to either Metacresol or glycerin.
Growth hormone should not be initiated to treat patients with acute critical illness due to complications following open heart or abdominal surgery, multiple accidental trauma or to patients having acute respiratory failure. Two placebo-controlled clinical trials in non-growth hormone-deficient adult patients (n=522) with these conditions revealed a significant increase in mortality (41.9% vs. 19.3%) among somatropin-treated patients (doses 5.3 to 8 mg/day) compared to those receiving placebo ( see WARNINGS ).
Growth hormone is contraindicated in patients with Prader-Willi syndrome who are severely obese or have severe respiratory impairment ( see WARNINGS ). Unless patients with Prader-Willi syndrome also have a diagnosis of growth hormone deficiency, Humatrope is not indicated for the long term treatment of pediatric patients who have growth failure due to genetically confirmed Prader-Willi syndrome.
WARNINGS
If sensitivity to the diluent should occur, the vials may be reconstituted with Bacteriostatic Water for Injection, USP or, Sterile Water for Injection, USP. When Humatrope is used with Bacteriostatic Water (Benzyl Alcohol preserved), the solution should be kept refrigerated at 2° to 8°C (36° to 46°F) and used within 14 days. Benzyl alcohol as a preservative in Bacteriostatic Water for Injection, USP has been associated with toxicity in newborns. When administering Humatrope to newborns, use the Humatrope diluent provided or if the patient is sensitive to the diluent, use Sterile Water for Injection, USP. When Humatrope is reconstituted with Sterile Water for Injection, USP in this manner, use only one dose per Humatrope vial and discard the unused portion. If the solution is not used immediately, it must be refrigerated [2° to 8°C (36° to 46°F)] and used within 24 hours.
Cartridges should be reconstituted only with the supplied diluent. Cartridges should not be reconstituted with the Diluent for Humatrope provided with Humatrope Vials, or with any other solution. Cartridges should not be used if the patient is allergic to Metacresol or glycerin.
See CONTRAINDICATIONS for information on increased mortality in patients with acute critical illnesses in intensive care units due to complications following open heart or abdominal surgery, multiple accidental trauma or with acute respiratory failure. The safety of continuing growth hormone treatment in patients receiving replacement doses for approved indications who concurrently develop these illnesses has not been established. Therefore, the potential benefit of treatment continuation with growth hormone in patients having acute critical illnesses should be weighed against the potential risk.
There have been reports of fatalities after initiating therapy with growth hormone in pediatric patients with Prader-Willi syndrome who had one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Male patients with one or more of these factors may be at greater risk than females. Patients with Prader-Willi syndrome should be evaluated for signs of upper airway obstruction and sleep apnea before initiation of treatment with growth hormone. If, during treatment with growth hormone, patients show signs of upper airway obstruction (including onset of or increased snoring) and/or new onset sleep apnea, treatment should be interrupted. All patients with Prader-Willi syndrome treated with growth hormone should also have effective weight control and be monitored for signs of respiratory infection, which should be diagnosed as early as possible and treated aggressively ( see CONTRAINDICATIONS ). Unless patients with Prader-Willi syndrome also have a diagnosis of growth hormone deficiency, Humatrope is not indicated for the long term treatment of pediatric patients who have growth failure due to genetically confirmed Prader-Willi syndrome.
PRECAUTIONS
General -- Therapy with Humatrope should be directed by physicians who are experienced in the diagnosis and management of patients with growth hormone deficiency, Turner syndrome, idiopathic short stature, or adult patients with either childhood-onset or adult-onset growth hormone deficiency.
Patients with preexisting tumors or with growth hormone deficiency secondary to an intracranial lesion should be examined routinely for progression or recurrence of the underlying disease process. In pediatric patients, clinical literature has demonstrated no relationship between somatropin replacement therapy and CNS tumor recurrence. In adults, it is unknown whether there is any relationship between somatropin replacement therapy and CNS tumor recurrence.
Patients should be monitored carefully for any malignant transformation of skin lesions.
For patients with diabetes mellitus, the insulin dose may require adjustment when somatropin therapy is instituted. Because human growth hormone may induce a state of insulin resistance, patients should be observed for evidence of glucose intolerance. Patients with diabetes or glucose in-tolerance should be monitored closely during somatropin therapy.
In patients with hypopituitarism (multiple hormonal deficiencies) standard hormonal replacement therapy should be monitored closely when somatropin therapy is administered. Hypothyroidism may develop during treatment with somatropin and inadequate treatment of hypothyroidism may prevent optimal response to somatropin.
Pediatric Patients ( see General Precautions ) -- Pediatric patients with endocrine disorders, including growth hormone deficiency, may develop slipped capital epiphyses more frequently. Any pediatric patient with the onset of a limp during growth hormone therapy should be evaluated.
Growth hormone has not been shown to increase the incidence of scoliosis. Progression of scoliosis can occur in children who experience rapid growth. Because growth hormone increases growth rate, patients with a history of scoliosis who are treated with growth hormone should be monitored for progression of scoliosis. Skeletal abnormalities including scoliosis are commonly seen in untreated Turner syndrome patients.
Patients with Turner syndrome should be evaluated carefully for otitis media and other ear disorders since these patients have an increased risk of ear or hearing disorders ( see Adverse Reactions ). Patients with Turner syndrome are at risk for cardiovascular disorders (e.g., stroke, aortic aneurysm, hypertension) and these conditions should be monitored closely.
Patients with Turner syndrome have an inherently increased risk of developing autoimmune thyroid disease. Therefore, patients should have periodic thyroid function tests and be treated as indicated ( see General Precautions ).
Intracranial hypertension (IH) with papilledema, visual changes, headache, nausea and/or vomiting has been reported in a small number of pediatric patients treated with growth hormone products. Symptoms usually occurred within the first 8 weeks of the initiation of growth hormone therapy. In all reported cases, IH-associated signs and symptoms resolved after termination of therapy or a reduction of the growth hormone dose. Funduscopic examination of patients is recommended at the initiation and periodically during the course of growth hormone therapy. Patients with Turner syndrome may be at increased risk for development of IH.
Adult Patients (see General Precautions ) -- Patients with epiphyseal closure who were treated with growth hormone replacement therapy in childhood should be re-evaluated according to the criteria in INDICATIONS AND USAGE before continuation of somatropin therapy at the reduced dose level recommended for growth hormone-deficient adults.
Experience with prolonged treatment in adults is limited.
Geriatric Use -- The safety and effectiveness of Humatrope in patients aged 65 and over has not been evaluated in clinical studies. Elderly patients may be more sensitive to the action of Humatrope and may be more prone to develop adverse reactions.
Drug Interactions -- Excessive glucocorticoid therapy may prevent optimal response to somatropin. If glucocorticoid replacement therapy is required, the glucocorticoid dosage and compliance should be monitored carefully to avoid either adrenal insufficiency or inhibition of growth promoting effects.
Limited published data indicate that growth hormone (GH) treatment increases cytochrome P450 (CP450) mediated antipyrine clearance in man. These data suggest that GH administration may alter the clearance of compounds known to be metabolized by CP450 liver enzymes (e.g., corticosteroids, sex steroids, anticonvulsants, cyclosporin). Careful monitoring is advisable when GH is administered in combination with other drugs known to be metabolized by CP450 liver enzymes.
Carcinogenesis, Mutagenesis, Impairment of Fertility -- Long-term animal studies for carcinogenicity and impairment of fertility with this human growth hormone (Humatrope) have not been performed. There has been no evidence to date of Humatrope-induced mutagenicity.
Pregnancy -- Pregnancy Category C -- Animal reproduction studies have not been conducted with Humatrope. It is not known whether Humatrope can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Humatrope should be given to a pregnant woman only if clearly needed.
Nursing Mothers -- There have been no studies conducted with Humatrope in nursing mothers. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Humatrope is administered to a nursing woman.
Information for Patients -- Patients being treated with growth hormone and/or their parents should be informed of the potential risks and benefits associated with treatment. Instructions on appropriate use should be given, including a review of the contents of the patient information insert. This information is intended to aid in the safe and effective administration of the medication. It is not a disclosure of all possible adverse or intended effects.
Patients and/or parents should be thoroughly instructed in the importance of proper needle disposal. A puncture resistant container should be used for the disposal of used needles and/or syringes (consistent with applicable state requirements). Needles and syringes must not be reused ( see Information for the Patient insert).
ADVERSE REACTIONS
Growth Hormone-Deficient Pediatric Patients
As with all protein pharmaceuticals, a small percentage of patients may develop antibodies to the protein. During the first 6 months of Humatrope therapy in 314 naive patients, only 1.6% developed specific antibodies to Humatrope (binding capacity >/=0.02 mg/L). None had antibody concentrations which exceeded 2 mg/L. Throughout 8 years of this same study, two patients (0.6%) had binding capacity >2 mg/L. Neither patient demonstrated a decrease in growth velocity at or near the time of increased antibody production. It has been reported that growth attenuation from pituitary-derived growth hormone may occur when antibody concentrations are >1.5 mg/L.
In addition to an evaluation of compliance with the treatment program and of thyroid status, testing for antibodies to human growth hormone should be carried out in any patient who fails to respond to therapy.
In studies with growth hormone-deficient pediatric patients, injection site pain was reported infrequently. A mild and transient edema, which appeared in 2.5% of patients, was observed early during the course of treatment.
Leukemia has been reported in a small number of pediatric patients who have been treated with growth hormone, including growth hormone of pituitary origin as well as of recombinant DNA origin (somatrem and somatropin). The relationship, if any, between leukemia and growth hormone therapy is uncertain.
Turner Syndrome Patients
In a randomized, concurrent controlled trial, there was a statistically significant increase in the occurrence of otitis media (43% vs. 26%), ear disorders (18% vs. 5%) and surgical procedures (45% vs. 27%) in patients receiving Humatrope compared with untreated control patients (Table 6). Other adverse events of special interest to Turner syndrome patients were not significantly different between treatment groups (Table 6). A similar increase in otitis media was observed in an 18-month placebo-controlled trial.
Table 6
Treatment-Emergent Events of Special Interest by Treatment Group in Turner SyndromeTreatment Group Adverse EventOverall hGH 1 Untreated 2 Significance Total Number of Patients136 74 62 Surgical procedure50 (36.8%) 33 (44.6%) 17 (27.4%) p</=0.05 Otitis media48 (35.3%) 32 (43.2%) 16 (25.8%) p</=0.05 Ear disorders16 (11.8%) 13 (17.6%) 3 (4.8%) p</=0.05 Bone disorder13 (9.6%) 6 (8.1%) 7 (11.3%) NS EdemaConjunctival1 (0.7%) 0 1 (1.6%) NS Non-specific3 (2.2%) 2 (2.7%) 1 (1.6%) NS Facial1 (0.7%) 1 (1.4%) 0 NS Peripheral6 (4.4%) 5 (6.8%) 1 (1.6%) NS Hyperglycemia0 0 0 NS Hypothyroidism15 (11.0%) 10 (13.5%) 5 (8.1%) NS Increased nevi 310 (7.4%) 8 (10.8%) 2 (3.2%) NS Lymphedema0 0 0 NS 1 Dose=0.3 mg/kg/wk.2 Open-label study.3 Includes any nevi coded to the following preferred terms: melanosis, skin hypertrophy, or skin benign neoplasm.NS=not significant.Patients with Idiopathic Short Stature
In the placebo-controlled study, the adverse events associated with Humatrope therapy were similar to those observed in other pediatric populations treated with Humatrope (Table 7). Mean serum glucose level did not change during Humatrope treatment. Mean fasting serum insulin levels increased 10% in the Humatrope treatment group at the end of treatment relative to baseline values but remained within the normal reference range. For the same duration of treatment the mean fasting serum insulin levels decreased by 2% in the placebo group. The incidence of above-range values for glucose, insulin, and HbA 1c were similar in the growth hormone and placebo-treated groups. No patient developed diabetes mellitus. Consistent with the known mechanism of growth hormone action, Humatrope-treated patients had greater mean increases, relative to baseline, in serum insulin-like growth factor-I (IGF-I) than placebo-treated patients at each study observation. However, there was no significant difference between the Humatrope and placebo treatment groups in the proportion of patients who had at least one serum IGF-I concentration more than 2.0 SD above the age- and gender-appropriate mean (Humatrope: 9 of 35 patients [26%]; placebo: 7 of 28 patients [25%]).
Table 7
Nonserious Clinically Significant Treatment-Emergent
Adverse Events by Treatment Group in Idiopathic
Short StatureTreatment Group Adverse EventHumatrope Placebo Total Number of
Patients37 31 Scoliosis7 (18.9%) 4 (12.9%) Otitis media6 (16.2%) 2 (6.5%) Hyperlipidemia3 (8.1%) 1 (3.2%) Gynecomastia2 (5.4%) 1 (3.2%) Hypothyroidism0 2 (6.5%) Aching joints0 1 (3.2%) Hip pain1 (2.7%) 0 Arthralgia4 (10.8%) 1 (3.2%) Arthrosis4 (10.8%) 2 (6.5%) Myalgia9 (24.3%) 4 (12.9%) Hypertension1 (2.7%) 0 The adverse events observed in the dose-response study (239 patients treated for 2 years) did not indicate a pattern suggestive of a growth hormone dose effect. Among Humatrope dose groups, mean fasting blood glucose, mean glycosylated hemoglobin, and the incidence of elevated fasting blood glucose concentrations were similar. One patient developed abnormalities of carbohydrate metabolism (glucose intolerance and high serum HbA 1c ) on treatment.
Adult Patients -- In clinical studies in which high doses of Humatrope were administered to healthy adult volunteers, the following events occurred infrequently: headache, localized muscle pain, weakness, mild hyperglycemia, and glucosuria.
In the first 6 months of controlled blinded trials during which patients received either Humatrope or placebo, adult-onset growth hormone-deficient adults who received Humatrope experienced a statistically significant increase in edema (Humatrope 17.3% vs. placebo 4.4%, p=0.043) and peripheral edema (11.5% vs. 0%, respectively, p=0.017). In patients with adult-onset growth hormone deficiency, edema, muscle pain, joint pain, and joint disorder were reported early in therapy and tended to be transient or responsive to dosage titration.
Two of 113 adult-onset patients developed carpal tunnel syndrome after beginning maintenance therapy without a low dose (0.00625 mg/kg/day) lead-in phase. Symptoms abated in these patients after dosage reduction.
All treatment-emergent adverse events with >/=5% overall incidence during 12 or 18 months of replacement therapy with Humatrope are shown in Table 8 (adult-onset patients) and in Table 9 (childhood-onset patients).
Adult patients treated with Humatrope who had been diagnosed with growth hormone deficiency in childhood reported side effects less frequently than those with adult-onset growth hormone deficiency.
Table 8
Treatment-Emergent Adverse Events with >/=5% Overall Incidence in Adult-Onset Growth Hormone-Deficient Patients Treated with Humatrope for 18 Months as Compared with 6-Month Placebo and 12-Month Humatrope Exposure18 Months Exposure
[Placebo (6 Months)/hGH
(12 Months)]
(N=46)18 Months hGH
Exposure
(N=52)Adverse Eventn % n % Edema a7 15.2 11 21.2 Arthralgia7 15.2 9 17.3 Paresthesia6 13.0 9 17.3 Myalgia6 13.0 7 13.5 Pain6 13.0 7 13.5 Rhinitis5 10.9 7 13.5 Peripheral edema b8 17.4 6 11.5 Back pain5 10.9 5 9.6 Headache5 10.9 4 7.7 Hypertension2 4.3 4 7.7 Acne0 0 3 5.8 Joint disorder1 2.2 3 5.8 Surgical procedure1 2.2 3 5.8 Flu syndrome3 6.5 2 3.9 Abbreviations: hGH=Humatrope; N=number of patients receiving treatment in the period stated; n=number of patients reporting each treatment-emergent adverse event.
a p=0.04 as compared to placebo (6 months).b p=0.02 as compared to placebo (6 months).Table 9
Treatment-Emergent Adverse Events with >/=5% Overall Incidence in Childhood-Onset Growth Hormone-Deficient Patients Treated with Humatrope for 18 Months as Compared with 6-Month Placebo and 12-Month Humatrope Exposure18 Months Exposure
[Placebo (6 Months)/hGH (12 Months)]
(N=35)18 Months hGH Exposure
(N=32)Adverse Eventn % n % Flu sndrome8 22.9 5 15.6 AST icreased a2 5.7 4 12.5 Headache4 11.4 3 9.4 Asthenia1 2.9 2 6.3 Cough increased0 0 2 6.3 Edema3 8.6 2 6.3 Hypesthesia0 0 2 6.3 Myalgia2 5.7 2 6.3 Pain3 8.6 2 6.3 Rhinitis2 5.7 2 6.3 ALT increased2 5.7 2 6.3 Respiratory disorder2 5.7 1 3.1 Gastritis2 5.7 0 0 Pharyngitis5 14.3 1 3.1 Abbreviations: hGH=Humatrope; N=number of patients receiving treatment in the period stated; n=number of patients reporting each treatment-emergent adverse event; ALT=alanine amino transferase, formerly SGPT; AST=aspartate amino transferase, formerly SGOT.
a p=0.03 as compared to placebo (6 months)Other adverse drug events that have been reported in growth hormone-treated patients include the following:
- Metabolic: Infrequent, mild and transient peripheral or generalized edema.
- Musculoskeletal: Rare carpal tunnel syndrome.
- Skin: Rare increased growth of pre-existing nevi. Patients should be monitored carefully for malignant transformation.
- Endocrine: Rare gynecomastia. Rare pancreatitis.
OVERDOSAGE
Acute overdosage could lead initially to hypoglycemia and subsequently to hyperglycemia. Long-term overdosage could result in signs and symptoms of gigantism/acromegaly consistent with the known effects of excess human growth hormone. (See recommended and maximal dosage instructions given below.)
DOSAGE AND ADMINISTRATION
Pediatric Patients
The Humatrope dosage and administration schedule should be individualized for each patient. Therapy should not be continued if epiphyseal fusion has occurred. Response to growth hormone therapy tends to decrease with time. However, failure to increase growth rate, particularly during the first year of therapy, should prompt close assessment of compliance and evaluation of other causes of growth failure such as hypothyroidism, under-nutrition and advanced bone age.
Growth hormone-deficient pediatric patients -- The recommended weekly dosage is 0.18 mg/kg (0.54 IU/kg) of body weight. The maximal replacement weekly dosage is 0.3 mg/kg (0.90 IU/kg) of body weight. It should be divided into equal doses given either on 3 alternate days, 6 times per week or daily. The subcutaneous route of administration is preferable; intramuscular injection is also acceptable. The dosage and administration schedule for Humatrope should be individualized for each patient.
Turner Syndrome -- A weekly dosage of up to 0.375 mg/kg (1.125 IU/kg) of body weight administered by subcutaneous injection is recommended. It should be divided into equal doses given either daily or on 3 alternate days.
Patients with idiopathic short stature -- A weekly dosage of up to 0.37 mg/kg of body weight administered by subcutaneous injection is recommended. It should be divided into equal doses given 6 to 7 times per week.
Adult Patients
Growth hormone-deficient adult patients -- The recommended dosage at the start of therapy is not more than 0.006 mg/kg/day (0.018 IU/kg/day) given as a daily subcutaneous injection. The dose may be increased according to individual patient requirements to a maximum of 0.0125 mg/kg/day (0.0375 IU/kg/day).
During therapy, dosage should be titrated if required by the occurrence of side effects or to maintain the IGF-I response below the upper limit of normal IGF-I levels, matched for age and sex. To minimize the occurrence of adverse events in patients with increasing age or excessive body weight, dose reductions may be necessary.
Reconstitution
Vial -- Each 5-mg vial of Humatrope should be reconstituted with 1.5 to 5 mL of Diluent for Humatrope. The diluent should be injected into the vial of Humatrope by aiming the stream of liquid against the glass wall. Following reconstitution, the vial should be swirled with a GENTLE rotary motion until the contents are completely dissolved. DO NOT SHAKE. The resulting solution should be inspected for clarity. It should be clear. If the solution is cloudy or contains particulate matter, the contents MUST NOT be injected.
Before and after injection, the septum of the vial should be wiped with rubbing alcohol or an alcoholic antiseptic solution to prevent contamination of the contents by repeated needle insertions. Sterile disposable syringes and needles should be used for administration of Humatrope. The volume of the syringe should be small enough so that the prescribed dose can be withdrawn from the vial with reasonable accuracy.
Cartridge -- Each cartridge of Humatrope should only be reconstituted using the diluent syringe and the diluent connector which accompany the cartridge and should not be reconstituted with the Diluent for Humatrope provided with Humatrope Vials. ( See WARNINGS section.) See Information for the Patient for comprehensive directions on Humatrope cartridge reconstitution.
The reconstituted solution should be inspected for clarity. It should be clear. If the solution is cloudy or contains particulate matter, the contents MUST NOT be injected.
The somatropin concentrations for the reconstituted Humatrope cartridges are as follows: 2.08 mg/mL for the 6 mg cartridge; 4.17 mg/mL for the 12 mg cartridge; and 8.33 mg/mL for the 24 mg cartridge.
This cartridge has been designed for use only with the Humatrope injection device. A sterile disposable needle should be used for each injection of Humatrope.
STABILITY AND STORAGE
Vials
Before Reconstitution -- Vials of Humatrope and Diluent for Humatrope are stable when refrigerated [2° to 8°C (36° to 46°F)]. Avoid freezing Diluent for Humatrope. Expiration dates are stated on the labels.
After Reconstitution -- Vials of Humatrope are stable for up to 14 days when reconstituted with Diluent for Humatrope or Bacteriostatic Water for Injection, USP and stored in a refrigerator at 2° to 8°C (36° to 46°F). Avoid freezing the reconstituted vial of Humatrope.
After Reconstitution with Sterile Water, USP -- Use only one dose per Humatrope vial and discard the unused portion. If the solution is not used immediately, it must be refrigerated [2° to 8°C (36° to 46°F)] and used within 24 hours.
Cartridges
Before Reconstitution -- Cartridges of Humatrope and Diluent for Humatrope are stable when refrigerated [2° to 8°C (36° to 46°F)]. Avoid freezing Diluent for Humatrope. Expiration dates are stated on the labels.
After Reconstitution -- Cartridges of Humatrope are stable for up to 28 days when reconstituted with Diluent for Humatrope and stored in a refrigerator at 2° to 8°C (36° to 46°F). Store the Humatrope injection device without the needle attached. Avoid freezing the reconstituted cartridge of Humatrope.
HOW SUPPLIED
Vials
5 mg (No. 7335) -- (6s) NDC 0002-7335-16, and 5-mL vials of Diluent for Humatrope (No. 7336)
Cartridges
Cartridge Kit (MS8089) NDC 0002-8089-01
6 mg cartridge (VL7554), and prefilled syringe of Diluent for Humatrope (VL7557)
Cartridge Kit (MS8090) NDC 0002-8090-01
12 mg cartridge (VL7555), and prefilled syringe of Diluent for Humatrope (VL7558)
Cartridge Kit (MS8091) NDC 0002-8091-01
24 mg cartridge (VL7556), and prefilled syringe of Diluent for Humatrope (VL7558)
Literature revised January 5, 2005
www.humatrope.com
PA 1644 AMP
Subscribe to the "News" RSS Feed 
TOP ۞
