-
Menopur for Subcutaneous Injection (Ferring)
DESCRIPTION
Menopur® (menotropins for injection, USP) is a preparation of gonadotropins, extracted from the urine of postmenopausal women, which has undergone additional steps for purification. Each vial of Menopur® contains 75 International Units (IU) of follicle-stimulating hormone (FSH) activity and 75 IU of luteinizing hormone (LH) activity, plus 21 mg lactose monohydrate and 0.005 mg Polysorbate 20 and Sodium Phosphate Buffer (Sodium Phosphate Dibasic, Heptahydrate and Phosphoric Acid) in a sterile, lyophilized form intended for reconstitution with sterile 0.9% Sodium Chloride Injection, USP. Menopur® is administered by subcutaneous (SC) injection.
The biological activity of Menopur® is determined using the USP bioassays for FSH (ovarian weight gain assay in female rats) and LH (seminal vesicle weight gain assay in male rats), modified to increase the accuracy and reproducibility of these assays. The FSH and LH activity assays are standardized using the Fourth International Standard for Urinary FSH and Urinary LH, November 2000, by the Expert Committee on Biological Standardization of the World Health Organization (WHO ECBS). Human Chorionic Gonadotropin (hCG) is detected in Menopur®.
Both FSH and LH are glycoproteins that are acidic and water soluble.
Therapeutic class: Infertility.
CLINICAL PHARMACOLOGY
Menopur®, administered for 7 to 20 days, produces ovarian follicular growth and maturation in women who do not have primary ovarian failure. In order to produce final follicular maturation and ovulation in the absence of an endogenous LH surge, hCG must be administered following Menopur® treatment, at a time when patient monitoring indicates sufficient follicular development has occurred.
PHARMACOKINETICS
Two open-label, randomized, controlled trials were conducted to assess the pharmacokinetics of Menopur®. Study 2003-02 compared single doses of SC administration of the US and European (EU) formulations of Menopur® in 57 healthy, pre-menopausal females who had undergone pituitary suppression. The study established that the two formulations are bioequivalent. Study 2000-03 assessed single and multiple doses of Menopur® administered SC and IM in a 3 phase cross-over design in 33 healthy, pre-menopausal females who had undergone pituitary suppression. The primary pharmacokinetic endpoints were FSH AUC and C max values. The results are summarized in Table 1.
Table 1: Mean (±SD) FSH Pharmacokinetic Parameters Following Menopur® Administration (Study 2000-03)PK ParametersSingle Dose
(225 IU)Multiple Dose
(225 IU × 1
day then
150 IU × 6
days)C max **/* (mIU/mL)SC IM SC IM 8.5
(2.5)7.8
(2.4)15.0
(3.6)12.5
(2.3)T max (hr)17.9
(5.8)27.5
(25.4)8.0
(3.0)9.0
(7.0)AUC **/* (hr-mIU/mL)726.2
(243.0)656.1
(233.7)622.7
(153.0)546.2
(91.2)**/* Single dose C max , AUC 120 and multiple dose C maxss , AUC ssAbsorption
The SC route of administration trends toward greater bioavailability than the IM route for single and multiple doses of Menopur®.
Distribution
Human tissue or organ distribution of FSH and LH has not been studied for Menopur®.
Metabolism
Metabolism of FSH and LH has not been studied for Menopur® in humans.
Elimination
The elimination half-lives for FSH in the multiple-dose phase were similar (11-13 hours) for Menopur® SC and Menopur® IM.
Pediatric Populations
Menopur® has not been studied in the pediatric population.
Geriatric Populations
Menopur® has not been studied in the geriatric population.
Special Populations
The safety and efficacy of Menopur® in renal and hepatic insufficiency have not been studied.
Drug Interactions
No drug/drug interaction studies have been conducted for Menopur® in humans.
CLINICAL STUDIES
The efficacy and safety of Menopur® have been established in one randomized, controlled, clinical study, 0399E, of women undergoing in vitro fertilization (IVF) or IVF plus intracytoplasmic injection to achieve pregnancy.
Study 0399E was a Phase 3, randomized, open-label, multicenter, multinational (in Europe and Israel), comparative clinical trial of ovulatory, infertile females undergoing ovarian stimulation to produce multiple follicles for IVF and embryo transfer (IVF/ET) after pituitary suppression with a GnRH agonist. A total of 373 patients were randomized to the Menopur® arm. Randomization was stratified by insemination technique [conventional in-vitro fertilization (IVF) vs. intra-cytoplasmic sperm injection (ICSI)]. Efficacy was assessed based on the primary efficacy parameter of continuing pregnancy. The initial daily dose of Menopur® was 225 IU SC for five days. Thereafter, the dose was individualized according to each patient's response, up to a maximum of 450 lU/day for a total maximum duration of stimulation of 20 days. Treatment outcomes are summarized in Table 2.
Table 2: Efficacy Outcomes for IVF Study 0399E (one cycle of treatment)ParameterMenopur®
SCn=373 Continuing Pregnancy (%) a87 (23) b Clinical Pregnancy (%)98 (26) c a Continuing pregnancy was defined as ultrasound visualization of a gestational sac with fetal heartbeat at >/=10 weeks after ET b Non-inferior to comparator recombinant human FSH based on a two-sided 95% confidence interval, intent-to-treat analysis c Secondary efficacy parameter. Study 0399E was not powered to demonstrate differences in this parameter INDICATIONS AND USAGE
Menopur® administered subcutaneously is indicated for the development of multiple follicles and pregnancy in the ovulatory patients participating in an ART program.
Selection of Patients
- A thorough gynecologic and endocrinologic evaluation, including an assessment of pelvic anatomy must be performed before treatment with Menopur®. Patients with tubal obstruction should receive Menopur® only if enrolled in an IVF program.
- Primary ovarian failure should be excluded by the determination of gonadotropin levels.
- Careful examination should be made to rule out the presence of an early pregnancy.
- Patients in late reproductive life have a greater predilection to endometrial carcinoma as well as a higher incidence of anovulatory disorders. A thorough diagnostic evaluation should always be performed in patients who demonstrate abnormal uterine bleeding or other signs of endometrial abnormalities before starting Menopur® therapy.
- Evaluation of the partner's fertility potential should be included in the workup.
CONTRAINDICATIONS
Menopur® is contraindicated in women who have:
- A high FSH level indicating primary ovarian failure.
- Uncontrolled thyroid and adrenal dysfunction.
- An organic intracranial lesion such as a pituitary tumor.
- Sex hormone dependent tumors of the reproductive tract and accessory organs.
- Abnormal uterine bleeding of undetermined origin.
- Ovarian cysts or enlargement not due to polycystic ovary syndrome.
- Prior hypersensitivity to menotropins or Menopur®.
- Menopur® is not indicated in women who are pregnant. There are limited human data on the effects of menotropins when administered during pregnancy.
WARNINGS
Menopur® is a drug that should only be used by physicians who are thoroughly familiar with infertility problems. It is a potent gonadotropic substance capable of Ovarian Hyperstimulation Syndrome (OHSS) in women with or without pulmonary or vascular complications. Gonadotropin therapy requires a certain time commitment by physicians and supportive health professionals, and its use requires the availability of appropriate monitoring facilities (see PRECAUTIONS - Laboratory Tests ) .
Overstimulation of the Ovary During Menopur® Therapy
Ovarian Enlargement: Mild to moderate uncomplicated ovarian enlargement which may be accompanied by abdominal distension and/or abdominal pain occurs in approximately 5 to 10 % of women treated with menotropins and hCG, and generally regresses without treatment within two or three weeks. The lowest dose consistent with expectation of good results and careful monitoring of ovarian response can further minimize the risk of overstimulation.
If the ovaries are abnormally enlarged on the last day of Menopur® therapy, hCG should not be administered in this course of treatment; this will reduce the chances of development of the Ovarian Hyperstimulation Syndrome (OHSS).
OHSS: OHSS is a medical event distinct from uncomplicated ovarian enlargement. OHSS may progress rapidly to become a serious medical event. It is characterized by an apparent dramatic increase in vascular permeability which can result in a rapid accumulation of fluid in the peritoneal cavity, thorax, and potentially, the pericardium. The early warning signs of development of OHSS are severe pelvic pain, nausea, vomiting, and weight gain. The following symptomatology has been seen with cases of OHSS: abdominal pain, abdominal distension, gastrointestinal symptoms including nausea, vomiting and diarrhea, severe ovarian enlargement, weight gain, dyspnea, and oliguria. Clinical evaluation may reveal hypovolemia, hemoconcentration, electrolyte imbalances, ascites, hemoperitoneum, pleural effusions, hydrothorax, acute pulmonary distress, and thromboembolic events (See " Pulmonary and Vascular Complications "). Transient liver function test abnormalities suggestive of hepatic dysfunction, which may be accompanied by morphologic changes on liver biopsy, have been reported in association with the OHSS.
In the IVF clinical study, 0399E, OHSS occurred in 7.2% of the 373 Menopur® treated women.
Cases of OHSS are more common, more severe and more protracted if pregnancy occurs. OHSS develops rapidly; therefore patients should be followed for at least two weeks after hCG administration. Most often, OHSS occurs after treatment has been discontinued and reaches its maximum at about seven to ten days following treatment. Usually, OHSS resolves spontaneously with the onset of menses. If there is evidence that OHSS may be developing prior to hCG administration (see PRECAUTIONS - Laboratory Tests ), the hCG should be withheld.
If severe OHSS occurs, treatment must be stopped and the patient should be hospitalized.
A physician experienced in the management of the syndrome, or who is experienced in the management of fluid and electrolyte imbalances, should be consulted.
Pulmonary and Vascular Complications
Serious pulmonary conditions (e.g. atelectasis, acute respiratory distress syndrome) have been reported. In addition, thromboembolic events both in association with, and separate from, the OHSS have been reported following menotropins therapy. Intravascular thrombosis and embolism, which may originate in venous or arterial vessels, can result in reduced blood flow to critical organs or the extremities. Sequelae of such events have included venous tbrombophlebitis, pulmonary embolism, pulmonary infarction, cerebral vascular occlusion (stroke), and arterial occlusion resulting in loss of limb. In rare cases, pulmonary complications and/or thromboembolic events have resulted in death.
Multiple Pregnancies
In the clinical trial multiple pregnancy as diagnosed by ultrasound occurred in 35.3% (n=30) of 85 total pregnancies.
The patient and her partner should be advised of the potential risk of multiple births before starting treatment.
PRECAUTIONS
General
Careful attention should be given to the diagnosis of infertility in the selection of candidates for Menopur® therapy (see " INDICATIONS AND USAGE - Selection of Patients ").
Information for Patients
Prior to therapy with Menopur®, patients should be informed of the duration of treatment and the monitoring of their condition that will be required. Possible adverse reactions (see ADVERSE REACTIONS section) and the risk of multiple births should also be discussed.
Laboratory Tests
The combination of both estradiol levels and ultrasonography are useful for monitoring the growth and development of follicles, timing hCG administration, as well as minimizing the risk of the OHSS and multiple gestations.
The clinical confirmation of ovulation, is determined by:
- A rise in basal body temperature;
- Increase in serum progesterone; and
- Menstruation following the shift in basal body temperature.
When used in conjunction with indices of progesterone production, sonographic visualization of the ovaries will assist in determining if ovulation has occurred. Sonographic evidence of ovulation may include the following:
- Fluid in the cul-de-sac;
- Ovarian stigmata; and
- Collapsed follicle.
Because of the subjectivity of the various tests for the determination of follicular maturation and ovulation, it cannot be overemphasized that the physician should choose tests with which he/she is thoroughly familiar.
Carcinogenesis and Mutagenesis
Long-term toxicity studies in animals have not been performed to evaluate the carcinogenic potential of menotropins.
Pregnancy
Pregnancy Category X: See CONTRAINDICATIONS section.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if menotropins are administered to a nursing woman.
Pediatric Patients
Safety and effectiveness in pediatric patients have not been established.
Geriatric Patients
Safety and effectiveness in geriatric patients have not been established.
ADVERSE REACTIONS
The safety of Menopur® was examined in 3 clinical studies that enrolled a total of 575 patients receiving Menopur® in the IVF and OI studies. All adverse events (without regard to causality assessment) occurring at an incidence of >/= 2 % in women treated with Menopur® are listed in Table 3.
Table 3: HIGHLY PURIFIED MENOTROPIN SC AND IM IN FEMALE PATIENTS UNDERGOING IVF AND OI ADVERSE EVENTS WITH ONSET ON OR AFTER GnRH ADMINISTRATION, COSTART CLASSIFICATION (FOR INCIDENCE OF 2% OR GREATER)BODY SYSTEM/PREFERRED TERM IVF *
n=499OI **
n=76N % N % BODY AS A WHOLEAbdomen Enlarged12 2.4 0 0.0 Abdominal cramps30 6.0 5 6.6 Abdominal fullness16 3.2 7 9.2 Abdominal pain88 17.6 7 9.2 Back pain16 3.2 0 0.0 Elevated estradiol12 2.4 0 0.0 Flu syndrome13 2.6 1 1.3 Flushing12 2.4 0 0.0 Headache170 34.1 12 15.8 Injection site pain27 5.4 0 0.0 Injection site reaction48 9.6 9 11.8 Malaise14 2.8 2 2.6 Pain16 3.2 2 2.6 CARDIOVASCULARMigraine12 2.4 0 0.0 DIGESTIVEConstipation8 1.6 0 0.0 Diarrhea14 2.8 2 2.6 Nausea60 12.0 6 7.9 Vomiting21 4.2 2 2.6 NERVOUSDizziness13 2.6 0 0.0 RESPIRATORYCough increased8 1.6 2 2.6 Respiratory disorder29 5.8 3 3.9 UROGENITALBreast tenderness9 1.8 2 2.6 Hot flash3 0.6 2 2.6 Menstrual disorder16 3.2 0 0.0 OHSS19 3.8 10 13.2 Pelvic cramps0 0.0 3 3.9 Pelvic discomfort2 0.4 2 2.6 Post retrieval pain32 6.4 0 0.0 Uterine spasm8 1.6 3 3.9 *INCLUDES IM AND SC SUBJECTS FROM PROTOCOLS MFK/IVF/O399E AND MENOPUR 2000-02.**INCLUDES IM AND SC SUBJECTS FROM PROTOCOL MENOPUR 2000-01.DRUG ABUSE AND DEPENDENCE
There have been no reports of abuse or dependence with menotropins.
OVERDOSAGE
Aside from possible ovarian hyperstimulation (see WARNINGS ), little is known concerning the consequences of acute overdosage with Menopur®.
DOSAGE AND ADMINISTRATION
-
Dosage:
Assisted Reproductive Technologies
The recommended initial dose of Menopur® for patients who have received a GnRH agonist for pituitary suppression is 225 IU. Based on clinical monitoring (including serum estradiol levels and vaginal ultrasound results) subsequent dosing should be adjusted according to individual patient response. Adjustments in dose should not be made more frequently than once every two days and should not exceed 150 IU per adjustment. The maximum daily dose of Menopur® given should not exceed 450 IU and dosing beyond 20 days is not recommended.
Once adequate follicular development is evident, hCG should be administered to induce final follicular maturation in preparation for oocyte retrieval. The administration of hCG must be withheld in cases where the ovaries are abnormally enlarged on the last day of therapy. This should reduce the chance of developing OHSS. -
Administration:
Dissolve the contents of one to six vials of Menopur® in one mL of sterile saline and ADMINISTER SUBCUTANEOUSLY immediately. Any unused reconstituted material should be discarded.
Parenteral drug products should be visually inspected for particulate matter and discoloration prior to administration, whenever solution and container permit.
The lower the abdomen (alternating sides) should be used for subcutaneous administration.
HOW SUPPLIED
Menopur® (menotropins for injection, USP) is supplied in sterile vials as a lyophilized, white to off-white powder or pellet.
Each vial of Menopur® is accompanied by a vial of sterile diluent containing 2 mL of 0.9 % Sodium Chloride Injection, USP:
75 IU FSH and 75 IU of LH activity, supplied as:
NDC 55566-7501-1 : Box of 5 vials + 5 vials diluent.
NDC 55566-7501-2 : Box of 5 vials + 5 vials diluent + 5 Q·Cap™ vial adapters.
STORAGE
Lyophilized powder may be stored refrigerated or at room temperature (3° to 25°C/37° to 77°F). Protect from light. Use immediately after reconstitution. Discard unused material.
DIRECTIONS FOR USING MENOPUR®
- Wash hands thoroughly with soap and water.
- Before injections, the septum tops of the vials should be wiped with an aseptic solution to prevent contamination of the contents.
- To prepare the Menopur® solution, inject 1 mL of Sterile Saline for Injection, USP into the vial of Menopur®. DO NOT SHAKE , but gently swirl until the solution is clear. Generally, the Menopur® dissolves immediately. Check the liquid in the container. If it is not clear or has particles in it, DO NOT USE IT .
- For patients requiring a single injection from multiple vials of Menopur®, up to 6 vials can be reconstituted with 1 mL of Sterile Saline for Injection, USP. This can be accomplished by reconstituting a single vial as described above (see step 3). Then draw the entire contents of the first vial into a syringe, and inject the contents into a second vial of lyophilized Menopur®. Gently swirl the second vial, as described above, once again checking to make sure the solution is clear and free of particles. This step can be repeated with 4 additional vials for a total of up to 6 vials of lyophilized Menopur® into 1 mL of diluent.
- Draw the reconstituted Menopur® into an empty, sterile syringe.
- Hold the syringe pointing upwards and gently tap the side to force any air bubbles to the top; then squeeze the plunger gently until all the air has been expelled and only Menopur® solution is left in the syringe.
- Menopur® works if it is injected SC . The recommended sites for SC injection are either side of the lower abdomen alternating between left and right sides of the lower abdomen below the naval. SC injection of Menopur® into the thigh is not recommended unless the lower abdomen is not useable because of scarring, surgical deformity or other medical conditions.
- The injection site should be swabbed with alcohol. Clean about two inches around the point where the needle will go in and let the alcohol dry for at least one minute before proceeding.
-
For
SC
injection, the needle should be inserted at a 90° angle to the skin surface.
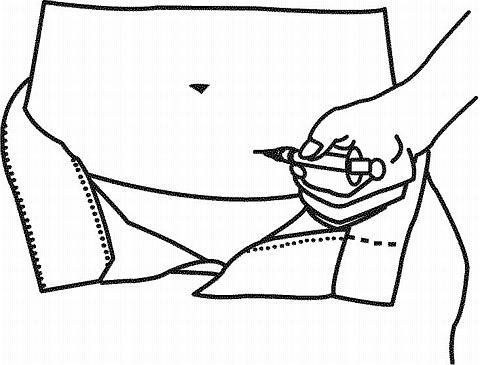
- If the needle is correctly positioned, it will be difficult to draw back on the plunger. Any blood drawn into the syringe means the needle tip has penetrated a vein or artery. If this happens, the needle should be withdrawn, cover the injection site with a swab containing alcohol and apply pressure; the site should stop bleeding in a minute or two. After withdrawing the needle, replace with a sterile needle and administer the injection.
- Once the needle is properly placed, depress the plunger slowly and steadily, so the solution is correctly injected and the skin or muscle tissue is not damaged.
- Withdraw the needle quickly and apply pressure to the site with a swab. If bleeding does not stop within a few minutes, place a clean piece of gauze and/or adhesive bandage over the site.
- Use the disposable syringe only once and dispose of it properly. Discard the used needle and syringe into your safety container. Do not reuse your injection materials.
Toll free number for providers and patients to call with questions:
1-(888)-FERRING (1-(888)-337-7464).
Rx only
Vials of sterile diluent of 0.9% Sodium Chloride Injection, USP manufactured for Ferring Pharmaceuticals Inc.
Manufactured for:
SUFFERN, NY 10901
By: CARDINAL HEALTH
Albuquerque, New Mexico 87107
6092-02
6-D6092FR-02
NOVEMBER 2004
Subscribe to the "News" RSS Feed 
TOP ۞
