-
Miacalcin Injection (Novartis)
Prescribing Information
The following prescribing information is based on official labeling in effect July 2005.
DESCRIPTION
Calcitonin is a polypeptide hormone secreted by the parafollicular cells of the thyroid gland in mammals and by the ultimobranchial gland of birds and fish.
Miacalcin® (calcitonin-salmon) Injection, Synthetic is a synthetic polypeptide of 32 amino acids in the same linear sequence that is found in calcitonin of salmon origin. This is shown by the following graphic formula:
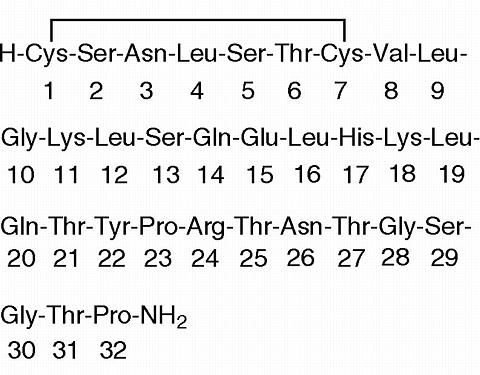
It is provided in sterile solution for intramuscular injection. Each milliliter contains; calcitonin-salmon 200 I.U., acetic acid, USP, 2.25 mg; phenol, USP, 5.0 mg; sodium acetate trihydrate, USP, 2.0 mg; sodium chloride, USP, 7.5 mg; water for injection, USP, qs to 1.0 mL.
The activity of Miacalcin Injection is stated in International Units based on bioassay in comparison with the International Reference Preparation of calcitonin-salmon for Bioassay, distributed by the National Institute for Biological Standards and Control, Holly Hill, London.
CLINICAL PHARMACOLOGY
Calcitonin acts primarily on bone, but direct renal effects and actions on the gastrointestinal tract are also recognized. Calcitonin-salmon appears to have actions essentially identical to calcitonins of mammalian origin, but its potency per mg is greater and it has a longer duration of action. The actions of calcitonin on bone and its role in normal human bone physiology are still incompletely understood.
Bone: Single injections of calcitonin cause a marked transient inhibition of the ongoing bone resorptive process. With prolonged use, there is a persistent, smaller decrease in the rate of bone resorption. Histologically, this is associated with a decreased number of osteoclasts and an apparent decrease in their resorptive activity. Decreased osteocytic resorption may also be involved. There is some evidence that initially bone formation may be augmented by calcitonin through increased osteoblastic activity. However, calcitonin will probably not induce a long-term increase in bone formation.
Animal studies indicate that endogenous calcitonin, primarily through its action on bone, participates with parathyroid hormone in the homeostatic regulation of blood calcium. Thus, high blood calcium levels cause increased secretion of calcitonin which, in turn, inhibits bone resorption. This reduces the transfer of calcium from bone to blood and tends to return blood calcium to the normal level. The importance of this process in humans has not been determined. In normal adults, who have a relatively low rate of bone resorption, the administration of exogenous calcitonin results in only a slight decrease in serum calcium. In normal children and in patients with generalized Paget's disease, bone resorption is more rapid and decreases in serum calcium are more pronounced in response to calcitonin.
Paget's Disease of Bone (osteitis deformans): Paget's disease is a disorder of uncertain etiology characterized by abnormal and accelerated bone formation and resorption in one or more bones. In most patients only small areas of bone are involved and the disease is not symptomatic. In a small fraction of patients, however, the abnormal bone may lead to bone pain and bone deformity, cranial and spinal nerve entrapment, or spinal cord compression. The increased vascularity of the abnormal bone may lead to high output congestive heart failure.
Active Paget's disease involving a large mass of bone may increase the urinary hydroxyproline excretion (reflecting breakdown of collagen-containing bone matrix) and serum alkaline phosphatase (reflecting increased bone formation).
Calcitonin-salmon, presumably by an initial blocking effect on bone resorption, causes a decreased rate of bone turnover with a resultant fall in the serum alkaline phosphatase and urinary hydroxyproline excretion in approximately 2/3 of patients treated. These biochemical changes appear to correspond to changes toward more normal bone, as evidenced by a small number of documented examples of: 1) radiologic regression of Pagetic lesions, 2) improvement of impaired auditory nerve and other neurologic function, 3) decreases (measured) in abnormally elevated cardiac output. These improvements occur extremely rarely, if ever, spontaneously (elevated cardiac output may disappear over a period of years when the disease slowly enters a sclerotic phase; in the cases treated with calcitonin, however, the decreases were seen in less than one year.)
Some patients with Paget's disease who have good biochemical and/or symptomatic responses initially, later relapse. Suggested explanations have included the formation of neutralizing antibodies and the development of secondary hyperparathyroidism, but neither suggestion appears to explain adequately the majority of relapses.
Although the parathyroid hormone levels do appear to rise transiently during each hypocalcemic response to calcitonin, most investigators have been unable to demonstrate persistent hypersecretion of parathyroid hormone in patients treated chronically with calcitonin-salmon.
Circulating antibodies to calcitonin after 2-18 months' treatment have been reported in about half of the patients with Paget's disease in whom antibody studies were done, but calcitonin treatment remained effective in many of these cases. Occasionally, patients with high antibody titers are found. These patients usually will have suffered a biochemical relapse of Paget's disease and are unresponsive to the acute hypocalcemic effects of calcitonin.
Hypercalcemia: In clinical trials, calcitonin-salmon has been shown to lower the elevated serum calcium of patients with carcinoma (with or without demonstrated metastases), multiple myeloma or primary hyperparathyroidism (lesser response). Patients with higher values for serum calcium tend to show greater reduction during calcitonin therapy. The decrease in calcium occurs about 2 hours after the first injection and lasts for about 6-8 hours. Calcitonin-salmon given every 12 hours maintained a calcium lowering effect for about 5-8 days, the time period evaluated for most patients during the clinical studies. The average reduction of 8-hour post-injection serum calcium during this period was about 9%.
Kidney: Calcitonin increases the excretion of filtered phosphate, calcium, and sodium by decreasing their tubular reabsorption. In some patients, the inhibition of bone resorption by calcitonin is of such magnitude that the consequent reduction of filtered calcium load more than compensates for the decrease in tubular reabsorption of calcium. The result in these patients is a decrease rather than an increase in urinary calcium.
Transient increases in sodium and water excretion may occur after the initial injection of calcitonin. In most patients, these changes return to pretreatment levels with continued therapy.
Gastrointestinal Tract: Increasing evidence indicates that calcitonin has significant actions on the gastrointestinal tract. Short-term administration results in marked transient decreases in the volume and acidity of gastric juice and in the volume and the trypsin and amylase content of pancreatic juice. Whether these effects continue to be elicited after each injection of calcitonin during chronic therapy has not been investigated.
Metabolism: The metabolism of calcitonin-salmon has not yet been studied clinically. Information from animal studies with calcitonin-salmon and from clinical studies with calcitonins of porcine and human origin suggest that calcitonin-salmon is rapidly metabolized by conversion to smaller inactive fragments, primarily in the kidneys, but also in the blood and peripheral tissues. A small amount of unchanged hormone and its inactive metabolites are excreted in the urine.
It appears that calcitonin-salmon cannot cross the placental barrier and its passage to the cerebrospinal fluid or to breast milk has not been determined.
INDICATIONS AND USAGE
Miacalcin® (calcitonin-salmon) Injection, Synthetic is indicated for the treatment of symptomatic Paget's disease of bone, for the treatment of hypercalcemia, and for the treatment of postmenopausal osteoporosis.
Paget's Disease: At the present time, effectiveness has been demonstrated principally in patients with moderate to severe disease characterized by polyostotic involvement with elevated serum alkaline phosphatase and urinary hydroxyproline excretion.
In these patients, the biochemical abnormalities were substantially improved (more than 30% reduction) in about 2/3 of patients studied, and bone pain was improved in a similar fraction. A small number of documented instances of reversal of neurologic deficits has occurred, including improvement in the basilar compression syndrome, and improvement of spinal cord and spinal nerve lesions. At present, there is too little experience to predict the likelihood of improvement of any given neurologic lesion. Hearing loss, the most common neurologic lesion of Paget's disease, is improved infrequently (4 of 29 patients studied audiometrically).
Patients with increased cardiac output due to extensive Paget's disease have had measured decreases in cardiac output while receiving calcitonin. The number of treated patients in this category is still too small to predict how likely such a result will be.
The large majority of patients with localized, especially monostotic disease do not develop symptoms and most patients with mild symptoms can be managed with analgesics. There is no evidence that the prophylactic use of calcitonin is beneficial in asymptomatic patients, although treatment may be considered in exceptional circumstances in which there is extensive involvement of the skull or spinal cord with the possibility of irreversible neurologic damage. In these instances, treatment would be based on the demonstrated effect of calcitonin on Pagetic bone, rather than on clinical studies in the patient population in question.
Hypercalcemia: Miacalcin Injection is indicated for early treatment of hypercalcemic emergencies, along with other appropriate agents, when a rapid decrease in serum calcium is required, until more specific treatment of the underlying disease can be accomplished. lt may also be added to existing therapeutic regimens for hypercalcemia such as intravenous fluids and furosemide, oral phosphate or corticosteroids, or other agents.
Postmenopausal Osteoporosis: Miacalcin Injection is indicated for the treatment of postmenopausal osteoporosis in females greater than 5 years postmenopause with low bone mass relative to healthy premenopausal females. Miacalcin Injection should be reserved for patients who refuse or cannot tolerate estrogens or in whom estrogens are contraindicated. Use of Miacalcin Injection is recommended in conjunction with adequate calcium and vitamin D intake to prevent the progressive loss of bone mass. No evidence currently exists to indicate whether or not Miacalcin Injection decreases the risk of vertebral crush fractures or spinal deformity. A recent controlled study, which was discontinued prior to completion because of questions regarding its design and implementation, failed to demonstrate any benefit of salmon calcitonin on fracture rate. No adequate controlled trials have examined the effect of salmon calcitonin injection on vertebral bone mineral density beyond 1 year of treatment. Two placebo-controlled studies with salmon calcitonin have shown an increase in total body calcium at 1 year, followed by a trend to decreasing total body calcium (still above baseline) at 2 years. The minimum effective dose of Miacalcin Injection for prevention of vertebral bone mineral density loss has not been established. It has been suggested that those postmenopausal patients having increased rates of bone turnover may be more likely to respond to anti-resorptive agents such as Miacalcin Injection.
CONTRAINDICATIONS
Clinical allergy to synthetic calcitonin-salmon.
WARNINGS
Allergic Reactions
Because calcitonin is protein in nature, the possibility of a systemic allergic reaction exists. Administration of calcitonin-salmon has been reported in a few cases to cause serious allergic-type reactions (e.g. bronchospasm, swelling of the tongue or throat, and anaphylactic shock), and in one case, death attributed to anaphylaxis. The usual provisions should be made for the emergency treatment of such a reaction should it occur. Allergic reactions should be differentiated from generalized flushing and hypotension.
For patients with suspected sensitivity to calcitonin, skin testing should be considered prior to treatment utilizing a dilute, sterile solution of Miacalcin® (calcitonin-salmon) Injection, Synthetic. Physicians may wish to refer patients who require skin testing to an allergist. A detailed skin testing protocol is available from the Medical Services Department of Novartis Pharmaceuticals Corporation.
The incidence of osteogenic sarcoma is known to be increased in Paget's disease. Pagetic lesions, with or without therapy, may appear by X-ray to progress markedly, possibly with some loss of definition of periosteal margins. Such lesions should be evaluated carefully to differentiate these from osteogenic sarcoma.
PRECAUTIONS
-
General
The administration of calcitonin possibly could lead to hypocalcemic tetany under special circumstances although no cases have yet been reported. Provisions for parenteral calcium administration should be available during the first several administrations of calcitonin. -
Laboratory Tests
Periodic examinations of urine sediment of patients on chronic therapy are recommended.
Coarse granular casts and casts containing renal tubular epithelial cells were reported in young adult volunteers at bed rest who were given calcitonin-salmon to study the effect of immobilization on osteoporosis. There was no other evidence of renal abnormality and the urine sediment became normal after calcitonin was stopped. Urine sediment abnormalities have not been reported by other investigators. -
Instructions for the Patient
Careful instruction in sterile injection technique should be given to the patient, and to other persons who may administer Miacalcin® (calcitonin-salmon) Injection, Synthetic. -
Carcinogenesis, Mutagenesis, and Impairment of Fertility
An increased incidence of pituitary adenomas has been observed in one-year toxicity studies in Sprague-Dawley rats administered calcitonin-salmon at dosages of 20 and 80 I.U./kg/day and in Fisher 344 rats given 80 I.U./kg/day. The relevance of these findings to humans is unknown. Calcitonin-salmon was not mutagenic in tests using Salmonella typhimurium, Escherichia coli, and Chinese Hamster V79 cells. -
Pregnancy: Teratogenic Effects
Category C
Calcitonin-salmon has been shown to cause a decrease in fetal birth weights in rabbits when given in doses 14-56 times the dose recommended for human use. Since calcitonin does not cross the placental barrier, this finding may be due to metabolic effects on the pregnant animal. There are no adequate and well-controlled studies in pregnant women. Miacalcin Injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. -
Nursing Mothers
It is not known whether this drug is excreted in human milk. As a general rule, nursing should not be undertaken while a patient is on this drug since many drugs are excreted in human milk. Calcitonin has been shown to inhibit lactation in animals. -
Pediatric Use
Disorders of bone in children referred to as juvenile Paget's disease have been reported rarely. The relationship of these disorders to adult Paget's disease has not been established and experience with the use of calcitonin in these disorders is very limited. There is no adequate data to support the use of Miacalcin Injection in children.
ADVERSE REACTIONS
Gastrointestinal System
Nausea with or without vomiting has been noted in about 10% of patients treated with calcitonin. It is most evident when treatment is first initiated and tends to decrease or disappear with continued administration.
Dermatologic/Hypersensitivity
Local inflammatory reactions at the site of subcutaneous or intramuscular injection have been reported in about 10% of patients. Flushing of face or hands occurred in about 2-5% of patients. Skin rashes, nocturia, pruritus of the ear lobes, feverish sensation, pain in the eyes, poor appetite, abdominal pain, edema of feet, and salty taste have been reported in patients treated with calcitonin-salmon. Administration of calcitonin-salmon has been reported in a few cases to cause serious allergic-type reactions (e.g. bronchospasm, swelling of the tongue or throat, and anaphylactic shock), and in one case, death attributed to anaphylaxis (see WARNINGS).
OVERDOSAGE
A dose of l000 I.U. subcutaneously may produce nausea and vomiting as the only adverse effects. Doses of 32 units per kg per day for 1-2 days demonstrate no other adverse effects.
Data on chronic high dose administration are insufficient to judge toxicity.
DOSAGE AND ADMINISTRATION
Paget's Disease: The recommended starting dose of Miacalcin® (calcitonin-salmon) Injection, Synthetic in Paget's disease is 100 I.U. (0.5 mL) per day administered subcutaneously (preferred for outpatient self-administration) or intramuscularly. Drug effect should be monitored by periodic measurement of serum alkaline phosphatase and 24-hour urinary hydroxyproline (if available) and evaluations of symptoms. A decrease toward normal of the biochemical abnormalities is usually seen, if it is going to occur, within the first few months. Bone pain may also decrease during that time. Improvement of neurologic lesions, when it occurs, requires a longer period of treatment, often more than one year.
In many patients, doses of 50 I.U. (0.25 mL) per day or every other day are sufficient to maintain biochemical and clinical improvement. At the present time, however, there are insufficient data to determine whether this reduced dose will have the same effect as the higher dose on forming more normal bone structure. It appears preferable, therefore, to maintain the higher dose in any patient with serious deformity or neurological involvement.
In any patient with a good response initially who later relapses, either clinically or biochemically, the possibility of antibody formation should be explored. The patient may be tested for antibodies by an appropriate specialized test or evaluated for the possibility of antibody formation by critical clinical evaluation.
Patient compliance should also be assessed in the event of relapse.
In patients who relapse, whether because of antibodies or for unexplained reasons, a dosage increase beyond 100 I.U. per day does not usually appear to elicit an improved response.
Hypercalcemia: The recommended starting dose of Miacalcin Injection in hypercalcemia is 4 I.U./kg body weight every 12 hours by subcutaneous or intramuscular injection. If the response to this dose is not satisfactory after one or two days, the dose may be increased to 8 l.U./kg every 12 hours. If the response remains unsatisfactory after two more days, the dose may be further increased to a maximum of 8 I.U./kg every 6 hours.
Postmenopausal Osteoporosis The minimum effective dose of Miacalcin Injection for the prevention of vertebral bone mineral density loss has not been established. Data from a single one-year placebo-controlled study with salmon calcitonin injection suggested that 100 I.U. (subcutaneously or intramuscularly) every other day might be effective in preserving vertebral bone mineral density. Baseline and interval monitoring of biochemical markers of bone resorption/turnover (e.g., fasting AM, second-voided urine hydroxyproline to creatinine ratio) and of bone mineral density may be useful in achieving the minimum effective dose. Patients should also receive supplemental calcium such as calcium carbonate 1.5 g daily and an adequate vitamin D intake (400 units daily). An adequate diet is also essential.
If the volume of Miacalcin Injection to be injected exceeds 2 mL, intramuscular injection is preferable and multiple sites of injection should be used.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
HOW SUPPLIED
Miacalcin® (calcitonin-salmon) Injection, Synthetic is available as a sterile solution in individual 2 mL vials containing 200 I.U. per mL (NDC 0078-0149-23).
Store in refrigerator between 2°C-8°C (36°F-46°F).
Manufactured by :
Novartis Pharma Stein AG
Stein, Switzerland
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, NJ 07936
T2002-84
REV: NOVEMBER 2002 890118101
-
General
Subscribe to the "News" RSS Feed 
TOP ۞
