-
MS Contin Tablets (Purdue Pharma)
*200 mg for use in opioid-tolerant patients only
OT01100A
301058-0A
DESCRIPTION
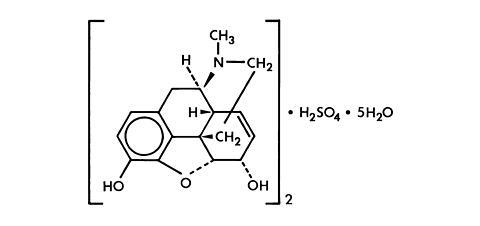
Chemically, morphine sulfate is 7,8-didehydro-4,5(alpha)-epoxy-17-methylmorphinan-3,6(alpha)-diol sulfate (2:1) (salt) pentahydrate and has the following structural formula:
MS CONTIN® Controlled-release Tablets 15 mg, 30 mg, 60 mg, 100 mg, and 200 mg contain the following inactive ingredients: cetostearyl alcohol, hydroxyethyl cellulose, hypromellose, magnesium stearate, polyethylene glycol, talc and titanium dioxide.
MS CONTIN® Controlled-release Tablets 15 mg also contains FD&C Blue No. 2, lactose and polysorbate 80.
MS CONTIN® Controlled-release Tablets 30 mg also contains D&C Red No. 7, FD&C Blue No. 1, lactose and polysorbate 80.
MS CONTIN® Controlled-release Tablets 60 mg also contains D&C Red No. 30, D&C Yellow No. 10, hydroxypropyl cellulose, and lactose.
MS CONTIN® Controlled-release Tablets 100 mg also contains black iron oxide.
MS CONTIN® Controlled-release Tablets 200 mg also contains D&C Yellow No. 10, FD&C Blue No. 1, and hydroxypropyl cellulose.
CLINICAL PHARMACOLOGY
Pharmacokinetics and Metabolism
MS CONTIN is a controlled-release tablet containing morphine sulfate. Following oral administration of a given dose of morphine, the amount ultimately absorbed is essentially the same whether the source is MS CONTIN or a conventional formulation. Morphine is released from MS CONTIN somewhat more slowly than from conventional oral preparations. Because of pre-systemic elimination (i.e., metabolism in the gut wall and liver) only about 40% of the administered dose reaches the central compartment.
Once absorbed, morphine is distributed to skeletal muscle, kidneys, liver, intestinal tract, lungs, spleen, and brain. Morphine also crosses the placental membranes and has been found in breast milk.
Although a small fraction (less than 5%) of morphine is demethylated, for all practical purposes, virtually all morphine is converted to glucuronide metabolites; among these, morphine-3-glucuronide is present in the highest plasma concentration following oral administration.
The glucuronide system has a very high capacity and is not easily saturated even in disease. Therefore, rate of delivery of morphine to the gut and liver should not influence the total and, probably, the relative quantities of the various metabolites formed. Moreover, even if rate affected the relative amounts of each metabolite formed, it should be unimportant clinically because morphine's metabolites are ordinarily inactive.
The following pharmacokinetic parameters show considerable inter-subject variation but are representative of average values reported in the literature. The volume of distribution (Vd) for morphine is 4 liters per kilogram, and its terminal elimination half-life is normally 2 to 4 hours.
Following the administration of conventional oral morphine products, approximately fifty percent of the morphine that will reach the central compartment intact reaches it within 30 minutes. Following the administration of an equal amount of MS CONTIN to normal volunteers, however, this extent of absorption occurs, on average, after 1.5 hours.
The possible effect of food upon the systemic bioavailability of MS CONTIN has not been systematically evaluated for all strengths. Data from at least one study suggests that concurrent administration of MS CONTIN with a fatty meal may cause a slight decrease in peak plasma concentration.
Variation in the physical/mechanical properties of a formulation of an oral morphine drug product can affect both its absolute bioavailability and its absorption rate constant (k a ). The formulation employed in MS CONTIN has not been shown to affect morphine's oral bioavailability, but does decrease its apparent k a . Other basic pharmacokinetic parameters (e.g., volume of distribution [Vd], elimination rate constant [k e ], clearance [Cl]), are unchanged as they are fundamental properties of morphine in the organism. However, in chronic use, the possibility that shifts in metabolite to parent drug ratios may occur cannot be excluded.
When immediate-release oral morphine or MS CONTIN is given on a fixed dosing regimen, steady-state is achieved in about a day.
For a given dose and dosing interval, the AUC and average blood concentration of morphine at steady-state (Css) will be independent of the specific type of oral formulation administered so long as the formulations have the same absolute bioavailability. The absorption rate of a formulation will, however, affect the maximum (C max ) and minimum (C min ) blood levels and the times of their occurrence.
Pharmacodynamics
The effects described below are common to all morphine-containing products.
Central Nervous System
The principal actions of therapeutic value of morphine are analgesia and sedation (i.e., sleepiness and anxiolysis).
The precise mechanism of the analgesic action is unknown. However, specific CNS opiate receptors and endogenous compounds with morphine-like activity have been identified throughout the brain and spinal cord and are likely to play a role in the expression of analgesic effects.
Morphine produces respiratory depression by direct action on brain stem respiratory centers. The mechanism of respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension, and to electrical stimulation.
Morphine depresses the cough reflex by direct effect on the cough center in the medulla. Antitussive effects may occur with doses lower than those usually required for analgesia. Morphine causes miosis, even in total darkness. Pinpoint pupils are a sign of narcotic overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen with worsening hypoxia.
Gastrointestinal Tract and Other Smooth Muscle
Gastric, biliary, and pancreatic secretions are decreased by morphine. Morphine causes a reduction in motility associated with an increase in tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone is increased to the point of spasm. The end result is constipation. Morphine can cause a marked increase in biliary tract pressure as a result of spasm of sphincter of Oddi.
Cardiovascular System
Morphine produces peripheral vasodilation which may result in orthostatic hypotension. Release of histamine can occur and may contribute to narcotic-induced hypotension. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, and sweating.
Plasma Level-Analgesia Relationships
In any particular patient, both analgesic effects and plasma morphine concentrations are related to the morphine dose. In non-tolerant individuals, plasma morphine concentration-efficacy relationships have been demonstrated and suggest that opiate receptors occupy effector compartments, leading to a lag-time, or hysteresis, between rapid changes in plasma morphine concentrations and the effects of such changes. The most direct and predictable concentration-effect relationships can, therefore, be expected at distribution equilibrium and/or steady-state conditions. In general, the minimum effective analgesic concentration in the plasma of non-tolerant patients ranges from approximately 5 to 20 ng/mL.
While plasma morphine-efficacy relationships can be demonstrated in non-tolerant individuals, they are influenced by a wide variety of factors and are not generally useful as a guide to the clinical use of morphine. The effective dose in opioid-tolerant patients may be 10-50 times as great (or greater) than the appropriate dose for opioid-naive individuals. Dosages of morphine should be chosen and must be titrated on the basis of clinical evaluation of the patient and the balance between therapeutic and adverse effects.
For any fixed dose and dosing interval, MS CONTIN will have at steady-state, a lower C max and a higher C min than conventional morphine. This is a potential advantage; a reduced fluctuation in morphine concentration during the dosing interval should keep morphine blood levels more centered within the theoretical "therapeutic window." (Fluctuation for a dosing interval is defined as [C max -C min ]/[Css-average].) On the other hand, the degree of fluctuation in serum morphine concentration might conceivably affect other phenomena. For example, reduced fluctuations in blood morphine concentrations might influence the rate of tolerance induction.
The elimination of morphine occurs primarily as renal excretion of 3-morphine glucuronide. A small amount of the glucuronide conjugate is excreted in the bile, and there is some minor enterohepatic recycling. Because morphine is primarily metabolized to inactive metabolites, the effects of renal disease on morphine's elimination are not likely to be pronounced. However, as with any drug, caution should be taken to guard against unanticipated accumulation if renal and/or hepatic function is seriously impaired.
INDICATIONS AND USAGE
MS CONTIN is a controlled-release oral morphine formulation indicated for the relief of moderate to severe pain. It is intended for use in patients who require repeated dosing with potent opioid analgesics over periods of more than a few days.
The MS CONTIN 200 mg tablet strength is a high dose, controlled-release, oral morphine formulation indicated for the relief of pain in opioid-tolerant patients only.
CONTRAINDICATIONS
MS CONTIN is contraindicated in patients with known hypersensitivity to the drug, in patients with respiratory depression in the absence of resuscitative equipment, and in patients with acute or severe bronchial asthma.
MS CONTIN is contraindicated in any patient who has or is suspected of having a paralytic ileus.
WARNINGS
(See also: CLINICAL PHARMACOLOGY )
Impaired Respiration
Respiratory depression is the chief hazard of all morphine preparations. Respiratory depression occurs most frequently in the elderly and debilitated patients as well as in those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation.
Morphine should be used with extreme caution in patients with chronic obstructive pulmonary disease or cor pulmonale, and in patients having a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression. In such patients, even usual therapeutic doses of morphine may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea.
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of morphine with carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions, or pre-existing increase in intracranial pressure. Morphine produces effects which may obscure neurologic signs of further increases in pressure in patients with head injuries.
Hypotensive Effect
MS CONTIN, like all opioid analgesics, may cause severe hypotension in an individual whose ability to maintain his blood pressure has already been compromised by a depleted blood volume, or a concurrent administration of drugs such as phenothiazines or general anesthetics. (See also: PRECAUTIONS; Drug Interactions .) MS CONTIN may produce orthostatic hypotension in ambulatory patients.
MS CONTIN, like all opioid analgesics, should be administered with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
Interactions with other CNS Depressants
MS CONTIN, like all opioid analgesics, should be used with great caution and in reduced dosage in patients who are concurrently receiving other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, other tranquilizers, and alcohol because respiratory depression, hypotension, and profound sedation or coma may result.
Interactions with Mixed Agonist/Antagonist Opioid Analgesics
From a theoretical perspective, agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol, and buprenorphine) should NOT be administered to a patient who has received or is receiving a course of therapy with a pure opioid agonist analgesic. In these patients, mixed agonist/antagonist analgesics may reduce the analgesic effect or may precipitate withdrawal symptoms.
Drug Dependence
Morphine can produce drug dependence and has a potential for being abused. Tolerance as well as psychological and physical dependence may develop upon repeated administration. Physical dependence, however, is not of paramount importance in the management of terminally ill patients or any patients in severe pain. Abrupt cessation or a sudden reduction in dose after prolonged use may result in withdrawal symptoms. After prolonged exposure to opioid analgesics, if withdrawal is necessary, it must be undertaken gradually. (See DRUG ABUSE AND DEPENDENCE .)
Infants born to mothers physically dependent on opioid analgesics may also be physically dependent and exhibit respiratory depression and withdrawal symptoms. (See DRUG ABUSE AND DEPENDENCE .)
Other
Although extremely rare, cases of anaphylaxis have been reported.
PRECAUTIONS (See also: CLINICAL PHARMACOLOGY )
Special precautions regarding MS CONTIN 200 mg Tablets
MS CONTIN 200 mg Tablets are for use only in opioid-tolerant patients requiring daily morphine equivalent dosages of 400 mg or more. Care should be taken in its prescription and patients should be instructed against use by individuals other than the patient for whom it was prescribed, as this may have severe medical consequences for that individual.
General
MS CONTIN® is intended for use in patients who require more than several days continuous treatment with a potent opioid analgesic. The controlled-release nature of the formulation allows it to be administered on a more convenient schedule than conventional immediate-release oral morphine products. (See CLINICAL PHARMACOLOGY: Pharmacokinetics and Metabolism .) However, MS CONTIN does not release morphine continuously over the course of a dosing interval. The administration of single doses of MS CONTIN on a q12h dosing schedule will result in higher peak and lower trough plasma levels than those that occur when an identical daily dose of morphine is administered using conventional oral formulations on a q4h regimen. The clinical significance of greater fluctuations in morphine plasma level has not been systematically evaluated. (See DOSAGE AND ADMINISTRATION .)
As with any potent opioid, it is critical to adjust the dosing regimen for each patient individually, taking into account the patient's prior analgesic treatment experience. Although it is clearly impossible to enumerate every consideration that is important to the selection of the initial dose and dosing interval of MS CONTIN, attention should be given to 1) the daily dose, potency, and characteristics of the opioid the patient has been taking previously (e.g., whether it is a pure agonist or mixed agonist/antagonist), 2) the reliability of the relative potency estimate used to calculate the dose of morphine needed [N.B. potency estimates may vary with the route of administration], 3) the degree of opioid tolerance, if any, and 4) the general condition and medical status of the patient.
Selection of patients for treatment with MS CONTIN should be governed by the same principles that apply to the use of morphine or other potent opioid analgesics. Specifically, the increased risks associated with its use in the following populations should be considered: the elderly or debilitated and those with severe impairment of hepatic, pulmonary, or renal function; myxedema or hypothyroidism; adrenocortical insufficiency (e.g., Addison's Disease); CNS depression or coma; toxic psychosis; prostatic hypertrophy, or urethral stricture; acute alcoholism; delirium tremens; kyphoscoliosis, or inability to swallow.
The administration of morphine, like all opioid analgesics, may obscure the diagnosis or clinical course in patients with acute abdominal conditions.
Morphine may aggravate pre-existing convulsions in patients with convulsive disorders. Morphine should be used with caution in patients about to undergo surgery of the biliary tract since it may cause spasm of the sphincter of Oddi. Similarly, morphine should be used with caution in patients with acute pancreatitis secondary to biliary tract disease.
Information for Patients
If clinically advisable, patients receiving MS CONTIN should be given the following instructions by the physician:
- Appropriate pain management requires changes in the dose to maintain best pain control. Patients should be advised of the need to contact their physician if pain control is inadequate, but not to change the dose of MS CONTIN without consulting their physician.
- Morphine may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating machinery). Patients started on MS CONTIN or whose dose has been changed should refrain from dangerous activity until it is established that they are not adversely affected.
- Morphine should not be taken with alcohol or other CNS depressants (sleep aids, tranquilizers) because additive effects including CNS depression may occur. A physician should be consulted if other prescription medications are currently being used or are prescribed for future use.
- For women of childbearing potential who become or are planning to become pregnant, a physician should be consulted regarding analgesics and other drug use.
- Upon completion of therapy, it may be appropriate to taper the morphine dose, rather than abruptly discontinue it.
- While psychological dependence ("addiction") to morphine used in the treatment of pain is very rare, morphine is one of a class of drugs known to be abused and should be handled accordingly.
- The MS CONTIN 200 mg Tablet is for use only in opioid-tolerant patients requiring daily morphine equivalent dosages of 400 mg or more. Special care must be taken to avoid accidental ingestion or the use by individuals (including children) other than the patient for whom it was originally prescribed, as such unsupervised use may have severe, even fatal, consequences.
Drug Interactions (See also: WARNINGS )
The concomitant use of other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, tranquilizers, and alcohol may produce additive depressant effects. Respiratory depression, hypotension, and profound sedation or coma may occur. When such combined therapy is contemplated, the dose of one or both agents should be reduced. Opioid analgesics, including MS CONTIN, may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression.
Carcinogenicity/Mutagenicity/Impairment of Fertility
Studies of morphine sulfate in animals to evaluate the drug's carcinogenic and mutagenic potential or the effect on fertility have not been conducted.
Pregnancy
Teratogenic Effects - CATEGORY C
Adequate animal studies on reproduction have not been performed to determine whether morphine affects fertility in males or females. There are no well-controlled studies in women, but marketing experience does not include any evidence of adverse effects on the fetus following routine (short-term) clinical use of morphine sulfate products. Although there is no clearly defined risk, such experience cannot exclude the possibility of infrequent or subtle damage to the human fetus.
MS CONTIN® should be used in pregnant women only when clearly needed. (See also: PRECAUTIONS: Labor and Delivery , and DRUG ABUSE AND DEPENDENCE .)
Nonteratogenic Effects
Infants born from mothers who have been taking morphine chronically may exhibit withdrawal symptoms.
Labor and Delivery
MS CONTIN is not recommended for use in women during and immediately prior to labor. Occasionally, opioid analgesics may prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilatation which tends to shorten labor.
Neonates whose mothers received opioid analgesics during labor should be observed closely for signs of respiratory depression. A specific narcotic antagonist, naloxone, should be available for reversal of narcotic-induced respiratory depression in the neonate.
Nursing Mothers
Low levels of morphine have been detected in the breast milk. Withdrawal symptoms can occur in breast-feeding infants when maternal administration of morphine sulfate is stopped. Ordinarily, nursing should not be undertaken while a patient is receiving MS CONTIN since morphine may be excreted in the milk.
Pediatric Use
Use of MS CONTIN has not been evaluated systematically in children.
Geriatric Use
Clinical studies of MS CONTIN did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The adverse reactions caused by morphine are essentially those observed with other opioid analgesics. They include the following major hazards: respiratory depression, apnea, and to a lesser degree, circulatory depression, respiratory arrest, shock, and cardiac arrest.
Most Frequently Observed
Constipation, lightheadedness, dizziness, sedation, nausea, vomiting, sweating, dysphoria, and euphoria.
Some of these effects seem to be more prominent in ambulatory patients and in those not experiencing severe pain. Some adverse reactions in ambulatory patients may be alleviated if the patient lies down.
Less Frequently Observed Reactions
Central Nervous System: Weakness, headache, agitation, tremor, uncoordinated muscle movements, seizure, alterations of mood (nervousness, apprehension, depression, floating feelings), dreams, muscle rigidity, transient hallucinations and disorientation, visual disturbances, insomnia, increased intracranial pressure
Gastrointestinal: Dry mouth, biliary tract spasm, laryngospasm, anorexia, diarrhea, cramps, taste alteration, constipation, ileus, intestinal obstruction, increases in hepatic enzymes.
Cardiovascular: Flushing of the face, chills, tachycardia, bradycardia, palpitation, faintness, syncope, hypotension, hypertension
Genitourinary: Urine retention or hesitance, reduced libido and/or potency
Dermatologic: Pruritus, urticaria, other skin rashes, edema, diaphoresis
Other: Antidiuretic effect, paresthesia, muscle tremor, blurred vision, nystagmus, diplopia, miosis, anaphylaxis
DRUG ABUSE AND DEPENDENCE
Opioid analgesics may cause psychological and physical dependence (see WARNINGS ). Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug or may be precipitated through the administration of drugs with narcotic antagonist activity, e.g., naloxone or mixed agonist/antagonist analgesics (pentazocine, etc.; see also OVERDOSAGE ). Physical dependence usually does not occur to a clinically significant degree until after several weeks of continued narcotic usage. Tolerance, in which increasingly large doses are required in order to produce the same degree of analgesia, is initially manifested by a shortened duration of analgesic effect, and, subsequently, by decreases in the intensity of analgesia.
In chronic-pain patients, and in narcotic-tolerant cancer patients, the administration of MS CONTIN should be guided by the degree of tolerance manifested. Physical dependence, per se, is not ordinarily a concern when one is dealing with opioid-tolerant patients whose pain and suffering is associated with an irreversible illness.
If MS CONTIN is abruptly discontinued, a moderate to severe abstinence syndrome may occur. The opioid agonist abstinence syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, gooseflesh, restless sleep or "yen", and mydriasis during the first 24 hours. These symptoms often increase in severity and over the next 72 hours may be accompanied by increasing irritability, anxiety, weakness, twitching and spasms of muscles; kicking movements; severe backache, abdominal and leg pains; abdominal and muscle cramps; hot and cold flashes, insomnia; nausea, anorexia, vomiting, intestinal spasm, diarrhea; coryza and repetitive sneezing; increase in body temperature, blood pressure, respiratory rate and heart rate. Because of excessive loss of fluids through sweating, vomiting, and diarrhea, there is usually marked weight loss, dehydration, ketosis, and disturbances in acid-base balance. Cardiovascular collapse can occur. Without treatment most observable symptoms disappear in 5-14 days; however, there appears to be a phase of secondary or chronic abstinence which may last for 2-6 months characterized by insomnia, irritability, and muscular aches.
If treatment of physical dependence of patients on MS CONTIN is necessary, the patient may be detoxified by gradual reduction of the dosage. Gastrointestinal disturbances or dehydration should be treated accordingly.
OVERDOSAGE
Acute overdosage with morphine is manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and, sometimes, bradycardia, hypotension and death.
In the treatment of overdosage, primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. The pure opioid antagonist, naloxone, is a specific antidote against respiratory depression which results from opioid overdose. Naloxone (usually 0.4 to 2.0 mg) should be administered intravenously; however, because its duration of action is relatively short, the patient must be carefully monitored until spontaneous respiration is reliably re-established. If the response to naloxone is suboptimal or not sustained, additional naloxone may be administered, as needed, or given by continuous infusion to maintain alertness and respiratory function; however, there is no information available about the cumulative dose of naloxone that may be safely administered.
Naloxone should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to morphine overdose. Naloxone should be administered cautiously to persons who are known or suspected to be physically dependent on MS CONTIN. In such cases, an abrupt or complete reversal of narcotic effects may precipitate an acute abstinence syndrome.
Note: In an individual physically dependent on opioids, administration of the usual dose of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. Use of a narcotic antagonist in such a person should be avoided. If necessary to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with care and by titration with smaller than usual doses of the antagonist.
Supportive measures (including oxygen, vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
DOSAGE AND ADMINISTRATION
(See also: CLINICAL PHARMACOLOGY , WARNINGS , and PRECAUTIONS sections)
MS CONTIN TABLETS ARE TO BE TAKEN WHOLE, AND ARE NOT TO BE BROKEN, CHEWED, OR CRUSHED.
TAKING BROKEN, CHEWED, OR CRUSHED MS CONTIN TABLETS COULD LEAD TO THE RAPID RELEASE AND ABSORPTION OF A POTENTIALLY TOXIC DOSE OF MORPHINE.
MS CONTIN is intended for use in patients who require more than several days continuous treatment with a potent opioid analgesic. The controlled-release nature of the formulation allows it to be administered on a more convenient schedule than conventional immediate-release oral morphine products. (See CLINICAL PHARMACOLOGY: Pharmacokinetics and Metabolism .) However, MS CONTIN does not release morphine continuously over the course of a dosing interval. The administration of single doses of MS CONTIN on a q12h dosing schedule will result in higher peak and lower trough plasma levels than those that occur when an identical daily dose of morphine is administered using conventional oral formulations on a q4h regimen. The clinical significance of greater fluctuations in morphine plasma level has not been systematically evaluated.
As with any potent opioid drug product, it is critical to adjust the dosing regimen for each patient individually, taking into account the patient's prior analgesic treatment experience. Although it is clearly impossible to enumerate every consideration that is important to the selection of initial dose and dosing interval of MS CONTIN, attention should be given to 1) the daily dose, potency, and precise characteristics of the opioid the patient has been taking previously (e.g., whether it is a pure agonist or mixed agonist/antagonist), 2) the reliability of the relative potency estimate used to calculate the dose of morphine needed [N.B. potency estimates may vary with the route of administration], 3) the degree of opioid tolerance, if any, and 4) the general condition and medical status of the patient.
The following dosing recommendations, therefore, can only be considered suggested approaches to what is actually a series of clinical decisions in the management of the pain of an individual patient.
Conversion from Conventional Oral Morphine to MS CONTIN
A patient's daily morphine requirement is established using immediate-release oral morphine (dosing every 4 to 6 hours). The patient is then converted to MS CONTIN in either of two ways: 1) by administering one-half of the patient's 24-hour requirement as MS CONTIN on an every 12-hour schedule; or, 2) by administering one-third of the patient's daily requirement as MS CONTIN on an every eight hour schedule. With either method, dose and dosing interval is then adjusted as needed (see discussion below). The 15 mg tablet should be used for initial conversion for patients whose total daily requirement is expected to be less than 60 mg. The 30 mg tablet strength is recommended for patients with a daily morphine requirement of 60 to 120 mg. When the total daily dose is expected to be greater than 120 mg, the appropriate combination of tablet strengths should be employed.
Conversion from Parenteral Morphine or Other Opioids (Parenteral or Oral) to MS CONTIN
MS CONTIN can be administered as the initial oral morphine drug product; in this case, however, particular care must be exercised in the conversion process. Because of uncertainty about, and intersubject variation in, relative estimates of opioid potency and cross tolerance, initial dosing regimens should be conservative; that is, an underestimation of the 24-hour oral morphine requirement is preferred to an overestimate. To this end, initial individual doses of MS CONTIN should be estimated conservatively. In patients whose daily morphine requirements are expected to be less than or equal to 120 mg per day, the 30 mg tablet strength is recommended for the initial titration period. Once a stable dose regimen is reached, the patient can be converted to the 60 mg or 100 mg tablet strength, or an appropriate combination of tablet strengths, if desired.
Estimates of the relative potency of opioids are only approximate and are influenced by route of administration, individual patient differences, and possibly, by an individual's medical condition. Consequently, it is difficult to recommend any fixed rule for converting a patient to MS CONTIN directly. The following general points should be considered, however.
- Parenteral to oral morphine ratio: Estimates of the oral to parenteral potency of morphine vary. Some authorities suggest that a dose of oral morphine only three times the daily parenteral morphine requirement may be sufficient in chronic use settings.
- Other parenteral or oral opioids to oral morphine: Because there is lack of systematic evidence bearing on these types of analgesic substitutions, specific recommendations are not possible.
Physicians are advised to refer to published relative potency data, keeping in mind that such ratios are only approximate. In general, it is safer to underestimate the daily dose of MS CONTIN required and rely upon ad hoc supplementation to deal with inadequate analgesia. (See discussion which follows.)
Use of MS CONTIN as the First Opioid Analgesic
There has been no systematic evaluation of MS CONTIN as an initial opioid analgesic in the management of pain. Because it may be more difficult to titrate a patient using a controlled-release morphine, it is ordinarily advisable to begin treatment using an immediate-release formulation.
Considerations in the Adjustment of Dosing Regimens
Whatever the approach, if signs of excessive opioid effects are observed early in a dosing interval, the next dose should be reduced. If this adjustment leads to inadequate analgesia, that is, "breakthrough" pain occurs late in the dosing interval, the dosing interval may be shortened. Alternatively, a supplemental dose of a short-acting analgesic may be given. As experience is gained, adjustments can be made to obtain an appropriate balance between pain relief, opioid side effects, and the convenience of the dosing schedule.
In adjusting dosing requirements, it is recommended that the dosing interval never be extended beyond 12 hours because the administration of very large single doses may lead to acute overdose. (N.B. MS CONTIN is a controlled-release formulation; it does not release morphine continuously over the dosing interval.)
For patients with low daily morphine requirements, the 15 mg tablet should be used.
Special Instructions for MS CONTIN® 200 mg Tablets
(For use in opioid-tolerant patients only.)
The MS CONTIN 200 mg tablet is for use only in opioid-tolerant patients requiring daily morphine equivalent dosages of 400 mg or more. It is recommended that this strength be reserved for patients that have already been titrated to a stable analgesic regimen using lower strengths of MS CONTIN or other opioids.
Conversion from MS CONTIN to Parenteral Opioids
When converting a patient from MS CONTIN to parenteral opioids, it is best to assume that the parenteral to oral potency is high. NOTE THAT THIS IS THE CONVERSE OF THE STRATEGY USED WHEN THE DIRECTION OF CONVERSION IS FROM THE PARENTERAL TO ORAL FORMULATIONS. IN BOTH CASES, HOWEVER, THE AIM IS TO ESTIMATE THE NEW DOSE CONSERVATIVELY. For example, to estimate the required 24-hour dose of morphine for IM use, one could employ a conversion of 1 mg of morphine IM for every 6 mg of morphine as MS CONTIN. Of course, the IM 24-hour dose would have to be divided by six and administered on a q4h regimen. This approach is recommended because it is least likely to cause overdose.
SAFETY AND HANDLING
MS CONTIN TABLETS ARE TO BE TAKEN WHOLE, AND ARE NOT TO BE BROKEN, CHEWED, OR CRUSHED. TAKING BROKEN, CHEWED, OR CRUSHED MS CONTIN TABLETS COULD LEAD TO THE RAPID RELEASE AND ABSORPTION OF A POTENTIALLY TOXIC DOSE OF MORPHINE.
The MS CONTIN 200 mg tablet strength is for use only in opioid-tolerant patients requiring daily morphine equivalent dosages of 400 mg or more. This strength is potentially toxic if accidentally ingested and patients and their families should be instructed to take special care to avoid accidental or intentional ingestion by individuals other than those for whom the medication was originally prescribed.
HOW SUPPLIED
NDC 59011-260-10: MS CONTIN (morphine sulfate controlled-release tablets) 15 mg are supplied in opaque plastic bottles containing 100 tablets.
NDC 59011-260-05: MS CONTIN (morphine sulfate controlled-release tablets) 15 mg are supplied in opaque plastic bottles containing 500 tablets.
NDC 59011-261-50: MS CONTIN (morphine sulfate controlled-release tablets) 30 mg are supplied in opaque plastic bottles containing 50 tablets.
NDC 59011-261-25: MS CONTIN (morphine sulfate controlled-release tablets) 30 mg are supplied in opaque plastic bottles containing 100 tablets.
NDC 59011-261-12: MS CONTIN (morphine sulfate controlled-release tablets) 30 mg are supplied in opaque plastic bottles containing 250 tablets.
NDC 59011-261-05: MS CONTIN (morphine sulfate controlled-release tablets) 30 mg are supplied in opaque plastic bottles containing 500 tablets.
NDC 59011-262-10: MS CONTIN (morphine sulfate controlled-release tablets) 60 mg are supplied in opaque plastic bottles containing 100 tablets.
NDC 59011-262-05: MS CONTIN (morphine sulfate controlled-release tablets) 60 mg are supplied in opaque plastic bottles containing 500 tablets.
NDC 59011-263-10: MS CONTIN (morphine sulfate controlled-release tablets) 100 mg are supplied in opaque plastic bottles containing 100 tablets.
NDC 59011-263-05: MS CONTIN (morphine sulfate controlled-release tablets) 100 mg are supplied in opaque plastic bottles containing 500 tablets.
NDC 59011-264-10: MS CONTIN (morphine sulfate controlled-release tablets) 200 mg are supplied in opaque plastic bottles containing 100 tablets.
15 mg: Each round, blue-colored, film-coated tablet bears the symbol PF on one side and M 15 on the other side.
30 mg: Each round, lavender-colored, film-coated tablet bears the symbol PF on one side and M 30 on the other side.
60 mg: Each round, orange-colored, film-coated tablet bears the symbol PF on one side and M 60 on the other side.
100 mg: Each round, gray-colored, film-coated tablet bears the symbol PF on one side and 100 on the other side.
200 mg: Each capsule-shaped, green-colored, film-coated tablet bears the symbol PF on one side and M 200 on the other side.
Store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F).
Dispense in a tight, light-resistant container.
CAUTION
DEA Order Form Required.
©2004 Purdue Pharma L.P.
Purdue Pharma L.P.
Stamford, CT 06901-3431
December 22, 2004
OT01100A
301058-0A
Subscribe to the "News" RSS Feed 
TOP ۞
