-
Neumega for Injection (Wyeth)
This product's label may have been revised after this insert was used in production. For further product information and current package insert, please visit www.wyeth.com or call our medical communications department toll-free at 1-800-934-5556.
BOXED WARNING
Allergic Reactions Including Anaphylaxis
Neumega has caused allergic or hypersensitivity reactions, including anaphylaxis. Administration of Neumega should be permanently discontinued in any patient who develops an allergic or hypersensitivity reaction (see WARNINGS , CONTRAINDICATIONS , ADVERSE REACTIONS and ADVERSE REACTIONS , Immunogenicity ).
DESCRIPTION
Interleukin eleven (IL-11) is a thrombopoietic growth factor that directly stimulates the proliferation of hematopoietic stem cells and megakaryocyte progenitor cells and induces megakaryocyte maturation resulting in increased platelet production. IL-11 is a member of a family of human growth factors which includes human growth hormone, granulocyte colony-stimulating factor (G-CSF), and other growth factors.
Oprelvekin, the active ingredient in Neumega, is produced in Escherichia coli (E. coli) by recombinant DNA technology. The protein has a molecular mass of approximately 19,000 daltons, and is non-glycosylated. The polypeptide is 177 amino acids in length and differs from the 178 amino acid length of native IL-11 only in lacking the amino-terminal proline residue. This alteration has not resulted in measurable differences in bioactivity either in vitro or in vivo.
Neumega is formulated in single-use vials containing 5 mg of oprelvekin (specific activity approximately 8 x 10 6 Units/mg) as a sterile, lyophilized powder with 23 mg Glycine, USP, 1.6 mg Dibasic Sodium Phosphate Heptahydrate, USP, and 0.55 mg Monobasic Sodium Phosphate Monohydrate, USP. When reconstituted with 1 mL of Sterile Water for Injection, USP, the resulting solution has a pH of 7.0 and a concentration of 5 mg/mL.
CLINICAL PHARMACOLOGY
The primary hematopoietic activity of Neumega is stimulation of megakaryocytopoiesis and thrombopoiesis. Neumega has shown potent thrombopoietic activity in animal models of compromised hematopoiesis, including moderately to severely myelosuppressed mice and nonhuman primates. In these models, Neumega improved platelet nadirs and accelerated platelet recoveries compared to controls.
Preclinical trials have shown that mature megakaryocytes which develop during in vivo treatment with Neumega are ultrastructurally normal. Platelets produced in response to Neumega were morphologically and functionally normal and possessed a normal life span.
IL-11 has also been shown to have non-hematopoietic activities in animals including the regulation of intestinal epithelium growth (enhanced healing of gastrointestinal lesions), the inhibition of adipogenesis, the induction of acute phase protein synthesis, inhibition of pro-inflammatory cytokine production by macrophages, and the stimulation of osteoclastogenesis and neurogenesis. Non-hematopoietic pathologic changes observed in animals include fibrosis of tendons and joint capsules, periosteal thickening, papilledema, and embryotoxicity (see PRECAUTIONS , Pediatric Use and PRECAUTIONS , Pregnancy Category C ).
IL-11 is produced by bone marrow stromal cells and is part of the cytokine family that shares the gp130 signal transducer. Primary osteoblasts and mature osteoclasts express mRNAs for both IL-11 receptor (IL-11R alpha) and gp130. Both bone-forming and bone-resorbing cells are potential targets of IL-11. (1)
Pharmacokinetics
The pharmacokinetics of Neumega have been evaluated in studies of healthy, adult subjects and cancer patients receiving chemotherapy. In a study in which a single 50 µg/kg subcutaneous dose was administered to eighteen healthy men, the peak serum concentration (C max ) of 17.4 ± 5.4 ng/mL (mean ± S.D.) was reached at 3.2 ± 2.4 hrs (T max ) following dosing. The terminal half-life was 6.9 ± 1.7 hrs. In a second study in which single 75 µg/kg subcutaneous and intravenous doses were administered to twenty-four healthy subjects, the pharmacokinetic profiles were similar between men and women. The absolute bioavailability of Neumega was >80%. In a study in which multiple, subcutaneous doses of both 25 and 50 µg/kg were administered to cancer patients receiving chemotherapy, Neumega did not accumulate and clearance of Neumega was not impaired following multiple doses.
In a dose escalation Phase 1 study, Neumega was also administered to 43 pediatric patients (ages 8 months to 18 years) and 1 adult patient receiving ICE (ifosfamide, carboplatin, etoposide) chemotherapy. Administered doses ranged from 25 to 125 µg/kg. Analysis of data from 40 pediatric patients showed that C max , T max , and terminal half-life were comparable to that in adults. The mean area under the concentration-time curve (AUC) for pediatric patients (8 months to 18 years), receiving 50 µg/kg was approximately half that achieved in healthy adults receiving 50 µg/kg. Available data suggest that clearance of oprelvekin decreases with increasing age.
In preclinical trials in rats, radiolabeled Neumega was rapidly cleared from the serum and distributed to highly perfused organs. The kidney was the primary route of elimination. The amount of intact Neumega in urine was low, indicating that the molecule was metabolized before excretion. In a clinical study, a single dose of Neumega was administered to subjects with severely impaired renal function (creatinine clearance <30 mL/min). The mean ± S.D. values for C max and AUC were 30.8 ± 8.6 ng/mL and 373 ± 106 ng*hr/mL, respectively. When compared with control subjects in this study with normal renal function, the mean C max was 2.2 fold higher and the mean AUC was 2.6 fold (95% confidence interval, 1.7%-3.8%) higher in the subjects with severe renal impairment. In the subjects with severe renal impairment, clearance was approximately 40% of the value seen in subjects with normal renal function. The average terminal half-life was similar in subjects with severe renal impairment and those with normal renal function.
A second clinical study of 24 subjects with varying degrees of renal function was also performed and confirmed the results observed in the first study. Single 50 µg/kg subcutaneous and intravenous doses were administered in a randomized fashion. As the degree of renal impairment increased, the Neumega AUC increased, although half-life remained unchanged. In the six patients with severe impairment, the mean ± S.D. C max and AUC were 23.6 ± 6.7 ng/mL and 373 ± 55.2 ng*hr/mL, respectively, compared with 13.1 ± 3.8 ng/mL and 195 ± 49.3 ng*hr/mL, respectively, in the six subjects with normal renal function. A comparable increase in exposure was observed after intravenous administration of Neumega.
The pharmacokinetic studies suggest that overall exposure to oprelvekin increases as renal function decreases, indicating that a 50% dose reduction of Neumega is warranted for patients with severe renal impairment (see PRECAUTIONS , Use in Patients with Renal Impairment and DOSAGE AND ADMINISTRATION ). No dosage reduction is required for smaller changes in renal function.
Pharmacodynamics
In a study in which Neumega was administered to non-myelosuppressed cancer patients, daily subcutaneous dosing for 14 days with Neumega increased the platelet count in a dose-dependent manner. Platelet counts began to increase relative to baseline between five and nine days after the start of dosing with Neumega. After cessation of treatment, platelet counts continued to increase for up to seven days then returned toward baseline within 14 days. No change in platelet reactivity as measured by platelet activation in response to ADP, and platelet aggregation in response to ADP, epinephrine, collagen, ristocetin and arachidonic acid has been observed in association with Neumega treatment.
In a randomized, double-blind, placebo-controlled study in normal volunteers, subjects receiving Neumega had a mean increase in plasma volume of >20%, and all subjects receiving Neumega had at least a 10% increase in plasma volume. Red blood cell volume decreased similarly (due to repeated phlebotomy) in the Neumega and placebo groups. As a result, whole blood volume increased approximately 10% and hemoglobin concentration decreased approximately 10% in subjects receiving Neumega compared with subjects receiving placebo. Mean 24 hour sodium excretion decreased, and potassium excretion did not increase, in subjects receiving Neumega compared with subjects receiving placebo.
CLINICAL STUDIES
Two randomized, double-blind, placebo-controlled trials in adults studied Neumega for the prevention of severe thrombocytopenia following single or repeated sequential cycles of various myelosuppressive chemotherapy regimens.
Study in Patients with Prior Chemotherapy-Induced Thrombocytopenia
One study evaluated the effectiveness of Neumega in eliminating the need for platelet transfusions in patients who had recovered from an episode of severe chemotherapy-induced thrombocytopenia (defined as a platelet count </=20,000/µL), and were to receive one additional cycle of the same chemotherapy without dose reduction. Patients had various underlying non-myeloid malignancies, and were undergoing dose-intensive chemotherapy with a variety of regimens. Patients were randomized to receive Neumega at a dose of 25 µg/kg or 50 µg/kg, or placebo. The primary endpoint was whether the patient required one or more platelet transfusions in the subsequent chemotherapy cycle. Ninety-three patients were randomized. Five patients withdrew from the study prior to receiving the study drug. As a result, eighty-eight patients were included in a modified intent-to-treat analysis. The results for the Neumega 50 µg/kg and placebo groups are summarized in Table 1. The placebo group includes one patient who underwent chemotherapy dose reduction and who avoided platelet transfusions.
TABLE 1
STUDY RESULTSPlacebo
n=30Neumega 50 µg/kg
n=29Number (%) of patients avoiding platelet transfusion 2 (7%) 8 (28%) Number (%) of patients requiring platelet transfusion 28 (93%) 21 (72%) Median (mean) number of platelet transfusion events 2.5 (3.3) 1 (2.2)
In the primary efficacy analysis, more patients avoided platelet transfusion in the Neumega 50 µg/kg arm than in the placebo arm (p = 0.04, Fisher's Exact test, 2-tailed). The difference in the proportion of patients avoiding platelet transfusions in the Neumega 50 µg/kg and placebo groups was 21% (95% confidence interval, 2%-40%). The results observed in patients receiving 25 µg/kg of Neumega were intermediate between those of the placebo and the 50 µg/kg groups.
Study in Patients Receiving Dose-Intensive Chemotherapy
A second study evaluated the effectiveness of Neumega in eliminating platelet transfusions over two dose-intensive chemotherapy cycles in breast cancer patients who had not previously experienced severe chemotherapy-induced thrombocytopenia. All patients received the same chemotherapy regimen (cyclophosphamide 3,200 mg/m 2 and doxorubicin 75 mg/m 2 ). All patients received concomitant filgrastim (G-CSF) in all cycles. The patients were stratified by whether or not they had received prior chemotherapy, and randomized to receive Neumega 50 µg/kg or placebo. The primary endpoint was whether or not a patient required one or more platelet transfusions in the two study cycles. Seventy-seven patients were randomized. Thirteen patients failed to complete both study cycles--eight of these had insufficient data to be evaluated for the primary endpoint. The results of this trial are summarized in Table 2.
TABLE 2
STUDY RESULTSOverall
n=77No Prior Chemotherapy
n=54Prior Chemotherapy
n=23Placebo
n=37Neumega
n=40Placebo
n=27Neumega
n=27Placebo
n=10Neumega
n=13Number (%) of patients avoiding platelet transfusion15 (41%) 26 (65%) 14 (52%) 19 (70%) 1 (10%) 7 (54%) Number (%) of patients requiring platelet transfusion16 (43%) 12 (30%) 9 (33%) 7 (26%) 7 (70%) 5 (38%) Number (%) of patients not evaluable6 (16%) 2 (5%) 4 (15%) 1 (4%) 2 (20%) 1 (8%)
This study showed a trend in favor of Neumega, particularly in the subgroup of patients with prior chemotherapy. Open-label treatment with Neumega has been continued for up to four consecutive chemotherapy cycles without evidence of any adverse effect on the rate of neutrophil recovery or red blood cell transfusion requirements. Some patients continued to maintain platelet nadirs >20,000/µL for at least four sequential cycles of chemotherapy without the need for transfusions, chemotherapy dose reduction, or changes in treatment schedules.
Platelet activation studies done on a limited number of patients showed no evidence of abnormal spontaneous platelet activation, or an abnormal response to ADP. In an unblinded, retrospective analysis of the two placebo-controlled studies, 19 of 69 patients (28%) receiving Neumega 50 µg/kg and 34 of 67 patients (51%) receiving placebo reported at least one hemorrhagic adverse event which involved bleeding.
Study in Patients Following Myeloablative Chemotherapy
In a randomized, double-blind, placebo-controlled, Phase 2 study conducted in 80 women with high-risk breast cancer who received 0 (n=26), 25 µg/kg (n=28), or 50 µg/kg (n=26) Neumega following myeloablative chemotherapy and autologous bone marrow transplantation, the incidence of platelet transfusions and time to neutrophil and platelet engraftment were similar in the Neumega and placebo-treated arms. The study showed a statistically significant increased incidence in edema, conjunctival bleeding, hypotension, and tachycardia in patients receiving Neumega as compared to placebo.
In long term follow-up of patients, the distribution of survival and progression-free survival times was similar between patients randomized to Neumega therapy and those randomized to receive placebo.
INDICATIONS AND USAGE
Neumega is indicated for the prevention of severe thrombocytopenia and the reduction of the need for platelet transfusions following myelosuppressive chemotherapy in adult patients with nonmyeloid malignancies who are at high risk of severe thrombocytopenia. Efficacy was demonstrated in patients who had experienced severe thrombocytopenia following the previous chemotherapy cycle. Neumega is not indicated following myeloablative chemotherapy (see WARNINGS , Increased Toxicity Following Myeloablative Therapy . The safety and effectiveness of Neumega have not been established in pediatric patients.
CONTRAINDICATIONS
Neumega is contraindicated in patients with a history of hypersensitivity to Neumega or any component of the pro-duct (see WARNINGS , Allergic Reactions Including Anaphylaxis ).
WARNINGS
Allergic Reactions Including Anaphylaxis
In the post-marketing setting, Neumega has caused allergic or hypersensitivity reactions, including anaphylaxis. The administration of Neumega should be attended by appropriate precautions in case allergic reactions occur. In addition, patients should be counseled about the symptoms for which they should seek medical attention (see PRECAUTIONS , Information for Patients ). Signs and symptoms reported included edema of the face, tongue, or larynx; shortness of breath; wheezing; chest pain; hypotension (including shock); dysarthria; loss of consciousness; mental status changes; rash; urticaria; flushing and fever. Reactions occurred after the first dose or subsequent doses of Neumega. Administration of Neumega should be permanently discontinued in any patient who develops an allergic or hypersensitivity reaction (see BOXED WARNING , CONTRAINDICATIONS , ADVERSE REACTIONS , and ADVERSE REACTIONS , Immunogenicity ).
Increased Toxicity Following Myeloablative Therapy
Neumega is not indicated following myeloablative chemotherapy. In a randomized, placebo-controlled Phase 2 study, the effectiveness of Neumega was not demonstrated (see CLINICAL STUDIES , Study in Patients Following Myeloablative Chemotherapy ). In this study, a statistically significant increased incidence in edema, conjunctival bleeding, hypotension, and tachycardia was observed in patients receiving Neumega as compared to placebo.
The following severe or fatal adverse reactions have been reported in post-marketing use in patients who received Neumega following bone marrow transplantation: fluid retention or overload (eg, facial edema, pulmonary edema), capillary leak syndrome, pleural and pericardial effusion, papilledema and renal failure.
Fluid Retention
Neumega is known to cause serious fluid retention that can result in peripheral edema, dyspnea on exertion, pulmonary edema, capillary leak syndrome, atrial arrhythmias, and exacerbation of pre-existing pleural effusions. Severe fluid retention, some cases resulting in death, was reported following recent bone marrow transplantation in patients who have received Neumega. Neumega is not indicated following myeloablative chemotherapy (see CLINICAL PHARMACOLOGY , Pharmacodynamics ; WARNINGS , Increased Toxicity Following Myeloablative Therapy ; WARNINGS , Cardiovascular Events ; and WARNINGS , Dilutional Anemia ). It should be used with caution in patients with clinically evident congestive heart failure, patients who may be susceptible to developing congestive heart failure, patients receiving aggressive hydration, patients with a history of heart failure who are well-compensated and receiving appropriate medical therapy, and patients who may develop fluid retention as a result of associated medical conditions or whose medical condition may be exacerbated by fluid retention.
Fluid retention is reversible within several days following discontinuation of Neumega. During dosing with Neumega, fluid balance should be monitored and appropriate medical management is advised.
Close monitoring of fluid and electrolyte status should be performed in patients receiving chronic diuretic therapy. Sudden deaths have occurred in oprelvekin-treated patients receiving chronic diuretic therapy and ifosfamide who developed severe hypokalemia (see ADVERSE REACTIONS ).
Pre-existing fluid collections, including pericardial effusions or ascites, should be monitored. Drainage should be considered if medically indicated.
Dilutional Anemia
Moderate decreases in hemoglobin concentration, hematocrit, and red blood cell count (~10% to 15%) without a decrease in red blood cell mass have been observed. These changes are predominantly due to an increase in plasma volume (dilutional anemia) that is primarily related to renal sodium and water retention. The decrease in hemoglobin concentration typically begins within three to five days of the initiation of Neumega, and is reversible over approximately a week following discontinuation of Neumega (see WARNINGS , Fluid Retention ).
Cardiovascular Events
Neumega use is associated with cardiovascular events including arrhythmias and pulmonary edema. Cardiac arrest has been reported, but the causal relationship to Neumega is uncertain. Use with caution in patients with a history of atrial arrhythmias, and only after consideration of the potential risks in relation to anticipated benefit. In clinical trials, cardiac events including atrial arrhythmias (atrial fibrillation or atrial flutter) occurred in 15% (23/157) of patients treated with Neumega at doses of 50 µg/kg. Arrhythmias were usually brief in duration; conversion to sinus rhythm typically occurred spontaneously or after rate-control drug therapy. Approximately one-half (11/24) of the patients who were rechallenged had recurrent atrial arrhythmias. Clinical sequelae, including stroke, have been reported in patients who experienced atrial arrhythmias while receiving Neumega.
The mechanism for induction of arrhythmias is not known. Neumega was not directly arrhythmogenic in animal models. In some patients, development of atrial arrhythmias may be due to increased plasma volume associated with fluid retention (see WARNINGS , Fluid Retention ).
Nervous System Events
Stroke has been reported in the setting of patients who develop atrial fibrillation/flutter while receiving Neumega (see WARNINGS , Cardiovascular Events ). Patients with a history of stroke or transient ischemic attack may also be at increased risk for these events.
Papilledema
Papilledema has been reported in 2% (10/405) of patients receiving Neumega in clinical trials following repeated cycles of exposure. The incidence was higher, 16% (7/43) in children than in adults, 1% (3/362). Nonhuman primates treated with Neumega at a dose of 1,000 µg/kg SC once daily for four to 13 weeks developed papilledema that was not associated with inflammation or any other histologic abnormality and was reversible after dosing was discontinued. Neumega should be used with caution in patients with pre-existing papilledema, or with tumors involving the central nervous system since it is possible that papilledema could worsen or develop during treatment (see ADVERSE REACTIONS ).
PRECAUTIONS
General
Dosing with Neumega should begin 6 to 24 hours following the completion of chemotherapy dosing. The safety and efficacy of Neumega given immediately prior to or concurrently with cytotoxic chemotherapy or initiated at the time of expected nadir have not been established (see DOSAGE AND ADMINISTRATION ).
The effectiveness of Neumega has not been evaluated in patients receiving chemotherapy regimens of greater than five days duration or regimens associated with delayed myelosuppression (eg, nitrosoureas, mitomycin-C).
Chronic Administration
Neumega has been administered safely using the recommended dosage schedule (see DOSAGE AND ADMINISTRATION ) for up to six cycles following chemotherapy. The safety and efficacy of chronic administration of Neumega have not been established. Continuous dosage (two to 13 weeks) in nonhuman primates produced joint capsule and tendon fibrosis and periosteal hyperostosis (see PRECAUTIONS , Pediatric Use ). The relevance of these findings to humans is unclear.
Information for Patients
Neumega should be used under the guidance and supervision of a health care professional. However, when the physician determines that Neumega may be used outside of the hospital or office setting, persons who will be administering Neumega should be instructed as to the proper dose, and the method for reconstituting and administering Neumega (see DOSAGE AND ADMINISTRATION ). If home use is prescribed, patients should be instructed in the importance of proper disposal and cautioned against the reuse of needles, syringes, drug product, and diluent. A puncture resistant container should be used by the patient for the disposal of used needles.
Patients should be informed of the serious and most common adverse reactions associated with Neumega administration, including those symptoms related to allergic or hypersensitivity reactions (see BOXED WARNING ). Patients should be advised to immediately seek medical attention if any of the following signs or symptoms develop: swelling of the face, tongue, or throat; difficulty breathing, swallowing or talking; shortness of breath; wheezing; chest pain; throat tightness; lightheadedness; loss of consciousness; confusion; drowsiness; rash; itching; hives; flushing and/or fever. Mild to moderate peripheral edema and shortness of breath on exertion can occur within the first week of treatment and may continue for the duration of administration of Neumega. Patients who have preexisting pleural or other effusions or a history of congestive heart failure should be advised to contact their physician for worsening of dyspnea (see ADVERSE REACTIONS and WARNINGS , Fluid Retention ). Most patients who receive Neumega develop anemia. Patients should be advised to contact their physician if symptoms attributable to atrial arrhythmia develop. Female patients of childbearing potential should be advised of the possible risks to the fetus of Neumega (see PRECAUTIONS , Pregnancy Category C ).
Laboratory Monitoring
A complete blood count should be obtained prior to chemotherapy and at regular intervals during Neumega therapy (see DOSAGE AND ADMINISTRATION ). Platelet counts should be monitored during the time of the expected nadir and until adequate recovery has occurred (post-nadir counts >/=50,000/µL).
Drug Interactions
Most patients in trials evaluating Neumega were treated concomitantly with filgrastim (G-CSF) with no adverse effect of Neumega on the activity of G-CSF. No information is available on the clinical use of sargramostim (GM-CSF) with Neumega in human subjects. However, in a study in nonhuman primates in which Neumega and GM-CSF were coadministered, there were no adverse interactions between Neumega and GM-CSF and no apparent difference in the pharmacokinetic profile of Neumega.
Drug interactions between Neumega and other drugs have not been fully evaluated. Based on in vitro and nonclinical in vivo evaluations of Neumega, drug-drug interactions with known substrates of P450 enzymes would not be predicted.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to assess the carcinogenic potential of Neumega. In vitro , Neumega did not stimulate the growth of tumor colony-forming cells harvested from patients with a variety of human malignancies. Neumega has been shown to be non-genotoxic in in vitro studies. These data suggest that Neumega is not mutagenic. Although prolonged estrus cycles have been noted at two to 20 times the human dose, no effects on fertility have been observed in rats treated with Neumega at doses up to 1000 µg/kg/day.
Pregnancy Category C
Neumega has been shown to have embryocidal effects in pregnant rats and rabbits when given in doses of 0.2 to 20 times the human dose. There are no adequate and well-controlled studies of Neumega in pregnant women. Neumega should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Neumega has been tested in studies of fertility, early embryonic development, and pre- and postnatal development in rats and in studies of organogenesis (teratogenicity) in rats and rabbits. Parental toxicity has been observed when Neumega is given at doses of two to 20 times the human dose (>/=100 µg/kg/day) in the rat and at 0.02 to 2.0 times the human dose (>/=1 µg/kg/day) in the rabbit. Findings in pregnant rats consisted of transient hypoactivity and dyspnea after administration (maternal toxicity), as well as prolonged estrus cycle, increased early embryonic deaths and decreased numbers of live fetuses. In addition, low fetal body weights and a reduced number of ossified sacral and caudal vertebrae (ie, retarded fetal development) occurred in rats at 20 times the human dose. Findings in pregnant rabbits consisted of decreased fecal/urine eliminations (the only toxicity noted at 1 µg/kg/day in dams) as well as decreased food consumption, body weight loss, abortion, increased embryonic and fetal deaths, and decreased numbers of live fetuses. No teratogenic effects of Neumega were observed in rabbits at doses up to 0.6 times the human dose (30 µg/kg/day).
Adverse effects in the first generation offspring of rats given Neumega at maternally toxic doses >/=2 times the human dose (>/=100 µg/kg/day) during both gestation and lactation included increased newborn mortality, decreased viability index on day 4 of lactation, and decreased body weights during lactation. In rats given 20 times the human dose (1000 µg/kg/day) during both gestation and lactation, maternal toxicity and growth retardation of the first generation offspring resulted in an increased rate of fetal death of the second generation offspring.
Nursing Mothers
It is not known if Neumega is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Neumega, a decision should be made whether to discontinue nursing or to discontinue Neumega, taking into account the importance of the drug to the mother.
Pediatric Use
A safe and effective dose of Neumega has not been established in children. In a Phase 1, single arm, dose-escalation study, 43 pediatric patients were treated with Neumega at doses ranging from 25 to 125 µg/kg/day following ICE chemotherapy. All patients required platelet transfusions and the lack of a comparator arm made the study design inadequate to assess efficacy. The projected effective dose (based on comparable AUC observed for the effective dose in healthy adults) in children appears to exceed the maximum tolerated pediatric dose of 50 µg/kg/day (see CLINICAL PHARMACOLOGY , Pharmacokinetics ). Papilledema was dose-limiting and occurred in 16% of children (see WARNINGS , Papilledema ).
The most common adverse events seen in pediatric studies included tachycardia (84%), conjunctival injection (57%), radiographic and echocardiographic evidence of cardiomegaly (21%) and periosteal changes (11%). These events occurred at a higher frequency in children than adults. The incidence of other adverse events was generally similar to those observed using Neumega at a dose of 50 µg/kg in the randomized studies in adults receiving chemotherapy (see ADVERSE REACTIONS ).
Studies in animals were predictive of the effect of Neumega on developing bone in children. In growing rodents treated with 100, 300, or 1000 µg/kg/day for a minimum of 28 days, thickening of femoral and tibial growth plates was noted, which did not completely resolve after a 28-day non-treatment period. In a nonhuman primate toxicology study of Neumega, animals treated for two to 13 weeks at doses of 10 to 1000 µg/kg showed partially reversible joint capsule and tendon fibrosis and periosteal hyperostosis. An asymptomatic, laminated periosteal reaction in the diaphyses of the femur, tibia, and fibula has been observed in one patient during pediatric studies involving multiple courses of Neumega treatment. The relationship of these findings to treatment with Neumega is unclear. No studies have been performed to assess the long-term effects of Neumega on growth and development.
Use in Patients with Renal Impairment
Neumega is eliminated primarily by the kidneys. The pharmacokinetics of Neumega were studied in subjects with varying degrees of renal dysfunction. AUC 0-(infinity) , C max , and absolute bioavailability were significantly increased in subjects with severe renal impairment (creatinine clearance < 30 mL/min) (see DOSAGE AND ADMINISTRATION ). There were no significant changes in the pharmacokinetic parameters in subjects with mild or moderate impairment. A significant decrease in the hemoglobin concentration was noted on Day 2 after a single dose of Neumega in subjects with all degrees of renal impairment. By Day 14, the hemoglobin was decreased only in patients with severe renal impairment. Fluid retention associated with Neumega treatment has not been studied in patients with renal impairment, but fluid balance should be carefully monitored in these patients (see WARNINGS , Fluid Retention ).
ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Three hundred twenty-four subjects, with ages ranging from eight months to 75 years, have been exposed to Neumega treatment in clinical studies. Subjects have received up to six (eight in pediatric patients) sequential courses of Neumega treatment, with each course lasting from one to 28 days. Apart from the sequelae of the underlying malignancy or cytotoxic chemotherapy, most adverse events were mild or moderate in severity and reversible after discontinuation of Neumega dosing.
In general, the incidence and type of adverse events were similar between Neumega 50 µg/kg and placebo groups. The most frequently reported serious adverse events were neutropenic fever, syncope, atrial fibrillation, fever and pneumonia. The most commonly reported adverse events were edema, dyspnea, tachycardia, conjunctival injection, palpitations, atrial arrhythmias, and pleural effusions. The most frequently reported adverse reactions resulting in clinical intervention (eg, discontinuation of Neumega, adjustment in dosage, or the need for concomitant medication to treat an adverse reaction symptom) were atrial arrhythmias, syncope, dyspnea, congestive heart failure, and pulmonary edema (see WARNINGS , Fluid Retention and WARNINGS , Cardiovascular Events ). Selected adverse events that occurred in >/=10% of Neumega-treated patients are listed in Table 3.
TABLE 3
SELECTED ADVERSE EVENTSBody System
Adverse EventPlacebo
n=67 (%)50 µg/kg
n=69 (%)Body as a WholeEdema *10 (15) 41 (59) Neutropenic fever28 (42) 33 (48) Headache24 (36) 28 (41) Fever19 (28) 25 (36) Cardiovascular SystemTachycardia *2 (3) 14 (20) Vasodilatation6 (9) 13 (19) Palpitations *2 (3) 10 (14) Syncope4 (6) 9 (13) Atrial fibrillation/
flutter *1 (1) 8 (12) Digestive SystemNausea/vomiting47 (70) 53 (77) Mucositis25 (37) 30 (43) Diarrhea22 (33) 30 (43) Oral moniliasis *1 (1) 10 (14) Nervous SystemDizziness19 (28) 26 (38) Insomnia18 (27) 23 (33) Respiratory SystemDyspnea *15 (22) 33 (48) Rhinitis21 (31) 29 (42) Cough increased15 (22) 20 (29) Pharyngitis11 (16) 17 (25) Pleural effusions *0 (0) 7 (10) Skin and AppendagesRash11 (16) 17 (25) Special SensesConjunctival
Injection *2 (3) 13 (19) *Occurred in significantly more Neumega-treated patients than in placebo-treated patients.
The following adverse events also occurred more frequently in cancer patients receiving Neumega than in those receiving placebo: amblyopia, paresthesia, dehydration, skin discoloration, exfoliative dermatitis, and eye hemorrhage; a statistically significant association of Neumega to these events has not been established. Other than a higher incidence of severe asthenia in Neumega treated patients (10 [14%] in Neumega patients versus two [3%] in placebo patients), the incidence of severe or life-threatening adverse events was comparable in the Neumega and placebo treatment groups.
Two patients with cancer treated with Neumega experienced sudden death that the investigator considered possibly or probably related to Neumega. Both deaths occurred in patients with severe hypokalemia (<3.0 mEq/L) who had received high doses of ifosfamide and were receiving daily doses of a diuretic (see WARNINGS , Cardiovascular Events ).
Other serious events associated with Neumega were papilledema and cardiovascular events including atrial arrhythmias and stroke. In addition, cardiomegaly was reported in children.
The following adverse events, occurring in >/=10% of patients, were observed at equal or greater frequency in placebo-treated patients: asthenia, pain, chills, abdominal pain, infection, anorexia, constipation, dyspepsia, ecchymosis, myalgia, bone pain, nervousness, and alopecia. The incidence of fever, neutropenic fever, flu-like symptoms, thrombocytosis, thrombotic events, the average number of units of red blood cells transfused per patient, and the duration of neutropenia <500 cells/µL were similar in the Neumega 50 µg/kg and placebo groups.
Immunogenicity
In clinical studies that evaluated the immunogenicity of Neumega, two of 181 patients (1%) developed antibodies to Neumega. In one of these two patients, neutralizing antibodies to Neumega were detected in an unvalidated assay. The clinical relevance of the presence of these antibodies is unknown. In the post-marketing setting, cases of allergic reactions, including anaphylaxis have been reported (see WARNINGS , Allergic Reactions Including Anaphylaxis ). The presence of antibodies to Neumega was not assessed in these patients.
The data reflect the percentage of patients whose test results were considered positive for antibodies to Neumega and are highly dependent on the sensitivity and specificity of the assay. Additionally the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, concomitant medications, and underlying disease. For these reasons, comparisons of the incidence of antibodies to Neumega with incidence of antibodies to other products may be misleading.
Abnormal Laboratory Values
The most common laboratory abnormality reported in patients in clinical trials was a decrease in hemoglobin concentration predominantly as a result of expansion of the plasma volume (see WARNINGS , Fluid Retention ).
The increase in plasma volume is also associated with a decrease in the serum concentration of albumin and several other proteins (eg, transferrin and gamma globulins). A parallel decrease in calcium without clinical effects has been documented. After daily SC injections, treatment with Neumega resulted in a two-fold increase in plasma fibrinogen. Other acute-phase proteins also increased. These protein levels returned to normal after dosing with Neumega was discontinued. Von Willebrand factor (vWF) concentrations increased with a normal multimer pattern in healthy subjects receiving Neumega.
Post-marketing Reports
The following adverse reactions have been reported during the post-marketing use of Neumega: allergic reactions, anaphylaxis/anaphylactoid reactions, papilledema, capillary leak syndrome, renal failure, and injection site reactions described as dermatitis, pain, and discoloration (see BOXED WARNING ; WARNINGS , Allergic Reactions IncludingAnaphylaxis ; WARNINGS , Increased Toxicity Following Myeloablative Therapy ; WARNINGS , Papilledema , and CONTRAINDICATIONS ).
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reactions, (2) frequency of reporting, or (3) strength of causal connection to Neumega.
OVERDOSAGE
Doses of Neumega above 125 µg/kg have not been administered to humans. While clinical experience is limited, doses of Neumega greater than 50 µg/kg may be associated with an increased incidence of cardiovascular events in adult patients (see WARNINGS , Fluid Retention and Cardiovascular Events ). If an overdose of Neumega is administered, Neumega should be discontinued, and the patient should be closely observed for signs of toxicity (see WARNINGS and ADVERSE REACTIONS ). Reinstitution of Neumega therapy should be based upon individual patient factors (eg, evidence of toxicity, continued need for therapy).
DOSAGE AND ADMINISTRATION
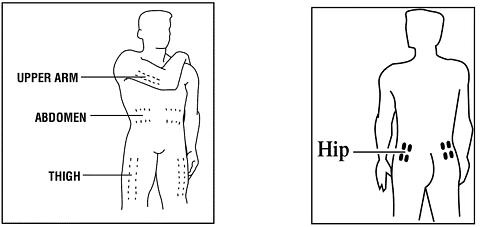
The recommended dose of Neumega in adults without severe renal impairment is 50 µg/kg given once daily. Neumega should be administered subcutaneously as a single injection in either the abdomen, thigh, or hip (or upper arm if not self-injecting). A safe and effective dose has not been established in children (see PRECAUTIONS , Pediatric Use ).
The recommended dose of Neumega in adults with severe renal impairment (creatinine clearance <30 mL/min) is 25 µg/kg. An estimate of the patient's creatinine clearance (CLcr) in mL/min is required. CLcr in mL/min may be estimated from a spot serum creatinine (mg/dL) determination using the following formula:
CLcr [apprx] [140 - age (years)] × weight (kg)
72 × serum creatinine (mg/dL)
{ × 0.85 for female patients} Dosing should be initiated six to 24 hours after the completion of chemotherapy. Platelet counts should be monitored periodically to assess the optimal duration of therapy. Dosing should be continued until the post-nadir platelet count is >/=50,000/µL. In controlled clinical trials, doses were administered in courses of 10 to 21 days. Dosing beyond 21 days per treatment course is not recommended.
Treatment with Neumega should be discontinued at least two days before starting the next planned cycle of chemotherapy.
Preparation of Neumega
- Neumega is a sterile, white, preservative-free, lyophilized powder for subcutaneous injection upon reconstitution. Neumega (5 mg vials) should be reconstituted aseptically with 1.0 mL of Sterile Water for Injection, USP (without preservative). The reconstituted Neumega solution is clear, colorless, isotonic, with a pH of 7.0, and contains 5 mg/mL of Neumega. The single-use vial should not be re-entered or reused. Any unused portion of either reconstituted Neumega solution or Sterile Water for Injection, USP should be discarded.
- During reconstitution, the Sterile Water for Injection, USP should be directed at the side of the vial and the contents gently swirled. EXCESSIVE OR VIGOROUS AGITATION SHOULD BE AVOIDED.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulate matter is present or the solution is discolored, the vial should not be used.
- Because neither Neumega powder for injection nor its accompanying diluent, Sterile Water for Injection, USP contains a preservative, Neumega should be used within 3 hours following reconstitution. Reconstituted Neumega may be refrigerated [2°C to 8°C (36°F to 46°F)] or at room temperature [up to 25°C (77°F)]. DO NOT FREEZE OR SHAKE THE RECONSTITUTED SOLUTION.
HOW SUPPLIED
Neumega is supplied as a sterile, white, preservative-free, lyophilized powder in vials containing 5 mg oprelvekin. Neumega is available in boxes containing one single-dose Neumega vial and one 1-mL vial of diluent for Neumega (Sterile Water for Injection, USP) - NDC 58394-004-01, and boxes containing seven single-dose Neumega vials and seven 1-mL vials of diluent for Neumega (Sterile Water for Injection, USP) - NDC 58394-004-02.
Storage
Lyophilized Neumega and diluent should be stored in a refrigerator at 2°C to 8°C (36°F to 46°F). Protect from light. DO NOT FREEZE. Reconstituted Neumega must be used within 3 hours of reconstitution and can be stored in the vial either at 2°C to 8°C (36°F to 46°F) or at room temperature up to 25°C (77°F).
REFERENCES
- Du, X. and Williams, D., Interleukin 11: review of molecular, cell biology and clinical use. Blood . 1997;89(11):3897-3908.
Wyeth ®
Wyeth Pharmaceuticals Inc.
Philadelphia, PA 19101
US Govt. License No. 3
W10439C007
ET01
Rev 06/04
Information for Patients
NEUMEGA ®
[nu-meg<a]
(oprelvekin)
Rx only
This product's label may have been revised after this insert was used in production. For further product information and current package insert, please visit www.wyeth.com or call our medical communications department toll-free at 1-800-934-5556.
This patient package insert contains information and directions for patients and their caregivers who are getting or giving injections of Neumega at home. You should read this patient information each time you pick up your prescription in case new information has been added. This patient package insert does not take the place of talking with your doctor or other healthcare provider. If you have any questions about your treatment with Neumega you should talk to your doctor.
What is Neumega?
Neumega is a medicine that stimulates your body to make platelets, which are a type of blood cell. Neumega is for people who have received certain types of chemotherapy and is used to help prevent the number of platelets circulating in the blood from dropping dangerously low. Too few platelets can cause serious problems and even death. Platelets are needed to help clot your blood when you are cut or injured. People with very low platelet counts are more likely to bruise and may not be able to control their bleeding if they are cut or injured. Platelets that have been donated by other people (platelet transfusions) are often given to patients with very low platelet counts. Neumega may reduce the need for platelet transfusions after chemotherapy. If your platelet levels are still too low after taking Neumega, your doctor may recommend that you receive a platelet transfusion.
What is the most important information I should know about Neumega?
Neumega may have side effects; some of these side effects may be serious. The most serious possible side effects of treatment with Neumega include:
-
Allergic Reactions
Neumega can cause serious allergic reactions in some patients. Signs that you are having a serious allergic reaction include: swelling of your face, tongue or throat; difficulty breathing, swallowing or talking; shortness of breath; wheezing; chest pain; a tightness in your throat; feeling lightheaded; loss of consciousness; confusion; drowsiness; rash; itching; hives; flushing and/or fever. You or your caregiver should call your doctor immediately if you develop any of these signs or symptoms. -
Heart Problems
Neumega can cause heart problems in some patients. If you feel like your heart is pounding, beating fast or skipping a beat, or you have chest pains or are short of breath, you should call your doctor immediately. If you have ever had heart problems, you should tell your doctor before you start treatment with Neumega.
If you are taking a water pill (diuretic), you should tell your doctor, because the diuretic can cause your body to lose potassium. This is very important, because Neumega can cause heart problems and these heart problems could be more serious when the potassium in your blood is too low. Your doctor will be checking your blood for the amount of potassium in it. If your potassium level is low, your doctor may prescribe a potassium replacement medication to correct it. -
Water Weight Gain
Neumega may cause you to retain water and gain weight from the extra fluid in your body. For some patients, water weight gain may cause serious problems that require medicine or hospitalization. A small amount of water weight gain will usually go away within several days after you stop taking Neumega. But, if you have a rapid weight gain over a few days, swelling of the legs and feet, dizziness, shortness of breath or chest pain, it could mean that you have a serious condition with fluid around the lungs and heart. If you have ever had heart failure or are taking medicine that may cause you to retain water, you should tell your doctor before you start treatment with Neumega. -
Children Receiving Neumega
Because Neumega is approved only for use in adults, you should talk to your child's doctor about the reasons why Neumega has been prescribed for your child. You should talk to your child's doctor about the risks and side effects of using this medication in children. One of the side effects seen in children taking Neumega is a serious eye condition called papilledema which is a form of swelling in the back of the eye. Many children may not show any signs of papilledema. If your child complains that they have a headache or are having difficulty seeing, call your child's doctor right away. Other side effects that have been seen in children are fast heartbeat, redness of the eye, changes to the heart, and changes to bones that can be seen on x-ray. -
Stop taking Neumega and call your doctor or healthcare provider immediately if you develop any of these symptoms:
- Shortness of breath or trouble breathing
- Chest pains
- Swelling in your face, hands, or feet
- Rapid weight gain over a few days
- You feel like your heart is pounding or beating out of your chest or skipping a beat, also referred to as palpitations
Before you start taking Neumega, you should tell your doctor the names of all of the medications you are taking including prescription and non-prescription drugs, vitamins, and nutritional supplements. If you have any of the following conditions or medical problems, tell your doctor or healthcare provider:
- You are pregnant or planning to become pregnant
- Breast feeding
- You have heart problems
- You have kidney disease
- You have eye problems
Who should not take Neumega?
Do not take Neumega if you have ever had or think you have had an allergic reaction to Neumega. Talk to your doctor if you have any questions about this information.
What are the other possible side effects of Neumega?
The most common, but less serious side effects, are:
- Slight water weight gain
- Some swelling in the arms and/or legs
- Shortness of breath when walking or moving around
- Anemia (low red blood cell count)
These side effects may be caused by water retention. For most people, the water weight gain will go away a few days after the last injection of Neumega. Make sure you have read and understand the section called "What is the most important information I should know about Neumega?" , because many of these side effects could develop into a more serious condition.
Other side effects that you should tell your doctor about are:
- Blurred vision, headaches, or redness of the eyes
- Any swelling or bruising that doesn't go away in the location where you have injected Neumega
If you have any other problems, whether or not you think they are related to Neumega, you should call your doctor.
What important information do I need to know about taking Neumega at home?
To see if Neumega is working, your doctor will ask you to have blood tests done to measure the number of platelets in your body. After starting Neumega, it may take 10 to 21 days for your platelet numbers to increase. The amount of time it takes to increase the number of platelets varies from patient to patient. Neumega may not work for everyone and you may still need platelet transfusions or have bleeding even if you take Neumega as directed by your doctor. You should always follow your doctor's instructions.
If your doctor has recommended that you receive Neumega at home, then you and/or your caregiver should be instructed on how to prepare Neumega, how much Neumega to use, how to inject it, how often it should be injected, and how to dispose of the unused portions of each bottle. Do not inject Neumega until you are comfortable with the steps to prepare and inject Neumega at home.
It is important that you do not take any more or less of the amount of Neumega that your doctor prescribed. Too much Neumega might put you at risk for irregular heartbeats and water retention (including fluid around the heart and lungs). If you accidentally take too much Neumega, you should call your doctor immediately.
You should always change the site of your injections each day to avoid soreness at any one site. Your injections should be given about the same time each day. If you miss an injection on one day, you should not try to add it on the next day. Tell your doctor that you missed a dose and continue as usual with your next scheduled dose. The section "How Do I Give Myself Neumega?" gives you step-by-step instructions for preparing and injecting your dose of Neumega.
How Do I Give Myself Neumega?
Preparing the Neumega for Injection
1.First, make sure that you have all of the supplies that you will need:
a) Four alcohol wipes.
b) Two cotton balls.
c) Two syringes (plastic tube with lines on it).
A 3 cc (or 3 mL) syringe for adding the Sterile Water for Injection, USP to the Neumega powder
A 1 cc (or 1 mL) syringe for giving the injection
d) Two needles.
One needle to use with the 3 cc syringe: 23 to 25 gauge, ¾ to 1 inch needle
One needle to use with the 1 cc syringe: 25 to 26 gauge, ½ to 1 inch needle
e) Bottle of Neumega powder.
f) Bottle of Sterile Water for Injection, USP.
g) A puncture proof container ("Sharps Container") for disposing needles and syringes.
2. You must use a new bottle of Neumega powder and a new bottle of Sterile Water for Injection, USP every time you give yourself a dose of Neumega.
Look for the expiration date printed on each bottle. DO NOT USE the Neumega powder or the Sterile Water for Injection, USP if the current month and year is after the month and year on the bottles; this means that the Neumega or Sterile Water for Injection, USP have expired. Notify your doctor that the Neumega and/or the Sterile Water for Injection, USP have expired and that you need replacement bottles. If the Neumega powder and the Sterile Water for Injection, USP have not expired, then continue with the steps that follow.
Wash your hands with soap and water.
3. Pick up the bottle labeled "Sterile Water for Injection, USP" and flip off the white protective cap. Wipe the rubber stopper on the top of the bottle with a sterile alcohol wipe. Leave the wipe on top of the bottle.
4. Pick up the bottle labeled "Neumega" and flip off the orange protective cap. Wipe the rubber stopper on the top of the bottle with a sterile alcohol wipe. Leave the wipe on top of the bottle.
5. Remove the 3 cc syringe from its package.
· The syringe has two parts:
1) A clear plastic tube with lines on the outside and
2) A plastic plunger that fits into the tube
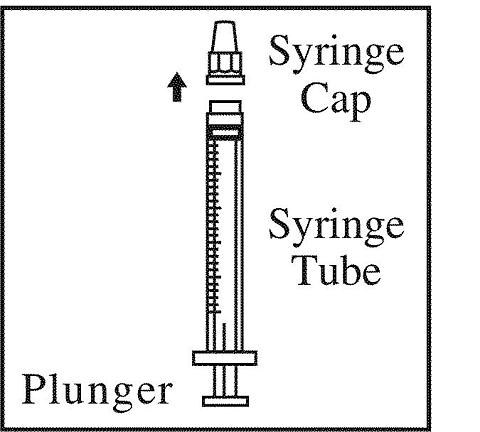
·Remove the protective cover (syringe cap) from the tip of this syringe. The needle attaches to this end of the syringe.
6. There are lines and numbers on the syringe, much like a measuring cup. The lines and numbers tell you how much fluid is in the syringe. If you hold the end of the plunger that sticks out, and move it in and out, you can adjust how much fluid you will pull into the syringe or inject out.
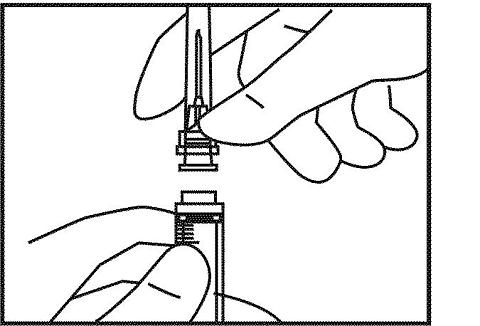
7. Remove the 23 to 25 gauge needle from its package. With the cap still on this needle, attach it to the 3 cc syringe. Remove the cap of this needle by gently pulling it off, but do not touch the needle with your hand or let it touch anything else. It is important to keep this needle sterile in order to prevent infection.
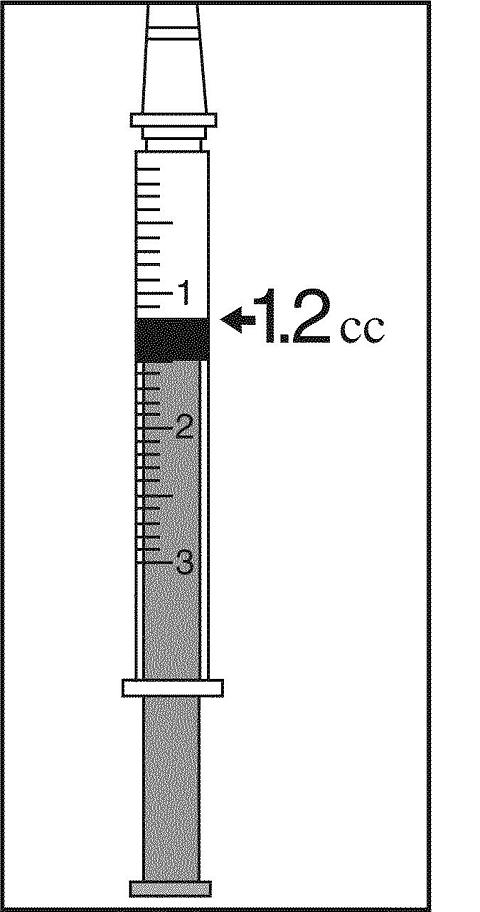
8. Holding the 3 cc syringe with the needle attached, carefully pull the plunger back until the end of the plunger that is inside the syringe is at the line that represents "1.2 cc" or 1.2 mL".
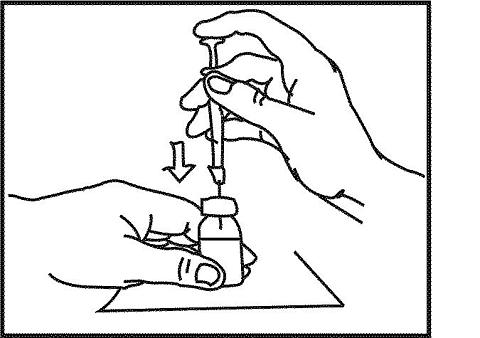
9. Take the bottle labeled "Sterile Water for Injection, USP" and remove the alcohol wipe. Do not touch the cleaned rubber stopper with your hands. Hold the bottle with one hand and push the needle through the center of the rubber stopper. Inject the air you pulled into the syringe (step 8) into the space at the top of the bottle. Do not inject the air into the water itself. Injecting the air directly into the sterile water will cause bubbles to form.
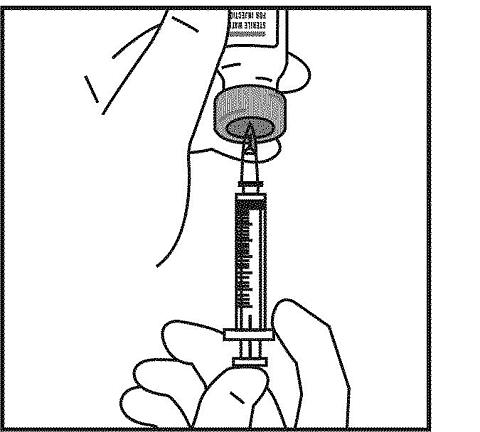
Keeping the needle inside the bottle, gently turn the bottle upside down. Slowly pull the plunger back to the black line marked "1.0 cc" or "1.0 mL" to remove 1.0 mL of Sterile Water for Injection, USP. To make sure that the tip of the needle stays in the fluid all of the time, you may need to pull back slightly on the syringe as you pull the Sterile Water for Injection, USP into the syringe.
10. After you have pulled 1.0 cc (or 1.0 mL) of Sterile Water for Injection, USP into the syringe, take the needle and syringe out of the bottle. Throw away the bottle labeled "Sterile Water for Injection, USP." DO NOT USE the bottle again, even if there is left over fluid inside. The Sterile Water for Injection, USP in the syringe will be added to the bottle with the Neumega powder.
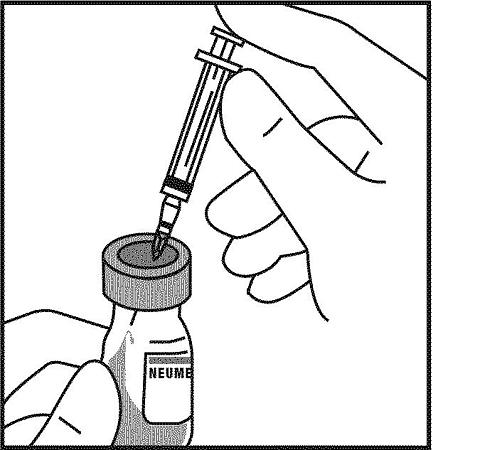
11. Take the bottle labeled "Neumega" and remove the alcohol wipe. Do not touch the cleaned rubber stopper with your hands. Holding the Neumega bottle with one hand, use the other hand to push the needle of the syringe that has the Sterile Water for Injection, USP through the middle of the rubber stopper. Press the plunger of the syringe SLOWLY. Gently aim the stream of Sterile Water for Injection, USP so that it runs down the inside wall of the bottle.
12. After injecting all of the Sterile Water for Injection, USP from the syringe into the Neumega bottle, take the needle and syringe out of the bottle. Dispose of this needle and syringe as described in step 7 of the section "Injecting Neumega". DO NOT RECAP NEEDLE.
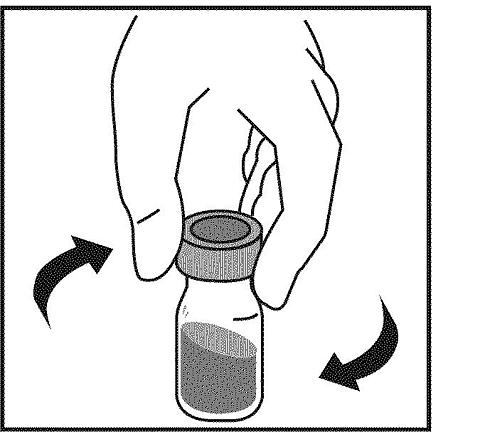
13. GENTLY SWIRL the bottle until all of the Neumega powder has dissolved and the fluid in the bottle is clear. DO NOT SHAKE THE BOTTLE. Shaking Neumega may damage the protein so it does not work properly.
Check the fluid inside the bottle. It should be clear and colorless without any powder or specks. DO NOT inject the Neumega if the fluid is cloudy or colored or if you see any particles. Call your doctor, nurse or pharmacist for instructions on what to do with a bottle of Neumega that you cannot use.
You should use the Neumega mixed with the Sterile Water for Injection, USP as soon after mixing it as possible. Do not let more than three (3) hours go by between the time you mix the Neumega and the water, and the time that you use it. The mixed Neumega and Sterile Water for Injection, USP can be stored in the Neumega bottle for up to three (3) hours either at room temperature or in the refrigerator. Remember to keep the bottle out of the light. DO NOT STORE THE NEUMEGA AND STERILE WATER FOR INJECTION, USP MIXTURE IN A SYRINGE.
14. After the Neumega powder is dissolved, wipe the rubber stopper on the top of the bottle again with a new sterile alcohol wipe.
15. Take the 1 cc syringe and the 25 to 26 gauge needle and remove them from their packages. Attach this needle to the 1 cc syringe as described in steps 6-8. This is the needle and syringe that you will use to inject the Neumega into your skin.
Fill the syringe with air by pulling the plunger back to the line or number on the syringe that your doctor or nurse has told you is the right one for the amount of Neumega that you are supposed to take.
16. Take the bottle of Neumega liquid and remove the alcohol wipe from the top. Do not touch the cleaned rubber stopper with your hands. Hold the bottle with one hand and push the needle through the center of the rubber stopper. Inject the air from the syringe into the bottle.
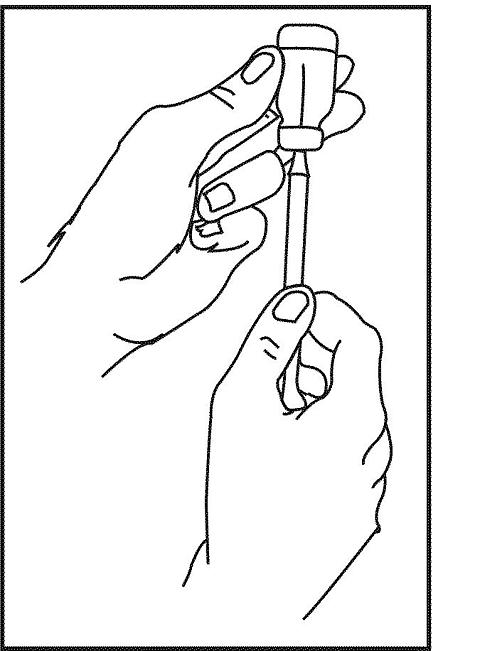
17. Turn the bottle and syringe upside down. Keep the tip of the needle in the fluid and slowly pull the plunger back. Stop when the fluid reaches the line or number that your doctor or nurse has told you is the right one for the amount of Neumega that you are supposed to take.
18. Check the syringe for bubbles. If you see bubbles in the syringe, push them back into the bottle by pushing in on the plunger. The fluid that is in the syringe should be clear and colorless, without any particles or bubbles.
Check to be sure that the fluid is still at the line or number that your doctor or nurse has told you is the right one for the amount of Neumega that you are supposed to take. If it is too little, you will need to pull the plunger back to the mark. If it is too much, you will need to push the plunger in to the mark. Once you are sure you have the right amount, you can go on to step 19.
19. Take the needle out of the bottle. Hold the syringe with the needle pointing straight up and gently tap the side of the syringe with your fingers to bring remaining air bubbles to the top of the syringe.
20. Still holding the syringe and needle pointing up, press the plunger in a little to push any air out through the needle. If a small drop of fluid comes out, that's okay. DO NOT RECAP NEEDLE. Do not lay the syringe down or allow it to touch a surface.
Injecting Neumega
1. Neumega can be injected into the skin of your upper legs (thighs), your abdomen (stomach), your hip, or your upper arms if not self-injecting. You should inject the Neumega into one of these different places of your body every time you use it.
2. Once you have decided where you will inject yourself, use your free hand to clean the skin with an alcohol wipe.
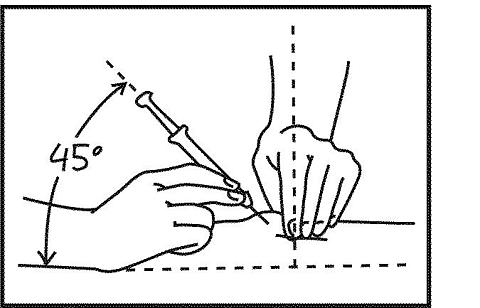
3. Take the 1 cc syringe containing the Neumega. Hold the syringe like a dart between the thumb and first finger just above the place where the needle attaches to the syringe. With your other hand, pinch your skin with your thumb and forefinger. This mound of skin is the place where you will inject the Neumega. Push the needle into the skin at a 45-degree angle. Gently let go of the pinched skin with one hand and keep holding the needle in the skin with the other hand.
4. GENTLY pull back on the plunger with your free hand. If you see blood come into the syringe, do not inject the Neumega. If this happens, take the syringe out of your skin, and discard this needle and syringe in a puncture proof container as outlined below in step 7 of this section. You will need to repeat all the above steps using new bottles of Sterile Water for Injection, USP and Neumega, with new syringes and needles and inject the Neumega at a new site.
5. If you do not see blood when you pull back the plunger, inject Neumega by slowly pushing the plunger all the way in.
6. Hold a cotton ball near the needle and pull the needle out of the skin. Press the cotton ball over the place where you made the injection for a few seconds. DO NOT RUB THE SITE.
7. DO NOT RECAP NEEDLES. Throw away the syringes with the needles on them into a puncture proof container ("Sharps Container"). A "Sharps Container" is a special box or other container for disposal of syringes and needles that your doctor or pharmacist can provide for you.
ALWAYS KEEP THE CONTAINER OUT OF THE REACH
OF CHILDREN.Ask your doctor, nurse, or pharmacist for instructions on how to properly dispose of a full container. There may be special state and local laws for disposal of used needles and syringes.
DO NOT THROW THE CONTAINERS IN
HOUSEHOLD TRASH. DO NOT RECYCLE.How should I store Neumega?
Both the bottles with the powdered Neumega and the Sterile Water for Injection, USP should be kept in a refrigerator. DO NOT FREEZE. The Neumega powder must be protected from light. Keeping the bottles in the box in the refrigerator until you are ready to use them will help protect them.
Every time you give yourself a dose of Neumega, you must use a new bottle of Neumega powder and a new bottle of Sterile Water for Injection, USP. There is an expiration date printed on the bottles of the Neumega powder and on the Sterile Water for Injection, USP. Do not use the Neumega or the Sterile Water for Injection, USP if it is past the month and year on the bottles.
After you mix the Neumega with the Sterile Water for Injection, USP, you must use it as soon as possible. Do not let more than three (3) hours go by between the time you mix the Neumega and the water, and the time that you use it. The Neumega and Sterile Water for Injection, USP mixture can be stored in the Neumega bottle for up to three (3) hours either at room temperature or in the refrigerator. Remember to keep the bottle out of the light. DO NOT STORE THE NEUMEGA AND STERILE WATER MIXTURE IN A SYRINGE.
After you give yourself an injection of Neumega, if there is any left over in the bottle, or in the syringe, it should be thrown away. DO NOT THROW THE CONTAINERS IN HOUSEHOLD TRASH. DO NOT RECYCLE. Throw away these bottles into the same "Sharps Container" you put the needles and syringes in.
General Advice About Prescription Medicines
Medicines are sometimes prescribed for purposes other than those listed here. If you have any questions or concerns about Neumega talk to your doctor. Do not use Neumega for a condition or person other than for whom it is prescribed.
Wyeth ®
Wyeth Pharmaceuticals Inc.
Philadelphia, PA 19101
US Govt. License No. 3
W10439C007
ET01
Rev 06/04
Subscribe to the "News" RSS Feed 
TOP ۞
