-
Prevacid NapraPAC 375, Prevacid NapraPAC 500 (TAP)
PREVACID ® NapraPAC™ (375 or 500) is a combination package containing NAPROSYN ® (naproxen) Tablets, a nonsteroidal anti-inflammatory drug (NSAID) with anal-gesic and antipyretic properties, and PREVACID ® (lansoprazole) Delayed-Release Capsules, a proton pump inhibitor (PPI). The information described in this labeling concerns only the use of these products as indicated in this combination package and does not include all individual use information. For information on use of the components when dispensed as individual medications outside this combination package, please see the package inserts for NAPROSYN Tablets and PREVACID Delayed-Release Capsules.
DESCRIPTION
PREVACID ® NapraPAC™ 375 is a combination package containing NAPROSYN 375 mg tablets and PREVACID 15 mg capsules. PREVACID ® NapraPAC™ 500 is a combination package containing NAPROSYN 500 mg tablets and PREVACID 15 mg capsules.
NAPROSYN
Naproxen is a member of the arylacetic acid group of nonsteroidal anti-inflammatory drugs (NSAID).
The chemical name for naproxen is (S)-6-methoxy-(alpha)-methyl-2-naphthaleneacetic acid. Its empirical formula is C 14 H 14 0 3 with a molecular weight of 230.26. Naproxen has the following structure:
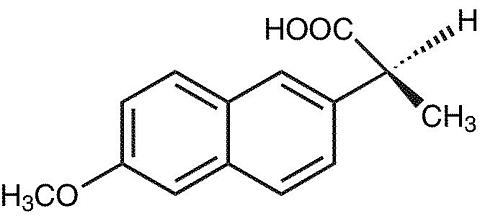
Naproxen is an odorless, white to off-white crystalline substance. It is lipid-soluble, practically insoluble in water at low pH and freely soluble in water at high pH. The octanol/water partition coefficient of naproxen at pH 7.4 is 1.6 to 1.8.
NAPROSYN tablets contain 250 mg, 375 mg or 500 mg of naproxen (active ingredient) and croscarmellose sodium, iron oxides, povidone and magnesium stearate (inactive ingredients).
PREVACID
The active ingredient in PREVACID capsules is a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl] sulfinyl] benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C 16 H 14 F 3 N 3 O 2 S with a molecular weight of 369.37. The structural formula is:
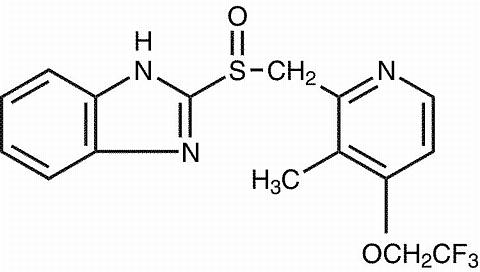
Lansoprazole is a white to brownish-white odorless crystalline powder which melts with decomposition at approximately 166°C. Lansoprazole is freely soluble in dimethylformamide; soluble in methanol; sparingly soluble in ethanol; slightly soluble in ethyl acetate, dichloromethane and acetonitrile; very slightly soluble in ether; and practically insoluble in hexane and water.
Lansoprazole is stable when exposed to light for up to two months. The rate of degradation of the compound in aqueous solution increases with decreasing pH. The degradation half-life of the drug substance in aqueous solution at 25[ordm ]C is approximately 0.5 hour at pH 5.0 and approximately 18 hours at pH 7.0.
PREVACID capsules contain enteric-coated granules consisting of lansoprazole (15 mg) [active ingredient], hydroxypropyl cellulose, low substituted hydroxypropyl cellulose, colloidal silicon dioxide, magnesium carbonate, methacrylic acid copolymer, starch, talc, sugar sphere, sucrose, polyethylene glycol, polysorbate 80, and titanium dioxide [inactive ingredients]. Components of the gelatin capsule include gelatin, titanium dioxide, D&C Red No. 28, FD&C Blue No. 1, FD&C Green No. 3, and FD&C Red No. 40 [inactiveingredients].
CLINICAL PHARMACOLOGY
Pharmacokinetics
NAPROSYN
Absorption
Naproxen is rapidly and completely absorbed from the gastrointestinal tract, with an in vivo bioavailability of 95%. After administration of naproxen tablets, peak plasma levels are attained in 2 to 4 hours. The elimination half-life of naproxen ranges from 12 to 17 hours. Steady-state levels of naproxen are reached in 4 to 5 days, and the degree of naproxen accumulation is consistent with this half-life.
Distribution
Naproxen has a volume of distribution of 0.16 L/kg. At therapeutic levels, naproxen is greater than 99% albumin-bound. At doses of naproxen greater than 500 mg/day, there is a less than dose-proportional increase in plasma levels due to an increase in clearance caused by saturation of plasma protein binding at higher doses (average trough C ss 36.5, 49.2 and 56.4 mg/L with 500, 1000 and 1500 mg daily doses of naproxen). However, the concentration of unbound naproxen continues to increase proportionally to dose.
Metabolism
Naproxen is extensively metabolized to 6-O-desmethyl naproxen, and both parent and metabolites do not induce metabolizing enzymes.
Elimination
The clearance of naproxen is 0.13 mL/min/kg. Approximately 95% of the naproxen from any dose is excreted in the urine, primarily as naproxen (less than 1%), 6-O-desmethyl naproxen (less than 1%) or their conjugates (66% to 92%). The plasma half-life of the naproxen anion in humans ranges from 12 to 17 hours. The corresponding half-lives of both naproxen's metabolites and conjugates are shorter than 12 hours, and their rates of excretion have been found to coincide closely with the rate of naproxen disappearance from the plasma. In patients with renal failure metabolites may accumulate.
Special Populations
Pediatric Use
The combination of naproxen and lansoprazole has not been studied in pediatric patients. (See CLINICAL PHARMACOLOGY , PREVACID Special Populations - Pediatric Use .)
Renal Insufficiency
Naproxen pharmacokinetics has not been determined in subjects with renal insufficiency. Given that naproxen, its metabolites and conjugates are primarily excreted by the kidney, the potential exists for naproxen to accumulate in the presence of renal insufficiency. (See CLINICAL PHARMACOLOGY , PREVACID Special Populations - Renal Insufficiency .)
PREVACID
PREVACID capsules contain an enteric-coated granule formulation of lansoprazole. Absorption of lansoprazole begins only after the granules leave the stomach. Absorption is rapid, with mean peak plasma levels of lansoprazole occurring after approximately 1.7 hours. Peak plasma concentrations of lansoprazole (C max ) and the area under the plasma concentration curve (AUC) of lansoprazole are approximately proportional in doses from 15 mg to 60 mg after single-dose oral administration. Lansoprazole does not accumulate and its pharmacokinetics are unaltered by multiple dosing.
Absorption
The absorption of lansoprazole is rapid, with mean C max occurring approximately 1.7 hours after oral dosing, and relatively complete with absolute bioavailability over 80%. In healthy subjects, the mean (± SD) plasma half-life was 1.5 (± 1.0) hours. Both C max and AUC are diminished by about 50-70% if the drug is given 30 minutes after food as opposed to the fasting condition. There is no significant food effect if the drug is given before meals.
Distribution
Lansoprazole is 97% bound to plasma proteins. Plasma protein binding is consistent over the concentration range of 0.05 to 5.0 µg/mL.
Metabolism
Lansoprazole is extensively metabolized in the liver. Two metabolites have been identified in measurable quantities in plasma (the hydroxylated sulfinyl and sulfone derivatives of lansoprazole). These metabolites have very little or no antisecretory activity. Lansoprazole is thought to be transformed into two active species which inhibit acid secretion by (H + ,K + )-ATPase within the parietal cell canaliculus, but are not present in the systemic circulation. The plasma elimination half-life of lansoprazole does not reflect its duration of suppression of gastric acid secretion. Thus, the plasma elimination half-life is less than 2 hours while the acid inhibitory effect lasts more than 24 hours.
Elimination
Following single-dose oral administration of PREVACID, virtually no unchanged lansoprazole was excreted in the urine. In one study, after a single oral dose of 14 C-lansoprazole, approximately one-third of the administered radiation was excreted in the urine and two-thirds was recovered in the feces. This implies a significant biliary excretion of the metabolites of lansoprazole.
Special Populations
Pediatric Use
The combination of lansoprazole and naproxen has not been studied in pediatric patients. (See CLINICAL PHARMACOLOGY , NAPROSYN Sp ecial Populations - Pediatric Use .)
Geriatric Use
The clearance of lansoprazole is decreased in the elderly, with elimination half-life increased approximately 50% to 100%. Because the mean half-life in the elderly remains between 1.9 to 2.9 hours, repeated once daily dosing does not result in accumulation of lansoprazole. Peak plasma levels were not increased in the elderly.
Gender
In a study comparing 12 male and 6 female human subjects, no gender differences were found in pharmacokinetics and intragastric pH results. (See PRECAUTIONS , PREVACID Use in Women .)
Renal Insufficiency
In patients with severe renal insufficiency, plasma protein binding decreased by 1.0%-1.5% after administration of 60 mg of lansoprazole. Patients with renal insufficiency had a shortened elimination half-life and decreased total AUC (free and bound). AUC for free lansoprazole in plasma, however, was not related to the degree of renal impairment, and C max and T max were not different from subjects with healthy kidneys. (See CLINICAL PHARMACOLOGY , NAPROSYN Special Populations-- Renal Insufficiency .)
Hepatic Insufficiency
In patients with various degrees of chronic hepatic disease, the mean plasma half-life of the drug was prolonged from 1.5 hours to 3.2-7.2 hours. An increase in mean AUC of up to 500% was observed at steady state in hepatically-impaired patients compared to healthy subjects. Dose re-duction in patients with severe hepatic disease should be considered.
Race
The pooled pharmacokinetic parameters of PREVACID from twelve U.S. Phase I studies (N=513) were compared to the mean pharmacokinetic parameters from two Asian studies (N=20). The mean AUCs of PREVACID in Asian subjects are approximately twice that seen in pooled U.S. data; however, the inter-individual variability is high. The C max values are comparable.
Pharmacodynamics
NAPROSYN
Naproxen is an NSAID with analgesic and antipyretic properties. The naproxen anion inhibits prostaglandin synthesis but beyond this its mode of action is unknown.
PREVACID
Mechanism of Action
Lansoprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that do not exhibit anticholinergic or histamine H2-receptor antagonist properties, but that suppress gastric acid secretion by specific inhibition of the (H + ,K + )-ATPase enzyme system at the secretory surface of the gastric parietal cell. Because this enzyme system is regarded as the acid (proton) pump within the parietal cell, lansoprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-related and leads to inhibition of both basal and stimulated gastric acid secretion irrespective of the stimulus.
Antisecretory Activity
After oral administration, lansoprazole was shown to significantly decrease the basal acid output and significantly increase the mean gastric pH and percent of time the gastric pH was >3 and >4. Lansoprazole also significantly reduced meal-stimulated gastric acid output and secretion volume, as well as pentagastrin-stimulated acid output. In patients with hypersecretion of acid, lansoprazole significantly reduced basal and pentagastrin- stimulated gastric acid secretion. Lansoprazole inhibited the normal increases in secretion volume, acidity and acid output induced by insulin.
In a crossover study that included lansoprazole 15 and 30 mg for five days, the following effects on intragastric pH were noted:
Table 1 Mean Antisecretory Effects After Single and Multiple Daily DosingPREVACIDBaseline15 mg30 mgParameterValueDay 1Day 5Day 1Day 5Mean 24-Hour pH2.12.7 +4.0 +3.6 *4.9 *Mean Nighttime pH1.92.43.0 +2.63.8 *% Time Gastric pH>31833 +59 +51 *72 *% Time Gastric pH>41222 +49 +41 *66 *NOTE: An intragastric pH of >4 reflects a reduction in gastric acid by 99%.
*(p<0.05) versus baseline and lansoprazole 15 mg.+ (p<0.05) versus baseline only.
After the initial dose in this study, increased gastric pH was seen within 1-2 hours with lansoprazole 30 mg and 2-3 hours with lansoprazole 15 mg. After multiple daily dosing, increased gastric pH was seen within the first hour postdosing with lansoprazole 30 mg and within 1-2 hours postdosing with lansoprazole 15 mg.
The inhibition of gastric acid secretion as measured by intragastric pH returns gradually to normal over two to four days after multiple doses. There is no indication of rebound gastric acidity.
Enterochromaffin-like (ECL) Cell Effects
During lifetime exposure of rats with up to 150 mg/kg/day of lansoprazole dosed 7 days per week, marked hypergastrinemia was observed followed by ECL cell proliferation and formation of carcinoid tumors, especially in female rats. (See PRECAUTIONS , PREVACID Carcinogenesis, Mutagenesis, Impairment of Fertility .)
Gastric biopsy specimens from the body of the stomach from approximately 150 patients treated continuously with lansoprazole for at least one year did not show evidence of ECL cell effects similar to those seen in rat studies. Longer term data are needed to rule out the possibility of an increased risk of the development of gastric tumors in patients receiving long-term therapy with lansoprazole.
Other Gastric Effects in Humans
Lansoprazole did not significantly affect mucosal blood flow in the fundus of the stomach. Due to the normal physiologic effect caused by the inhibition of gastric acid secretion, a decrease of about 17% in blood flow in the antrum, pylorus, and duodenal bulb was seen. Lansoprazole significantly slowed the gastric emptying of digestible solids. Lansoprazole increased serum pepsinogen levels and decreased pepsin activity under basal conditions and in response to meal stimulation or insulin injection. As with other agents that elevate intragastric pH, increases in gastric pH were associated with increases in nitrate-reducing bacteria and elevation of nitrite concentration in gastric juice in patients with gastric ulcer. No significant increase in nitrosamine concentrations was observed.
Serum Gastrin Effects
In over 2100 patients, median fasting serum gastrin levels increased 50% to 100% from baseline but remained within normal range after treatment with lansoprazole given orally in doses of 15 mg to 60 mg. These elevations reached a plateau within two months of therapy and returned to pretreatment levels within four weeks after discontinuation of therapy.
Endocrine Effects
Human studies for up to one year have not detected any clinically significant effects on the endocrine system. Hormones studied include testosterone, luteinizing hormone (LH), follicle stimulating hormone (FSH), sex hormone binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEA-S), prolactin, cortisol, estradiol, insulin, aldosterone, parathormone, glucagon, thyroid stimulating hormone (TSH), triiodothyronine (T 3 ), thyroxine (T 4 ), and somatotropic hormone (STH). Lansoprazole in oral doses of 15 to 60 mg for up to one year had no clinically significant effect on sexual function. In addition, lansoprazole in oral doses of 15 to 60 mg for two to eight weeks had no clinically significant effect on thyroid function.
In 24-month carcinogenicity studies in Sprague-Dawley rats with daily dosages up to 150 mg/kg, proliferative changes in the Leydig cells of the testes, including benign neoplasm, were increased compared to control rates.
Other Effects
No systemic effects of lansoprazole on the central nervous system, lymphoid, hematopoietic, renal, hepatic, cardiovascular or respiratory systems have been found in humans. No visual toxicity was observed among 56 patients who had extensive baseline eye evaluations, were treated with up to 180 mg/day of lansoprazole and were observed for up to 58 months. Other rat-specific findings after lifetime exposure included focal pancreatic atrophy, diffuse lymphoid hyperplasia in the thymus, and spontaneous retinal atrophy.
CLINICAL STUDIES
PREVACID ® NapraPAC™ (375 or 500)
Risk Reduction of NSAID-Associated Gastric Ulcer
A large U.S., multicenter, double-blind, placebo- and misoprostol-controlled (misoprostol blinded only to the endoscopist) study was conducted in patients who required chronic use of an NSAID and had a history of an endoscopically documented gastric ulcer. Patients took one or more NSAIDs during the study. Concomitant aspirin use (</= 325 mg) was allowed. The proportion of patients remaining free from gastric ulcer at 4, 8, and 12 weeks was significantly higher with 15 or 30 mg of PREVACID than placebo (see Table 2). A total of 537 patients were enrolled in the study, and 535 patients were treated. Patients ranged in age from 23 to 89 years (median age 60 years), with 65% female patients and 35% male patients. Race was distributed as follows: 90% Caucasian, 6% Black, 4% other. Concomitant aspirin was used in 20% of the patients. The 30 mg dose of PREVACID demonstrated no additional benefit in risk reduction of the NSAID-associated gastric ulcer than the 15 mg dose.
Table 2 NSAID-Associated Gastric Ulcer Risk Reduction Rates% of Patients Remaining Gastric Ulcer-Free 1WeekPREVACID
15 mg QD
(N=121)PREVACID
30 mg QD
(N=116)Misoprostol
200 µg QID
(N=106)Placebo
(N=112)490% 92% 96% 66% 886% 88% 95% 60% 1280% 82% 93% 51% 1 % = Life Table Estimate
(p<0.001) PREVACID 15 mg QD versus placebo; PREVACID 30 mg QD versus placebo; and misoprostol 200 µg QID versus placebo.
(p<0.05) Misoprostol 200 µg QID versus PREVACID 15 mg QD; and misoprostol 200 µg QID versus PREVACID 30 mg QD
A retrospective subset analysis of 119 patients whose NSAID was naproxen only or naproxen and aspirin only, was performed. Again, the proportion of patients remaining free from gastric ulcer at 4, 8, and 12 weeks was significantly higher with 15 or 30 mg of PREVACID than placebo (see Table 3). Patients ranged in age from 37 to 84 years (median age 58 years) with 61% female patients and 39% male patients. Race was distributed as follows: 88% Caucasian, 8% Black, 4% other. Concomitant aspirin was used in 15% of the patients. The 30 mg dose of PREVACID demonstrated no additional benefit in risk reduction of the NSAID-associated gastric ulcer than the 15 mg dose.
Table 3 Gastric Ulcer Risk Reduction Rates in Patients whose NSAID was Naproxen Only
or Naproxen and Aspirin Only% of Patients Remaining Gastric Ulcer-Free 1 Week PREVACID
15 mg QD
(N=37)PREVACID
30 mg QD
(N=24)Misoprostol
200 µg QID
(N=28)Placebo
(N=30)4 91% 83% 88% 52% 8 89% 83% 88% 52% 12 89% 83% 83% 33% 1 % = Life Table Estimate
(p<0.001) PREVACID 15 mg QD versus placebo; PREVACID 30 mg QD versus placebo; and misoprostol 200 µg QID versus placebo.
For patients who received PREVACID the highest total daily dose of naproxen was as follows: 5 patients took < 750 mg/daily, 54 patients took 750 - 1000 mg daily. Only 2 patients who received PREVACID took greater than 1000 mg of naproxen.
NAPROSYN
General Information
Naproxen has been studied in patients with rheumatoid arthritis, osteoarthritis and ankylosing spondylitis. Improvement in patients treated for rheumatoid arthritis was demonstrated by a reduction in joint swelling, a reduction in duration of morning stiffness, a reduction in disease activity as assessed by both the investigator and patient, and by increased mobility as demonstrated by a reduction in walking time. Generally, response to naproxen has not been found to be dependent on age, sex, severity or duration of rheumatoid arthritis.
In patients with osteoarthritis, the therapeutic action of naproxen has been shown by a reduction in joint pain or tenderness, an increase in range of motion in knee joints, increased mobility as demonstrated by a reduction in walking time, and improvement in capacity to perform activities of daily living impaired by the disease.
In a clinical trial comparing standard formulations of naproxen 375 mg bid (750 mg a day) vs 750 mg bid (1500 mg/day), 9 patients in the 750 mg group terminated prematurely because of adverse events. Nineteen patients in the 1500 mg group terminated prematurely because of adverse events. Most of these adverse events were gastrointestinal events.
In clinical studies in patients with rheumatoid arthritis or osteoarthritis, naproxen has been shown to be comparable to aspirin and indomethacin in controlling the aforementioned measures of disease activity, but the frequency and severity of the milder gastrointestinal adverse effects (nausea, dyspepsia, heartburn) and nervous system adverse effects (tinnitus, dizziness, lightheadedness) were less in naproxen-treated patients than in those treated with aspirin or indomethacin.
In patients with ankylosing spondylitis, naproxen has been shown to decrease night pain, morning stiffness and pain at rest. In double-blind studies the drug was shown to be as effective as aspirin, but with fewer side effects.
Naproxen may be used safely in combination with gold salts and/or corticosteroids; however, in controlled clinical trials, when added to the regimen of patients receiving corticosteroids, it did not appear to cause greater improvement over that seen with corticosteroids alone. Whether naproxen has a "steroid-sparing" effect has not been adequately studied. When added to the regimen of patients receiving gold salts, naproxen did result in greater improvement. Its use in combination with salicylates is not recommended because there is evidence that aspirin increases the rate of excretion of naproxen and data are inadequate to demonstrate that naproxen and aspirin produce greater improvement over that achieved with aspirin alone. In addition, as with other NSAIDs, the combination may result in higher frequency of adverse events than demonstrated for either product alone.
In 51 Cr blood loss and gastroscopy studies with normal volunteers, daily administration of 1000 mg of naproxen has been demonstrated to cause statistically significantly less gastric bleeding and erosion than 3250 mg of aspirin.
INDICATIONS AND USAGE
PREVACID ® NapraPAC™ (375 or 500) is indicated for reducing the risk of NSAID-associated gastric ulcers in patients with a history of documented gastric ulcer who require the use of an NSAID for treatment of the signs and symptoms of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. (See CLINICAL STUDIES and DOSAGE AND ADMINISTRATION .) Controlled studies did not extend beyond 12 weeks.
CONTRAINDICATIONS
PREVACID ® NapraPAC™ (375 or 500) is contraindicated in patients with known hypersensitivity or allergic reactions to any component of the formulations of PREVACID, NAPROSYN, or over-the-counter products containing naproxen.
Naproxen is also contraindicated in patients in whom aspirin or other nonsteroidal anti-inflammatory/analgesic drugs induce the syndrome of asthma, rhinitis, and nasal polyps. Both types of reactions have the potential of being fatal. Anaphylactoid reactions to naproxen, whether of the true allergic type or the pharmacologic idiosyncratic (eg, aspirin hypersensitivity syndrome) type, usually but not always occur in patients with a known history of such reactions. Therefore, careful questioning of patients for such things as asthma, nasal polyps, urticaria, and hypotension associated with nonsteroidal anti-inflammatory drugs before starting therapy is important. In addition, if such symptoms occur during therapy, treatment should be discontinued.
WARNINGS
NAPROSYN
Risk of GI Ulceration, Bleeding and Perforation With NSAID Therapy
Serious gastrointestinal toxicity such as bleeding, ulceration and perforation can occur at any time, with or without warning symptoms, in patients treated chronically with NSAID therapy. Although minor upper gastrointestinal problems, such as dyspepsia, are common, usually developing early in therapy, physicians should remain alert for ulceration and bleeding in patients treated chronically with NSAIDs even in the absence of previous GI tract symptoms. In patients observed in clinical trials of several months to 2 years' duration, symptomatic upper GI ulcers, gross bleeding or perforation appear to occur in approximately 1% of patients treated for 3 to 6 months and in about 2% to 4% of patients treated for 1 year.
Physicians should inform patients about the signs and/or symptoms of serious GI toxicity and what steps to take if they occur.
Studies to date with naproxen have not identified any subset of patients not at risk of developing peptic ulceration and bleeding. Except for a prior history of serious GI events and other risk factors known to be associated with peptic ulcer disease, such as alcoholism, smoking, etc., no risk factors (e.g., age, sex) have been associated with increased risk. Elderly or debilitated patients seem to tolerate ulceration or bleeding less well than other individuals and most spontaneous reports of fatal GI events are in this population. Studies to date are inconclusive concerning the relative risk of various NSAIDs in causing such reactions. High doses of any NSAID probably carry a greater risk of these reactions, although controlled clinical trials showing this do not exist in most cases. In considering the use of relatively large doses (within the recommended dosage range), sufficient benefit should be anticipated to offset the potential increased risk of GI toxicity.
Physicians should consider alternative treatment to NSAIDs in patients who have experienced a serious gastrointestinal toxicity associated with NSAID use. For patients who require the use of an NSAID, coadministration of PREVACID 15 mg Delayed-Release Capsules with NSAIDs has been proven effective to reduce the risk of gastric ulcers associated with NSAID use in patients with a previous history of documented gastric ulcers. (See CLINICAL STUDIES , PREVACID ® NapraPAC™ (375 or 500) , Risk Reduction of NSAID-Associated Gastric Ulcer .)
PRECAUTIONS
General
NAPROSYN
NAPROXEN-CONTAINING PRODUCTS SUCH AS NAPROSYN ® (NAPROXEN) TABLETS, EC-NAPROSYN ® (NAPROXEN) DELAYED-RELEASE TABLETS, ANAPROX ® /ANAPROX DS ® (NAPROXEN SODIUM) TABLETS, NAPROSYN ® (NAPROXEN) SUSPENSION, ALEVE ® (NAPROXEN SODIUM), AND OTHER NAPROXEN PRODUCTS INCLUDING PREVACID ® NapraPAC™ (375 or 500), SHOULD NOT BE USED CONCOMITANTLY SINCE THEY ALL CIRCULATE IN THE PLASMA AS THE NAPROXEN ANION.
NAPROSYN cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. If the steroid dose is reduced or eliminated during therapy, the steroid dosage should be reduced slowly and the patient should be observed closely for any evidence of adverse effects, including adrenal insufficiency and exacerbation of symptoms of arthritis.
Patients with initial hemoglobin values of 10 grams or less who are to receive long-term therapy should have hemoglobin values determined periodically.
The antipyretic and anti-inflammatory activities of the drug may reduce fever and inflammation, thus diminishing their utility as diagnostic signs in detecting complications of presumed noninfectious, noninflammatory painful conditions.
Because of adverse eye findings in animal studies with drugs of this class, it is recommended that ophthalmic studies be carried out if any change or disturbance in vision occurs.
PREVACID
Symptomatic response to therapy with lansoprazole does not preclude the presence of gastric malignancy.
Information for Patients
Each convenience package of PREVACID ® NapraPAC™ (375 or 500) contains sufficient product for seven days of treatment. Each daily dose consists of one PREVACID 15 mg capsule and two NAPROSYN tablets, either 375 mg or 500 mg. Take the PREVACID capsule and one NAPROSYN tablet in the morning before eating with a glass of water. Take the second NAPROSYN tablet in the evening with a glass of water.
NAPROSYN, like other drugs of this class, is not free of side effects. The side effects of this formulation can cause discomfort and, rarely, there are more serious side effects, such as gastrointestinal bleeding, which may result in hospitalization and even fatal outcomes. PREVACID when taken with naproxen has been shown to reduce the risk of NSAID-associated gastric ulcers in patients with a history of ulcer.
NSAIDs (Nonsteroidal Anti-Inflammatory Drugs) are often essential agents in the management of arthritis and have a major role in the treatment of pain. Naproxen as NAPROSYN is indicated for the treatment of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis.
Physicians may wish to discuss with their patients the potential risks (see WARNINGS , PRECAUTIONS and ADVERSE REACTIONS ) and likely benefits of naproxen treatment, particularly when it is used for less serious conditions where treatment without NSAIDs may represent an acceptable alternative to both the patient and physician.
Caution should be exercised by patients whose activities require alertness if they experience drowsiness, dizziness, vertigo or depression during therapy with naproxen.
Renal Effects
NAPROSYN
As with other nonsteroidal anti-inflammatory drugs, long-term administration of naproxen to animals has resulted in renal papillary necrosis and other abnormal renal pathology. In humans, there have been reports of acute interstitial nephritis, hematuria, proteinuria and occasionally nephrotic syndrome associated with naproxen-containing products and other NSAIDs since they have been marketed.
A second form of renal toxicity has been seen in patients taking naproxen as well as other nonsteroidal anti-inflammatory drugs. In patients with prerenal conditions leading to a reduction in renal blood flow or blood volume, where the renal prostaglandins have a supportive role in the maintenance of renal perfusion, administration of a nonsteroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and the elderly. Discontinuation of nonsteroidal anti- inflammatory therapy is typically followed by recovery to the pretreatment state.
Naproxen and its metabolites are eliminated primarily by the kidneys; therefore, the drug should be used with caution in patients with significantly impaired renal function, and the monitoring of serum creatinine and/or creatinine clearance is advised in these patients. Caution should be used if the drug is given to patients with creatinine clearance of less than 20 mL/minute because accumulation of naproxen metabolites has been seen in such patients.
Chronic alcoholic liver disease and probably other diseases with decreased or abnormal plasma proteins (albumin) reduce the total plasma concentration of naproxen, but the plasma concentration of unbound naproxen is increased. Caution is advised when high doses are required and some adjustment of dosage may be required in these patients. It is prudent to use the lowest effective dose.
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly. Caution is advised when high doses are required and some adjustment of dosage may be required in elderly patients. As with other drugs used in the elderly, it is prudent to use the lowest effective dose.
Hepatic Function
NAPROSYN
As with other nonsteroidal anti-inflammatory drugs, borderline elevations of one or more liver tests may occur in up to 15% of patients. These abnormalities may progress, may remain essentially unchanged, or may be transient with continued therapy. The SGPT (ALT) test is probably the most sensitive indicator of liver dysfunction. Meaningful (3 times the upper limit of normal) elevations of SGPT or SGOT (AST) occurred in controlled clinical trials in less than 1% of patients. A patient with symptoms and/or signs suggesting liver dysfunction or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of more severe hepatic reaction while on therapy with naproxen. Severe hepatic reactions, including jaundice and cases of fatal hepatitis, have been reported with naproxen as with other nonsteroidal anti-inflammatory drugs. Although such reactions are rare, if abnormal liver tests persist or worsen, if clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (eg, eosinophilia, rash, etc.), naproxen should be discontinued.
Fluid Retention and Edema
NAPROSYN
Peripheral edema has been observed in some patients receiving naproxen.
Laboratory Tests
NAPROSYN
Because serious GI tract ulceration and bleeding can occur without warning symptoms, physicians should follow patients chronically treated with naproxen for signs and symptoms of ulceration and bleeding and should inform them of the importance of this follow-up and what they should do if certain signs and symptoms do appear (see WARNINGS , NAPROSYN Risk of GI Ulcerations, Bleeding and Perforation With NSAID Therapy ).
Drug Interactions
NAPROSYN
The use of NSAIDs in patients who are receiving ACE inhibitors may potentiate renal disease states (see PRECAUTIONS , NAPROSYN Renal Effects ).
In vitro studies have shown that naproxen anion, because of its affinity for protein, may displace from their binding sites other drugs that are also albumin-bound (see CLINICAL PHARMACOLOGY , NAPROSYN Pharmacokinetics ).
Theoretically, the naproxen anion itself could likewise be displaced. Short-term controlled studies failed to show that taking the drug significantly affects prothrombin times when administered to individuals on coumarin-type anticoagulants. Caution is advised nonetheless, since interactions have been seen with other nonsteroidal agents of this class. Similarly, patients receiving the drug and a hydantoin, sulfonamide or sulfonylurea should be observed for signs of toxicity to these drugs (see CLINICAL STUDIES , NAPROSYN General Information ).
Concomitant administration of naproxen and aspirin is not recommended because naproxen is displaced from its binding sites during the concomitant administration of aspirin, resulting in lower plasma concentrations and peak plasma levels.
The natriuretic effect of furosemide has been reported to be inhibited by some drugs of this class. Inhibition of renal lithium clearance leading to increases in plasma lithium concentrations has also been reported. Naproxen and other nonsteroidal anti-inflammatory drugs can reduce the antihypertensive effect of propranolol and other beta-blockers.
Probenecid given concurrently increases naproxen anion plasma levels and extends its plasma half-life significantly.
Caution should be used if naproxen is administered concomitantly with methotrexate. Naproxen, and other nonsteroidal anti-inflammatory drugs have been reported to reduce the tubular secretion of methotrexate in an animal model, possibly increasing the toxicity of methotrexate.
PREVACID
Lansoprazole is metabolized through the cytochrome P 450 system, specifically through the CYP3A and CYP2C19 isozymes. Studies have shown that lansoprazole does not have clinically significant interactions with other drugs metabolized by the cytochrome P 450 system, such as warfarin, antipyrine, indomethacin, ibuprofen, phenytoin, propranolol, prednisone, diazepam, or clarithromycin in healthy subjects. These compounds are metabolized through various cytochrome P 450 isozymes including CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A. When lansoprazole was administered concomitantly with theophylline (CYP1A2, CYP3A), a minor increase (10%) in the clearance of theophylline was seen. Because of the small magnitude and the direction of the effect on theophylline clearance, this interaction is unlikely to be of clinical concern. Nonetheless, individual patients may require additional titration of their theophylline dosage when lansoprazole is started or stopped to ensure clinically effective blood levels.
In a study of healthy subjects neither the pharmacokinetics of warfarin enantiomers nor prothrombin time were affected following single or multiple 60 mg doses of lansoprazole. However, there have been reports of increased International Normalized Ratio (INR) and prothrombin time in patients receiving proton pump inhibitors, including lansoprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with proton pump inhibitors and warfarin concomitantly may need to be monitored for increases in INR and prothrombin time.
Lansoprazole has also been shown to have no clinically significant interaction with amoxicillin.
In a single-dose crossover study examining lansoprazole 30 mg and omeprazole 20 mg each administered alone and concomitantly with sucralfate 1 gram, absorption of the proton pump inhibitors was delayed and their bioavailability was reduced by 17% and 16%, respectively, when administered concomitantly with sucralfate. Therefore, proton pump inhibitors should be taken at least 30 minutes prior to sucralfate. In clinical trials, antacids were administered concomitantly with PREVACID Delayed-Release Capsules; this did not interfere with its effect.
Lansoprazole causes a profound and long-lasting inhibition of gastric acid secretion; therefore, it is theoretically possible that lansoprazole may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability (e.g., ketoconazole, ampicillin esters, iron salts, digoxin).
Drug/Laboratory Test Interactions
NAPROSYN
Naproxen may decrease platelet aggregation and prolong bleeding time. This effect should be kept in mind when bleeding times are determined.
The administration of naproxen may result in increased urinary values for 17-ketogenic steroids because of an interaction between the drug and/or its metabolites with m-dinitrobenzene used in this assay. Although 17-hydroxy-corticosteroid measurements (Porter-Silber test) do not appear to be artifactually altered, it is suggested that therapy with naproxen be temporarily discontinued 72 hours before adrenal function tests are performed if the Porter-Silber test is to be used.
Naproxen may interfere with some urinary assays of 5-hydroxy indoleacetic acid (5HIAA).
Carcinogenesis, Mutagenesis, Impairment of Fertility
NAPROSYN
A 2-year study was performed in rats to evaluate the carcinogenic potential of naproxen at rat doses of 8, 16, and 24 mg/kg/day (50, 100, and 150 mg/m 2 ). The maximum dose used was 0.28 times the systemic exposure to humans at the recommended dose. No evidence of tumorigenicity was found.
PREVACID
In two 24-month carcinogenicity studies, Sprague-Dawley rats were treated orally with doses of 5 to 150 mg/kg/day, about 1 to 40 times the exposure on a body surface (mg/m 2 ) basis, of a 50-kg person of average height (1.46 m 2 body surface area) given the recommended human dose of 30 mg/day (22.2 mg/m 2 ). Lansoprazole produced dose-related gastric enterochromaffin-like (ECL) cell hyperplasia and ECL cell carcinoids in both male and female rats. It also increased the incidence of intestinal metaplasia of the gastric epithelium in both sexes. In male rats, lansoprazole produced a dose-related increase of testicular interstitial cell adenomas. The incidence of these adenomas in rats receiving doses of 15 to 150 mg/kg/day (4 to 40 times the recommended human dose based on body surface area) exceeded the low background incidence (range = 1.4 to 10%) for this strain of rat. Testicular interstitial cell adenoma also occurred in 1 of 30 rats treated with 50 mg/kg/day (13 times the recommended human dose based on body surface area) in a 1-year toxicity study.
In a 24-month carcinogenicity study, CD-1 mice were treated orally with doses of 15 to 600 mg/kg/day, 2 to 80 times the recommended human dose based on body surface area. Lansoprazole produced a dose-related increased incidence of gastric ECL cell hyperplasia. It also produced an increased incidence of liver tumors (hepatocellular adenoma plus carcinoma). The tumor incidences in male mice treated with 300 and 600 mg/kg/day (40 to 80 times the recommended human dose based on body surface area) and female mice treated with 150 to 600 mg/kg/day (20 to 80 times the recommended human dose based on body surface area) exceeded the ranges of background incidences in historical controls for this strain of mice. Lansoprazole treatment produced adenoma of rete testis in male mice receiving 75 to 600 mg/kg/day (10 to 80 times the recommended human dose based on body surface area).
Lansoprazole was not genotoxic in the Ames test, the ex vivo rat hepatocyte unscheduled DNA synthesis (UDS) test, the in vivo mouse micronucleus test or the rat bone marrow cell chromosomal aberration test. It was positive in in vitro human lymphocyte chromosomal aberration assays.
Lansoprazole at oral doses up to 150 mg/kg/day (40 times the recommended human dose based on body surface area) was found to have no effect on fertility and reproductive performance of male and female rats.
Pregnancy: Teratogenic Effects
Pregnancy Category B
PREVACID ® NapraPAC™ (375 or 500)
There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, PREVACID ® NapraPAC™ (375 or 500) should not be used during pregnancy unless clearly needed.
NAPROSYN
Reproduction studies have been performed in rats at 20 mg/kg/day (125 mg/m 2 /day, 0.23 times the human systemic exposure), rabbits at 20 mg/kg/day (220 mg/m 2 /day, 0.27 times the human systemic exposure), and mice at 170 mg/kg/day (510 mg/m 2 /day, 0.28 times the human systemic exposure) with no evidence of impaired fertility or harm to the fetus due to the drug. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, naproxen should not be used during pregnancy unless clearly needed.
PREVACID
Teratology studies have been performed in pregnant rats at oral doses up to 150 mg/kg/day (40 times the recommended human dose based on body surface area) and pregnant rabbits at oral doses up to 30 mg/kg/day (16 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to lansoprazole.
Nonteratogenic Effects
NAPROSYN
There is some evidence to suggest that when inhibitors of prostaglandin synthesis are used to delay preterm labor there is an increased risk of neonatal complications such as necrotizing enterocolitis, patent ductus arteriosus and intracranial hemorrhage. Naproxen treatment given in late pregnancy to delay parturition has been associated with persistent pulmonary hypertension, renal dysfunction and abnormal prostaglandin E levels in preterm infants. Because of the known effect of drugs of this class on the human fetal cardiovascular system (closure of ductus arteriosus), use during third trimester should be avoided.
Nursing Mothers
PREVACID ® NapraPAC™ (375 or 500)
No studies were conducted in this population with PREVACID ® NapraPAC™, however, because of the possible adverse effects of prostaglandin-inhibiting drugs on neonates, use of PREVACID ® NapraPAC™ (375 or 500) in nursing mothers should be avoided.
NAPROSYN
The naproxen anion has been found in the milk of lactating women at a concentration of approximately 1% of that found in plasma. Because of the possible adverse effects of prostaglandin-inhibiting drugs on neonates, use in nursing mothers should be avoided.
PREVACID
Lansoprazole or its metabolites are excreted in the milk of rats. It is not known whether lansoprazole is excreted in human milk. Because many drugs are excreted in human milk, because of the potential for serious adverse reactions in nursing infants from lansoprazole, and because of the potential for tumorigenicity shown for lansoprazole in rat carcinogenicity studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
PREVACID ® NapraPAC™ (375 or 500)
The safety and effectiveness of PREVACID ® NapraPAC™ (375 or 500) in pediatric patients have not been established.
Geriatric Use
NAPROSYN
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly. Caution is advised when high doses are required and some adjustment of dosage may be required in elderly patients. As with other drugs used in the elderly, it is prudent to use the lowest effective dose.
PREVACID
The incidence rates of adverse events and laboratory test abnormalities are also similar to those seen in younger patients. For elderly patients, dosage and administration of lansoprazole need not be altered.
Use in Women
PREVACID
Over 4,000 women were treated with lansoprazole. Ulcer healing rates in females were similar to those in males. The incidence rates of adverse events were also similar to those seen in males.
ADVERSE REACTIONS
NAPROSYN
The following adverse reactions are divided into three parts based on frequency and whether or not the possibility exists of a causal relationship between naproxen and these adverse events. In those reactions listed as "Probable Causal Relationship" there is at least 1 case for each adverse reaction where there is evidence to suggest that there is a causal relationship between drug usage and the reported event.
Adverse reactions reported in controlled clinical trials in 960 patients treated for rheumatoid arthritis or osteoarthritis are listed below. In general, reactions in patients treated chronically were reported 2 to 10 times more frequently than they were in short-term studies in the 962 patients treated for mild to moderate pain or for dysmenorrhea. The most frequent complaints reported related to the gastrointestinal tract.
A clinical study found gastrointestinal reactions to be more frequent and more severe in rheumatoid arthritis patients taking daily doses of 1500 mg naproxen compared to those taking 750 mg naproxen.
The following adverse reactions are divided into three parts based on frequency and causal relationship.
Incidence greater than 1% (Probable Causal Relationship): Gastrointestinal - constipation * , heartburn * , abdominal pain * , nausea * , dyspepsia, diarrhea, stomatitis; Central Nervous System - headache * , dizziness * , drowsiness * , lightheadedness, vertigo; Dermatologic - itching (pruritus) * , skin eruptions * , ecchymoses * , sweating, purpura; Special Senses - tinnitus * , hearing disturbances, visual disturbances; Cardiovascular - edema * , dyspnea * , palpitations; General - thirst
*Incidence of reported reaction between 3% and 9%. Those reactions occurring in less than 3% of the patients are unmarked.
Incidence less than 1% (Probable Causal Relationship): The following adverse reactions were reported less frequently than 1% during controlled clinical trials and through voluntary reports since marketing. Those reactions observed through voluntary reporting since marketing of NAPROSYN are underlined.
Gastrointestinal - abnormal liver function tests , colitis , gastrointestinal bleeding and/or perforation , hematemesis , jaundice, pancreatitis, melena, vomiting; Renal - glomerular nephritis , hematuria , hyperkalemia , interstitial nephritis , nephrotic syndrome , renal disease , renal failure , renal papillary necrosis ; Hematologic - agranulocytosis, eosinophilia , granulocytopenia , leukopenia , thrombocytopenia; Central Nervous System - depression , dream abnormalities , inability to concentrate, insomnia , malaise , myalgia , muscle weakness ; Dermatologic - alopecia , photosensitive dermatitis , urticaria , skin rashes, photosensitivity reactions resembling porphyria cutanea tarda , epidermolysis bullosa ; Special Senses - hearing impairment ; Cardiovascular - congestive heart failure ; Respiratory - eosinophilic pneumonitis ; General - anaphylactoid reactions , angioneurotic edema , menstrual disorders , pyrexia (chills and fever)
Incidence less than 1% (Causal Relationship Unknown): These observations are being listed to serve as alerting information to the physician. H ematologic - aplastic anemia , hemolytic anemia ; Central Nervous System - aseptic meningitis , cognitive dysfunction ; Dermatologic - epidermal necrolysis , erythema multiforme , Stevens-Johnson syndrome ; Gastrointestinal - nonpeptic gastrointestinal ulceration , ulcerative stomatitis ; Cardiovascular - vasculitis ; General - hyperglycemia , hypoglycemia
PREVACID
Clinical
Worldwide, over 10,000 patients have been treated with lansoprazole in Phase 2-3 clinical trials involving various dosages and durations of treatment. The adverse reaction profiles for PREVACID Delayed-Release Capsules and PREVACID for Delayed-Release Oral Suspension are similar. In general, lansoprazole treatment has been well-tolerated in both short-term and long-term trials.
The following adverse events were reported by the treating physician to have a possible or probable relationship to drug in 1% or more of PREVACID-treated patients and occurred at a greater rate in PREVACID-treated patients than placebo-treated patients in Table 4.
Table 4 Incidence of Possibly or Probably Treatment-Related Adverse Events in Short-Term, Placebo-Controlled StudiesBody System/Adverse
EventPREVACID
(N= 2768)
%Placebo
(N= 1023)
%Body as a WholeAbdominal Pain2.1 1.2 Digestive SystemConstipation1.0 0.4 Diarrhea3.8 2.3 Nausea1.3 1.2
Headache was also seen at greater than 1% incidence but was more common on placebo. The incidence of diarrhea was similar between patients who received placebo and patients who received lansoprazole 15 mg and 30 mg, but higher in the patients who received lansoprazole 60 mg (2.9%, 1.4%, 4.2%, and 7.4%, respectively).
The most commonly reported possibly or probably treatment-related adverse event during maintenance therapy was diarrhea.
In the risk reduction study of PREVACID for NSAID-associated gastric ulcers, the incidence of diarrhea for patients treated with PREVACID was 5%, misoprostol 22%, and placebo 3%.
Additional adverse experiences occurring in <1% of patients or subjects in domestic trials are shown below. Refer to Postmarketing for adverse reactions occurring since the drug was marketed.
Body as a Whole --abdomen enlarged, allergic reaction, asthenia, back pain, candidiasis, carcinoma, chest pain (not otherwise specified), chills, edema, fever, flu syndrome, halitosis, infection (not otherwise specified), malaise, neck pain, neck rigidity, pain, pelvic pain; Cardiovascular System - angina, arrhythmia, bradycardia, cerebrovascular accident/cerebral infarction, hypertension/hypotension, migraine, myocardial infarction, palpitations, shock (circulatory failure), syncope, tachycardia, vasodilation; Digestive System - abnormal stools, anorexia, bezoar, cardiospasm, cholelithiasis, colitis, dry mouth, dyspepsia, dysphagia, enteritis, eructation, esophageal stenosis, esophageal ulcer, esophagitis, fecal discoloration, flatulence, gastric nodules/fundic gland polyps, gastritis, gastroenteritis, gastrointestinal anomaly, gastrointestinal disorder, gastrointestinal hemorrhage, glossitis, gum hemorrhage, hematemesis, increased appetite, increased salivation, melena, mouth ulceration, nausea and vomiting, nausea and vomiting and diarrhea, oral moniliasis, rectal disorder, rectal hemorrhage, stomatitis, tenesmus, thirst, tongue disorder, ulcerative colitis, ulcerative stomatitis; Endocrine System - diabetes mellitus, goiter, hypothyroidism; Hemic and Lymphatic System - anemia, hemolysis, lymphadenopathy; Metabolic and Nutritional Disorders - gout, dehydration, hyperglycemia/hypoglycemia, peripheral edema, weight gain/loss; Musculoskeletal System - arthralgia, arthritis, bone disorder, joint disorder, leg cramps, musculoskeletal pain, myalgia, myasthenia, synovitis; Nervous System - abnormal dreams, agitation, amnesia, anxiety, apathy, confusion, convulsion, depersonalization, depression, diplopia, dizziness, emotional lability, hallucinations, hemiplegia, hostility aggravated, hyperkinesia, hypertonia, hypesthesia, insomnia, libido decreased/increased, nervousness, neurosis, paresthesia, sleep disorder, somnolence, thinking abnormality, tremor, vertigo; Respiratory System - asthma, bronchitis, cough increased, dyspnea, epistaxis, hemoptysis, hiccup, laryngeal neoplasia, pharyngitis, pleural disorder, pneumonia, respiratory disorder, upper respiratory inflammation/infection, rhinitis, sinusitis, stridor; Skin and Appendages - acne, alopecia, contact dermatitis, dry skin, fixed eruption, hair disorder, maculopapular rash, nail disorder, pruritus, rash, skin carcinoma, skin disorder, sweating, urticaria; Special Senses - abnormal vision, blurred vision, conjunctivitis, deafness, dry eyes, ear disorder, eye pain, otitis media, parosmia, photophobia, retinal degeneration, taste loss, taste perversion, tinnitus, visual field defect; Urogenital System - abnormal menses, breast enlargement, breast pain, breast tenderness, dysmenorrhea, dysuria, gynecomastia, impotence, kidney calculus, kidney pain, leukorrhea, menorrhagia, menstrual disorder, penis disorder, polyuria, testis disorder, urethral pain, urinary frequency, urinary tract infection, urinary urgency, urination impaired, vaginitis.
Postmarketing
On-going Safety Surveillance: Additional adverse experiences have been reported since lansoprazole has been marketed. The majority of these cases are foreign-sourced and a relationship to lansoprazole has not been established. Because these events were reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events are listed below by COSTART body system.
Body as a Whole --anaphylactoid-like reaction; Digestive System - hepatotoxicity, pancreatitis, vomiting; Hemic and Lymphatic System - agranulocytosis, aplastic anemia, hemolytic anemia, leukopenia, neutropenia, pancytopenia, thrombocytopenia, and thrombotic thrombocytopenic purpura; Skin and Appendages - severe dermatologic reactions including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis (some fatal); Special Senses - speech disorder; Urogenital System - urinary retention.
Laboratory Values
The following changes in laboratory parameters for lansoprazole were reported as adverse events:
Abnormal liver function tests, increased SGOT (AST), increased SGPT (ALT), increased creatinine, increased alkaline phosphatase, increased globulins, increased GGTP, increased/decreased/abnormal WBC, abnormal AG ratio, abnormal RBC, bilirubinemia, eosinophilia, hyperlipemia, increased/decreased electrolytes, increased/decreased cholesterol, increased glucocorticoids, increased LDH, increased/decreased/abnormal platelets, and increased gastrin levels. Urine abnormalities such as albuminuria, glycosuria, and hematuria were also reported. Additional isolated laboratory abnormalities were reported.
In the placebo controlled studies, when SGOT (AST) and SGPT (ALT) were evaluated, 0.4% (4/978) placebo patients and 0.4% (11/2677) lansoprazole patients had enzyme elevations greater than three times the upper limit of normal range at the final treatment visit. None of these lansoprazole patients reported jaundice at any time during the study.
OVERDOSAGE
PREVACID ® NapraPAC™ (375 or 500)
In case of an overdose, patients should contact a physician, poison control center, or emergency room. There are no data suggesting increased toxicity of the combination of naproxen and lansoprazole compared with individual components.
NAPROSYN
Significant naproxen overdosage may be characterized by drowsiness, heartburn, indigestion, nausea or vomiting. In the event that ANAPROX/ANAPROX-DS, ALEVE, or naproxen sodium is taken with PREVACID ® NapraPAC™ (375 or 500), earlier and higher blood levels may be anticipated due to the rapid absorption of naproxen sodium. A few patients have experienced seizures, but it is not clear whether or not these were drug-related. It is not known what dose of the drug would be life-threatening. The oral LD 50 of the drug is 543 mg/kg in rats, 1234 mg/kg in mice, 4110 mg/kg in hamsters, and greater than 1000 mg/kg in dogs.
Should a patient ingest a large number of tablets, accidentally or purposefully, the stomach may be emptied and usual supportive measures employed. In animals 0.5 g/kg of activated charcoal was effective in reducing plasma levels of naproxen. Hemodialysis does not decrease the plasma concentration of naproxen because of the high degree of its protein binding.
PREVACID
Oral doses up to 5000 mg/kg in rats (approximately 1300 times the 30 mg human dose based on body surface area) and mice (about 675.7 times the 30 mg human dose based on body surface area) did not produce deaths or any clinical signs.
Lansoprazole is not removed from the circulation by hemodialysis. In one reported case of overdose, the patient consumed 600 mg of lansoprazole with no adverse reaction.
DOSAGE AND ADMINISTRATION
Risk Reduction of NSAID-Associated Gastric Ulcers in the Treatment of Osteoarthritis, Rheumatoid Arthritis, Ankylosing Spondylitis
Each convenience package of PREVACID ® NapraPAC™ (375 or 500) contains sufficient product for seven days of treatment. Each daily dose consists of one PREVACID 15 mg capsule and two NAPROSYN tablets, either 375 mg or 500 mg. Take the PREVACID capsule and one NAPROSYN tablet in the morning before eating with a glass of water. Take the second NAPROSYN tablet in the evening with a glass of water.
The maximum daily naproxen dose of PREVACID ® NapraPAC™ (375 or 500) is 1000 mg. Controlled studies for PREVACID ® NapraPAC™ did not extend beyond 12 weeks.
For PREVACID ® NapraPAC™ (375 or 500), no adjustment of the 15 mg PREVACID component is necessary in patients with renal insufficiency or for the elderly. Dosage adjustment for the NAPROSYN component should be considered for patients with renal insufficiency, liver disease or the elderly (see PRECAUTIONS - Renal Effects and Hepatic Function ).
PREVACID Delayed-Release Capsules should be swallowed whole. The capsule should not be chewed or crushed.
HOW SUPPLIED
PREVACID ® NapraPAC™ 375 is supplied as a weekly blister card packaged as a monthly (28 days) course of therapy. Each weekly blister card contains:
NAPROSYN
- fourteen pink, biconvex oval tablets, engraved with NPR LE 375 on one side.
PREVACID
- seven opaque, hard gelatin, pink and green PREVACID 15 mg capsules, with the TAP logo and "PREVACID 15" imprinted on the capsules.
PREVACID ® NapraPAC™ 500 is supplied as a weekly blister card packaged as a monthly (28 days) course of therapy. Each weekly blister card contains:
NAPROSYN
- fourteen yellow, capsule-shaped tablets, engraved with NPR LE 500 on one side and scored on the other.
PREVACID
- seven opaque, hard gelatin, pink and green PREVACID 15 mg capsules, with the TAP logo and "PREVACID 15" imprinted on the capsules.
NDC 0300-1545-07 Weekly Blister Card, 375 mg
NDC 0300-1546-07 Weekly Blister Card, 500 mg
NDC 0300-1545-30 One Month Administration Pack, 375 mg
NDC 0300-1546-30 One Month Administration Pack, 500 mg
Storage: Protect from light and moisture.
Store at 25°C (77°F), excursions permitted to 15-30°C (59-86°F). [See USP Controlled Room Temperature]
Store and dispense in original container.
Rx only
U.S. Patent No. 6,047,829
Distributed by TAP Pharmaceuticals Inc.
Lake Forest, Illinois 60045, U.S.A.
ALEVE ® is a registered trademark of Bayer-Roche L.L.C.
ANAPROX ® /ANAPROX DS ® , EC-NAPROSYN ® , NAPROSYN ® , and NAPROSYN ® SUSPENSION are registered trademarks of and NapraPAC™ is a trademark of Syntex Pharmaceuticals International Ltd., A Bermuda Corporation
PREVACID ® is a registered trademark of TAP Pharmaceuticals Inc.
3646-R3, Rev. November, 2003
© 2003 Tap Pharmaceutical Products Inc.
Subscribe to the "News" RSS Feed 
TOP ۞
