-
Prochieve 4% Gel, Prochieve 8% Gel (Columbia)
DESCRIPTION
Prochieve® (progesterone gel) is a bioadhesive vaginal gel containing micronized progesterone in an emulsion system, which is contained in single use, one piece polyethylene vaginal applicators. The carrier vehicle is an oil in water emulsion containing the water swellable, but insoluble polymer, polycarbophil. The progesterone is partially soluble in both the oil and water phase of the vehicle, with the majority of the progesterone existing as a suspension. Physically, Prochieve® has the appearance of a soft, white to off-white gel.
The active ingredient, progesterone, is present in either a 4% or an 8% concentration (w/w). The chemical name for progesterone is pregn-4-ene-3,20-dione. It has an empirical formula of C 21 H 30 O 2 and a molecular weight of 314.5. The structural formula is:
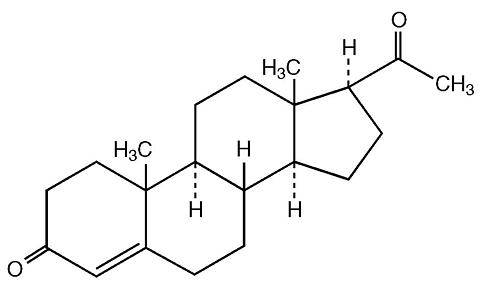
Progesterone exists in two polymorphic forms. Form 1, which is the form used in Prochieve®, exists as white orthorhombic prisms with a melting point of 127-131°C.
Each applicator delivers 1.125 grams of Prochieve® gel containing either 45 mg (4% gel) or 90 mg (8% gel) of progesterone in a base containing glycerin, mineral oil, polycarbophil, carbomer 934P, hydrogenated palm oil glyceride, sorbic acid, purified water and may contain sodium hydroxide.
CLINICAL PHARMACOLOGY
Progesterone is a naturally occurring steroid that is secreted by the ovary, placenta, and adrenal gland. In the presence of adequate estrogen, progesterone transforms a proliferative endometrium into a secretory endometrium. Progesterone is essential for the development of decidual tissue, and the effect of progesterone on the differentiation of glandular epithelia and stroma has been extensively studied. Progesterone is necessary to increase endometrial receptivity for implantation of an embryo. Once an embryo is implanted, progesterone acts to maintain the pregnancy. Normal or near-normal endometrial responses to oral estradiol and intramuscular progesterone have been noted in functionally agonadal women through the sixth decade of life. Progesterone administration decreases the circulatory levels of gonadotropins.
Pharmacokinetics
Absorption
Due to the sustained release properties of Prochieve®, progesterone absorption is prolonged with an absorption half-life of approximately 25-50 hours, and an elimination half-life of 5-20 minutes. Therefore, the pharmacokinetics of Prochieve® are rate-limited by absorption rather than by elimination.
The bioavailability of progesterone in Prochieve® was determined relative to progesterone administered intramuscularly. In a single dose crossover study, 20 healthy, estrogenized postmenopausal women received 45 mg or 90 mg progesterone vaginally in Prochieve® 4% or Prochieve® 8%, or 45 mg or 90 mg progesterone intramuscularly. The pharmacokinetic parameters (mean ± standard deviation) are shown in Table 1.
TABLE 1
Single Dose Relative BioavailabilityPROCHIEVE® 4% 45 mg
Intramuscular
ProgesteronePROCHIEVE® 8% 90 mg
Intramuscular
ProgesteroneC max (ng/mL)13.15 ± 6.49 39.06 ± 13.68 14.87 ± 6.32 53.76 ± 14.9 C avg 0-24 (ng/mL)6.94 ± 4.24 22.41 ± 4.92 6.98 ± 3.21 28.98 ± 8.75 AUC 0-96 (ng·hr/mL)288.63 ± 273.72 806.26 ± 102.75 296.78 ± 129.90 1378.91 ± 176.39 T max (hr)5.6 ± 1.84 8.2 ± 6.43 6.8 ± 3.3 9.2 ± 2.7 t 1/2 (hr)55.13 ± 28.04 28.05 ± 16.87 34.8 ± 11.3 19.6 ± 6.0 F (%)27.6 19.8 C max - maximum progesterone serum concentration
C avg 0-24 - average progesterone serum concentration over 24 hours
AUC 0-96 - area under the drug concentration versus time curve from 0-96 hours post dose
T max - time to maximum progesterone concentration
t 1/2 - elimination half-life
F - relative bioavailabilityThe multiple dose pharmacokinetics of Prochieve® 4% and Prochieve® 8% administered every other day and Prochieve® 8% administered daily or twice daily for 12 days were studied in 10 healthy, estrogenized postmenopausal women in two separate studies. Steady state was achieved within the first 24 hours after initiation of treatment. The pharmacokinetic parameters (mean ± standard deviation) after the last administration of Prochieve® 4% or 8% derived from these studies are shown in Table 2.
TABLE 2
Multiple Dose PharmacokineticsAssisted Reproductive Technology Secondary Amenorrhea Daily Dosing
8%Twice Daily
Dosing 8%Every Other
Day Dosing 4%Every Other
Day Dosing 8%C max (ng/mL)15.97 ± 5.05 14.57 ± 4.49 13.21 ± 9.46 13.67 ± 3.58 C avg (ng/mL)8.99 ± 3.53 11.6 ± 3.47 4.05 ± 2.85 6.75 ± 2.83 T max (hr)5.40 ± 0.97 3.55 ± 2.48 6.67 ± 3.16 7.00 ± 2.88 AUC 0-t (ng·hr/mL)391.98 ± 153.28 138.72 ± 41.58 242.15 ± 167.88 438.36 ± 223.36 t 1/2 (hr)45.00 ± 34.70 25.91 ± 6.15 49.87 ± 31.20 39.08 ± 12.88 Distribution
Progesterone is extensively bound to serum proteins (~96-99%), primarily to serum albumin and corticosteroid binding globulin.
Metabolism
The major urinary metabolite of oral progesterone is 5(beta)-pregnan-3(alpha), 20(alpha)-diol glucuronide which is present in plasma in the conjugated form only. Plasma metabolites also include 5(beta)-pregnan-3(alpha)-ol-20-one (5(beta)-pregnanolone) and 5(alpha)-pregnan-3(alpha)-ol-20-one (5(alpha)-pregnanolone).
Excretion
Progesterone undergoes both biliary and renal elimination. Following an injection of labeled progesterone, 50-60% of the excretion of progesterone metabolites occurs via the kidney; approximately 10% occurs via the bile and feces, the second major excretory pathway. Overall recovery of labeled material accounts for 70% of an administered dose, with the remainder of the dose not characterized with respect to elimination. Only a small portion of unchanged progesterone is excreted in the bile.
Clinical Studies
Assisted Reproductive Technology
In a single-center, open-label study (COL1620-007US), 99 women (aged 28-47 years) with either partial (n=84) or premature ovarian failure (n=15) who were candidates to receive a donor oocyte transfer as an Assisted Reproductive Technology ("ART") procedure were randomized to recieve either Prochieve® 8% twice daily (n=68) or intramuscular progesterone 100 mg daily (n=31). The study was divided into three phases (Pilot, Donor Egg and Treatment). The first phase of the study consisted of a test Pilot Cycle to ensure that the administration of transdermal estradiol and progesterone would adequately prime the endometrium to receive the donor egg. The second phase was the Donor Egg Cycle during which a fertilized oocyte was implanted. Prochieve® 8% was administered beginning the evening of Day 14 of the Pilot and Donor Egg cycles. Subjects with partial ovarian function also underwent a Pre-Pilot Cycle and a Pre-Donor Egg Cycle during which time they were administered only leuprolide acetate to suppress remaining ovarian function. The Pre-Pilot Cycle, Pilot Cycle, Pre-Donor Egg Cycle, and Donor Egg Cycle each lasted approximately 34 days. The third phase of the study consisted of a 10-week treatment period to maintain a pregnancy until placental autonomy was achieved.
Sixty-one women received Prochieve® 8% as part of the Pilot Cycle to determine their endometrial response. Of the 55 evaluable endometrial biopsies in the Prochieve® 8% group performed on Day 25-27, all were histologically "in-phase", consistent with luteal phase biopsy specimens of menstruating women at comparable time intervals. Fifty-four women who received Prochieve® 8% and had a histologically "in-phase" biopsy received a donor oocyte transfer. Among these 54 Prochieve®-treated women, clinical pregnancies (assessed about week 10 after transfer by clinical examination, ultrasound and/or (beta)-hCG levels) occurred in 26 women (48%). In these 26 women, 17 women (65%) delivered a total of 25 newborns, seven women (27%) had spontaneous abortions and two women (8%) had elective abortions.
In a second study (COL1620-F01), Prochieve® 8% was used in luteal phase support of women with tubal or idiopathic infertility due to endometriosis and normal ovulatory cycles, undergoing in vitro fertilization ("IVF") procedures. All women received a GnRH analog to suppress endogenous progesterone, human menopausal gonadotropins, and human chorionic gonadotropin. In this multi-center, open-label study, 139 women (aged 22-38 years) received Prochieve® 8% once daily beginning within 24 hours of embryo transfer and continuing through Day 30 post-transfer. Clinical pregnancies assessed at Day 90 post-transfer were seen in 36 (26%) of women. Thirty-two women (23%) delivered newborns and four women (3%) had spontaneous abortions. (See PRECAUTIONS , subsection Pregnancy )
Secondary Amenorrhea
In three parallel, open-label studies (COL1620-004US, COL1620-005US, COL1620-009US), 127 women (aged 18-44) with hypothalamic amenorrhea or premature ovarian failure were randomized to receive either Prochieve® 4% (n=62) or Prochieve® 8% (n=65). All women were treated with either conjugated estrogens 0.625 mg daily (n=100) or transdermal estradiol (delivering 50 mcg/day) twice weekly (n=27).
Estrogen therapy was continuous for the entire three 28-day cycle studies. At Day 15 of the second cycle (six weeks after initiating estrogen replacement), women who demonstrated adequate response to estrogen therapy (by ultrasound) and who continued to be amenorrheic received Prochieve® every other day for six doses (Day 15 through Day 25 of the cycle).
In cycle 2, Prochieve® 4% induced bleeding in 79% of women and Prochieve® 8% induced bleeding in 77% of women. In the third cycle, estrogen was continued and Prochieve® was administered every other day beginning on Day 15 for six doses. On Day 24 an endometrial biopsy was performed. In 53 women who received Prochieve® 4%, biopsy results were as follows: 7% proliferative, 40% late secretory, 19% mid secretory, 13% early secretory, 7% atrophic, 6% menstrual endometrium, 6% inactive endometrium and 2% negative endometrium. In 54 women who received Prochieve® 8%, biopsy results were as follows: 44% late secretory, 19% mid secretory, 11% early secretory, 19% atrophic, 5% menstrual endometrium and 2% "oral contraceptive like" endometrium.
INDICATIONS AND USAGE
Assisted Reproductive Technology
Prochieve® 8% is indicated for progesterone supplementation or replacement as part of an Assisted Reproductive Technology ("ART") treatment for infertile women with progesterone deficiency.
Secondary Amenorrhea
Prochieve® 4% is indicated for the treatment of secondary amenorrhea. Prochieve® 8% is indicated for use in women who have failed to respond to treatment with Prochieve® 4%.
CONTRAINDICATIONS
Prochieve® should not be used in individuals with any of the following conditions:
- Known sensitivity to Prochieve® (progesterone or any of the other ingredients)
- Undiagnosed vaginal bleeding
- Liver dysfunction or disease
- Known or suspected malignancy of the breast or genital organs
- Missed abortion
- Active thrombophlebitis or thromboembolic disorders, or a history of hormone-associated thrombophlebitis or thromboembolic disorders
WARNINGS
The physician should be alert to the earliest manifestations of thrombotic disorders (thrombophlebitis, cerebrovascular disorders, pulmonary embolism, and retinal thrombosis). Should any of these occur or be suspected, the drug should be discontinued immediately.
Progesterone and progestins have been used to prevent miscarriage in women with a history of recurrent spontaneous pregnancy losses. No adequate evidence is available to show that they are effective for this purpose.
PRECAUTIONS
General
- The pretreatment physical examination should include special reference to breast and pelvic organs, as well as Papanicolaou smear.
- In cases of breakthrough bleeding, as in all cases of irregular vaginal bleeding, nonfunctional causes should be considered. In cases of undiagnosed vaginal bleeding, adequate diagnostic measures should be undertaken.
- Because progestogens may cause some degree of fluid retention, conditions which might be influenced by this factor (e.g., epilepsy, migraine, asthma, cardiac or renal dysfunction) require careful observation.
- The pathologist should be advised of progesterone therapy when relevant specimens are submitted.
- Patients who have a history of psychic depression should be carefully observed and the drug discontinued if the depression recurs to a serious degree.
- A decrease in glucose tolerance has been observed in a small percentage of patients on estrogen-progestin combination drugs. The mechanism of this decrease is not known. For this reason, diabetic patients should be carefully observed while receiving progestin therapy.
Information for Patients
The product should not be used concurrently with other local intravaginal therapy. If other local intravaginal therapy is to be used concurrently, there should be at least a 6-hour period before or after Prochieve® administration. Small, white globules may appear as a vaginal discharge possibly due to gel accumulation, even several days after usage.
Drug Interactions
No drug interactions have been assessed with Prochieve®.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Nonclinical toxicity studies to determine the potential of Prochieve® to cause carcinogenicity or mutagenicity have not been performed. The effect of Prochieve® on fertility has not been evaluated in animals.
Pregnancy (See CLINICAL PHARMACOLOGY , subsection Clinical Studies )
Prochieve® 8% has been used to support embryo implantation and maintain pregnancies through its use as part of ART treatment regimens in two clinical studies (studies COL1620-007US and COL1620-F01). In the first study (COL1620-007US), 54 Prochieve®-treated women had donor oocyte transfer procedures, and clinical pregnancies occurred in 26 women (48%). The outcomes of these 26 pregnancies were as follows: one woman had an elective termination of pregnancy at 19 weeks due to congenital malformations (omphalocele) associated with a chromosomal abnormality; one woman pregnant with triplets had an elective termination of her pregnancy; seven women had spontaneous abortions; and 17 women delivered 25 apparently normal newborns.
In the second study (COL1620-F01), Prochieve® 8% was used in the luteal phase support of women undergoing in vitro fertilization ("IVF") procedures. In this multi-center, open-label study, 139 women received Prochieve® 8% once daily beginning within 24 hours of embryo transfer and continuing through Day 30 post-transfer.
Clinical pregnancies assessed at Day 90 post-transfer were seen in 36 (26%) of women. Thirty-two women (23%) delivered newborns and four women (3%) had spontaneous abortions. Of the 47 newborns delivered, one had a teratoma associated with a cleft palate; one had respiratory distress syndrome; 44 were apparently normal and one was lost to follow-up.
Geriatric Use
The safety and effectiveness in geriatric patients (over age 65) have not been established.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Nursing Mothers
Detectable amounts of progestins have been identified in the milk of mothers receiving them. The effect of this on the nursing infant has not been determined.
ADVERSE REACTIONS
Assisted Reproductive Technology
In a study of 61 women with ovarian failure undergoing a donor oocyte transfer procedure receiving Prochieve® 8% twice daily, treatment-emergent adverse events occurring in 5% or more of the women are shown in Table 3.
TABLE 3
Treatment-Emergent Adverse Events in >/=5% of Women Receiving Prochieve® 8% Twice Daily
Study COL1620-007US (n=61)Body as a WholeBloating7% Cramps NOS15% Pain8% Central and Peripheral Nervous SystemDizziness5% Headache13% Gastro-Intestinal SystemNausea7% Reproductive, FemaleBreast Pain13% Moniliasis Genital5% Vaginal Discharge7% Skin and AppendagesPruritus Genital5% In a second clinical study of 139 women using Prochieve® 8% once daily for luteal phase support while undergoing an in vitro fertilization procedure, treatment-emergent adverse events reported in >/=5% of the women are shown in Table 4.
TABLE 4
Treatment-Emergent Adverse Events in >/=5% of Women
Receiving Prochieve® 8% Once Daily
Study COL1620-F01 (n=139)Body as a WholeAbdominal Pain12% Perineal Pain Female17% Central and Peripheral Nervous SystemHeadache17% Gastro-Intestinal SystemConstipation27% Diarrhea8% Nausea22% Vomiting5% Musculo-Skeletal SystemArthralgia8% PsychiatricDepression11% Libido Decreased10% Nervousness16% Somnolence27% Reproductive, FemaleBreast Enlargement40% Dyspareunia6% Urinary SystemNocturia13% Secondary Amenorrhea
In three studies, 127 women with secondary amenorrhea received estrogen replacement therapy and Prochieve® 4% or 8% every other day for six doses. Treatment emergent adverse events during estrogen and Prochieve® treatment that occurred in 5% or more of women are shown in Table 5.
TABLE 5
Treatment-Emergent Adverse Events in >/=5% of Women
Receiving Estrogen Treatment and Prochieve®
Every Other Day
Studies COL1620-004US, COL1620-005US,
COL1620-009USEstrogen
+Prochieve® 4%
n=62Estrogen
+Prochieve® 8%
n=65Body as a WholeAbdominal Pain3 (5%) 6 (9%) Appetite Increased3 (5%) 5 (8%) Bloating8 (13%) 8 (12%) Cramps NOS12 (19%) 17 (26%) Fatigue13 (21%) 14 (22%) Central and Peripheral Nervous SystemHeadache12 (19%) 10 (15%) Gastro-Intestinal SystemNausea5 (8%) 4 (6%) Musculo-Skeletal SystemBack Pain5 (8%) 2 (3%) Myalgia5 (8%) 0 (0%) PsychiatricDepression12 (19%) 10 (15%) Emotional Lability14 (23%) 14 (22%) Sleep Disorder11 (18%) 12 (18%) Reproductive, FemaleVaginal Discharge7 (11%) 2 (3%) Resistance MechanismUpper Respiratory
Tract Infection3 (5%) 5 (8%) Skin and AppendagesPruritus genital1 (2%) 4 (6%) Additional adverse events reported in women at a frequency <5% in Prochieve® ART and secondary amenorrhea studies and not listed in the tables above include:
Autonomic Nervous System --mouth dry, sweating increased
Body as a Whole --abnormal crying, allergic reaction, allergy, appetite decreased, asthenia, edema, face edema, fever, hot flushes, influenza-like symptoms, water retention, xerophthalmia
Cardiovascular, General --syncope
Central and Peripheral Nervous System --migraine, tremor
Gastro-Intestinal --dyspepsia, eructation, flatulence, gastritis, toothache
Metabolic and Nutritional --thirst
Musculo-Skeletal System --cramps legs, leg pain, skeletal pain
Neoplasm --benign cyst
Platelet, Bleeding Clotting --purpura
Psychiatric --aggressive reactions, forgetfulness, insomnia
Red Blood Cell --anemia
Reproductive, Female --dysmenorrhea, premenstrual tension, vaginal dryness
Resistance Mechanism --infection, pharyngitis, sinusitis, urinary tract infection
Respiratory System --asthma, dyspnea, hyperventilation, rhinitis
Skin and Appendages --acne, pruritis, rash, seborrhea, skin discoloration, skin disorder, urticaria
Urinary System --cystitis, dysuria, micturition frequency
Vision Disorders --conjunctivitis
OVERDOSAGE
There have been no reports of overdosage with Prochieve®. In the case of overdosage, however, discontinue Prochieve®, treat the patient symptomatically, and institute supportive measures.
As with all prescription drugs, this medicine should be kept out of the reach of children.
DOSAGE AND ADMINISTRATION
Assisted Reproductive Technology
Prochieve® 8% is administered vaginally at a dose of 90 mg once daily in women who require progesterone supplementation. Prochieve® 8% is administered vaginally at a dose of 90 mg twice daily in women with partial or complete ovarian failure who require progesterone replacement. If pregnancy occurs, treatment may be continued until placental autonomy is achieved, up to 10-12 weeks.
Secondary Amenorrhea
Prochieve® 4% is administered vaginally every other day up to a total of six doses. For women who fail to respond, a trial of Prochieve® 8% every other day up to a total of six doses may be instituted.
It is important to note that a dosage increase from the 4% gel can only be accomplished by using the 8% gel. Increasing the volume of gel administered does not increase the amount of progesterone absorbed.
SEE Prochieve® PATIENT INFORMATION SHEET - HOW TO USE Prochieve®. Note: The PATIENT INFORMATION SHEET contains special instructions for using the applicator at altitudes above 2500 feet in order to avoid a partial release of Prochieve® before vaginal insertion.
HOW SUPPLIED
Prochieve® is available in the following strengths:
4% gel (45 mg) in a single use, one piece, disposable, white polyethylene vaginal applicator with a twist-off top. Each applicator contains 1.45 g of gel and delivers 1.125 g of gel.
NDC-55056-0406-1 - 6 Single-use prefilled applicators.
8% gel (90 mg) in a single use, one piece, disposable, white polyethylene vaginal applicator with a twist-off top. Each applicator contains 1.45 g of gel and delivers 1.125 g of gel.
NDC-55056-0806-1 - 6 Single-use prefilled applicators.
NDC-55056-0818-1 - 18 Single-use prefilled applicators
Each applicator is wrapped and sealed in a foil overwrap.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Rx only.
U.S. Patent Number 5,543,150.
Manufactured for: Columbia Laboratories, Inc. Livingston, NJ 07039
Manufactured by: Fleet Laboratories Ltd., Watford, United Kingdom
40905010004 Revised November 2004
PATIENT INFORMATION
Prochieve® 8%
(progesterone gel)
For Vaginal Use Only
FOR PROGESTERONE SUPPLEMENTATION OR REPLACEMENT AS PART OF AN ASSISTED REPRODUCTIVE TECHNOLOGY ("ART") TREATMENT FOR INFERTILE WOMEN WITH PROGESTERONE DEFICIENCY
Please read this information carefully before you start to use Prochieve® and each time your prescription is renewed, in case anything has changed. This leaflet does not take the place of discussions with your doctor. If you still have any questions, ask your doctor or health-care provider.
What Prochieve® is
Prochieve® is a specially formulated gel that you insert in your vagina. It contains the natural female hormone called progesterone. Prochieve® 8% is used as part of a program for women who are undergoing fertility treatment.
Understanding the role of Prochieve® in your infertility treatment
Progesterone is one of the hormones essential for maintaining a pregnancy. If you are undergoing ART treatment and your doctor has determined your body does not produce enough progesterone on its own, Prochieve® may be prescribed to provide the progesterone you need.
The progesterone in Prochieve® will help prepare the lining of your uterus so that it is ready to receive and nourish a fertilized egg. If pregnancy occurs, Prochieve® may be supplemented for 10-12 weeks until production of progesterone by the placenta is adequate.
When you should not use Prochieve®
- If you are allergic to progesterone, progesterone-like drugs, or any of the inactive ingredients in the gel (ask a pharmacist if you are not sure about the inactive ingredients in Prochieve®).
- If you have unusual vaginal bleeding which has not been evaluated by a doctor.
- If you have a liver disease.
- If you have known or suspected cancer of the breast or genital organs.
- If you have a miscarriage and your physician suspects some tissue is still in the uterus.
- If you have or have had blood clots in the legs, lungs, eyes, or elsewhere.
Risks of Prochieve®
- Risk to the fetus. Birth defects have been reported in the offspring of women who were using Prochieve® during early pregnancy. These included an abdominal wall defect and a cleft palate. A causal association has been neither confirmed nor refuted. You should check with your doctor about the risks to your unborn child of any medication used during pregnancy.
- Blood clots and related health problems. Blood clots have been reported with the use of estrogens and progestational drugs (alone or in combination). If blood clots do form in your bloodstream, they can cut off the blood supply to vital organs, causing serious problems. These problems may include a stroke (by cutting off blood to part of the brain), a heart attack (by cutting off blood to part of the heart), a pulmonary embolus (by cutting off blood to part of the lungs), or other problems. Any of these conditions may cause death or serious long-term disability. Call your doctor immediately if you suspect you have any of these conditions. He or she may advise you to stop using this drug.
PRECAUTIONS
Be alert for unusual signs and symptoms. If any of these warning signals (or any other unusual symptoms) happen while you are using Prochieve®, call your doctor immediately:
- Abnormal bleeding from the vagina.
- Pains in the calves or chest, a sudden shortness of breath or coughing blood indicating possible clots in the legs, heart, or lungs.
- Severe headache or vomiting, dizziness, faintness, or changes in vision or speech, weakness or numbness of an arm or leg indicating possible clots in the brain or eye.
- Breast lumps, which could be associated with fibrocystic disorders, fibroadenoma, or breast cancer. (Ask your doctor or health-care provider to show you how to examine your breasts monthly.)
- Yellowing of the skin and/or white of the eyes indicating possible liver problems.
You should also notify your doctor if you experience depression, worsening of your diabetic condition, or fluid retention.
Possible side effects of Prochieve®
In addition to the risks listed above, the following side effects have been reported with Prochieve® used either for progesterone supplementation or for replacement as part of an ART treatment for infertile women with progesterone deficiency. Consult your doctor if you experience any of the side effects mentioned below, or other side effects.
SIDE EFFECTS REPORTED AT A FREQUENCY OF 5% OR GREATER
- abdominal pain; perineal pain (the perineum is the area between the vagina and the rectum)
- headache
- constipation; diarrhea; nausea
- joint pain
- depression; decreased libido; nervousness; sleepiness *
- breast enlargement
- excessive urination at night
SIDE EFFECTS REPORTED AT A FREQUENCY RANGING FROM 1% TO 5%
- allergy; bloating; cramps; fatigue; pain
- dizziness *
- vomiting
- mood swings
- breast pain
- difficult or painful intercourse; genital itching; genital yeast infection; vaginal discharge
- urinary tract infection
SIDE EFFECTS REPORTED AT A FREQUENCY OF LESS THAN 1%
- fever; flu-like symptoms
- water retention †
- gastrointestinal discomfort; gas; abdominal swelling
- back pain; leg pain
- insomnia
- sinusitis; upper respiratory tract infection
- asthma
- acne; itching
- painful or difficult urination; frequent urination
*If you experience dizziness or sleepiness, do not drive or operate machinery.
† This may worsen some conditions such as asthma, epilepsy, migraine, heart disease, or kidney disease.
How Prochieve® works
Prochieve® has been formulated to be administered through the vagina. The moisturizing gel in Prochieve® forms a coating on the walls of the vagina which allows for absorption of progesterone through the vaginal tissue. Small, white globules may appear as a vaginal discharge possibly due to gel accumulation, even several days after usage. Prochieve® contains no irritating perfumes or dyes.
Other information
- Your doctor has prescribed this drug for you and you alone. Do not give this drug to anyone else.
- This medication was prescribed for your particular medical condition. Do not use it for another condition.
- Keep this and all drugs out of the reach of children.
How to use Prochieve®
The dosage is one application of the 8% gel (90 mg of progesterone) vaginally, daily or twice daily as directed by your doctor. If you become pregnant, your doctor may decide to continue treatment for up to 10 to 12 weeks.
Prochieve® is to be applied directly from the specially designed sealed applicator into the vagina. The applicator is designed to deliver a premeasured dose of Prochieve®. A small amount of gel will be left in the tube after usage. Do not be concerned because you will still be receiving the appropriate, measured dosage.
-
Remove the applicator from the sealed wrapper. DO NOT remove the twist-off tab at this time.
For use at altitudes above 2500 feet, see special instructions below
-
Hold the applicator between the thumb and forefinger along the seam on the sides of the bulb. Shake down vigorously 3 to 4 times (like a thermometer) to ensure that the contents are at the thin end of the applicator.
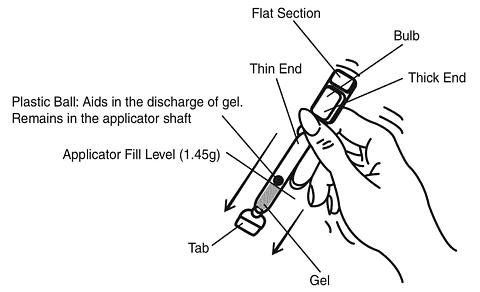
-
Hold the applicator by the flat section of the bulb. Twist off the tab at the thin end and throw away. DO NOT squeeze the bulb while twisting the tab. This could force some gel to be released before it is inserted.
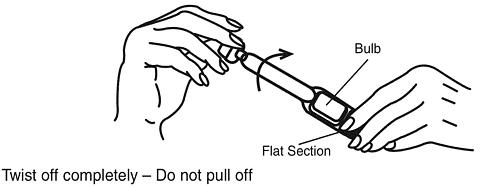
-
The applicator may be inserted into the vagina while you are in a sitting position or when lying on your back with your knees bent.
Gently insert the thin end well into the vagina.
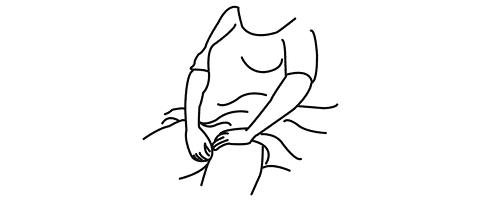
-
Squeeze the bulb of the applicator to deposit the gel into the vagina. Remove the applicator and throw it away in a waste container. Do not be concerned if a small amount of gel is left in the applicator. You will still be receiving the appropriate, measured dosage.
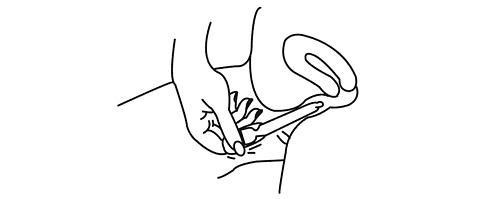
SPECIAL INSTRUCTIONS FOR USE AT ALTITUDES ABOVE 2500 FEET
-
Remove the applicator from the sealed wrapper. DO NOT remove the twist-off tab at this time.
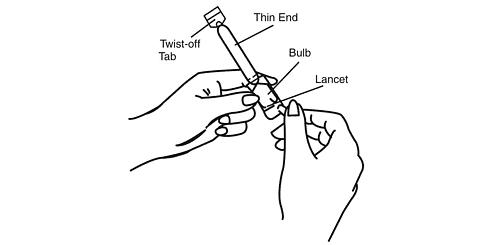
Hold the applicator on both sides of the bulb, as shown. Using a lancet or a stick pin, make a single puncture in the flat part of the bulb. This will relieve the difference in air pressure between the inside and outside of the applicator caused by high altitudes. It will not affect the amount of gel administered.
You will still be receiving the appropriate, measured dosage.
-
See Step 2 above.
-
See Step 3 above.
-
See Step 4 above.
-
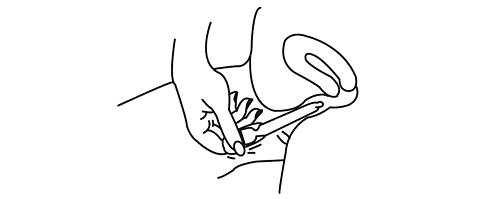
Place your thumb or finger over the puncture that you made in the bulb of the applicator.
Squeeze the
bulb
of the applicator to deposit the gel into the vagina. Remove the applicator and throw it away in a waste container. Do not be concerned if a small amount of gel is left in the applicator. You will still be receiving the appropriate, measured dosage.
Prochieve® coats the vaginal lining to provide long-lasting release of progesterone. Small, white globules may appear as a vaginal discharge possibly due to gel accumulation, even several days after usage. It is not unusual, but if you are concerned, discuss this with your doctor.
If you forget a dose of Prochieve®, use it as soon as you remember, but do not use more than the recommended daily dose. Prochieve® should not be used at the same time that you are using other vaginal therapy.
This leaflet provides the most important information about Prochieve®. If you want to read more, ask your doctor or pharmacist about the professional leaflet. You may need their help to understand some of the information.
How Supplied
Prochieve® is available as 8% gel (90 mg of progesterone).
Each box of the 8% gel contains either six or eighteen single use, disposable vaginal applicators with a twist-off tab. Each applicator is wrapped and sealed in a foil overwrap.
Prochieve® should be stored at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Do not use Prochieve® after the expiration date which is printed on the box.
Manufactured for: Columbia Laboratories, Inc., Livingston, NJ 07039
Manufactured by: Fleet Laboratories Ltd., Watford, United Kingdom
40905010004 Revised November 2004
PATIENT INFORMATION
Prochieve® 4% and
Prochieve® 8%
(progesterone gel)
For Vaginal Use Only
FOR THE TREATMENT OF SECONDARY AMENORRHEA (ABSENCE OF MENSES IN WOMEN WHO HAVE PREVIOUSLY HAD A MENSTRUAL PERIOD)
Please read this information carefully before you start to use Prochieve® and each time your prescription is renewed, in case anything has changed. This leaflet does not take the place of discussions with your doctor. If you still have any questions, ask your doctor or health-care provider.
What Prochieve® is
Prochieve® is a specially formulated gel that you insert in your vagina. It contains the natural female hormone called progesterone. The 4% gel is used for women whose menstrual cycle has stopped. The 8% gel is to be used when the 4% gel has not worked.
Understanding the role of Prochieve® in the treatment of your menstrual irregularities
Progesterone is one of the hormones essential for regular menstrual periods. If your doctor has determined your body does not produce enough progesterone on its own, Prochieve® may be prescribed to provide the progesterone you need.
When you do not produce enough progesterone, menstrual irregularities can occur. Prochieve® can provide you with the progesterone needed during a normal menstrual cycle.
When you should not use Prochieve®
- If you are allergic to progesterone, progesterone-like drugs, or any of the inactive ingredients in the gel (ask a pharmacist if you are not sure about the inactive ingredients in Prochieve®).
- If you have unusual vaginal bleeding which has not been evaluated by a doctor.
- If you have a liver disease.
- If you have known or suspected cancer of the breast or genital organs.
- If you have a miscarriage and your physician suspects some tissue is still in the uterus.
- If you have or have had blood clots in the legs, lungs, eyes, or elsewhere.
Risks of Prochieve®
- Risk to the fetus. Birth defects have been reported in the offspring of women who were using Prochieve® during early pregnancy. These included an abdominal wall defect and a cleft palate. A causal association has been neither confirmed nor refuted. You should check with your doctor about the risks to your unborn child of any medication used during pregnancy.
- Blood clots and related health problems. Blood clots have been reported with the use of estrogens and progestational drugs (alone or in combination). If blood clots do form in your bloodstream, they can cut off the blood supply to vital organs, causing serious problems. These problems may include a stroke (by cutting off blood to part of the brain), a heart attack (by cutting off blood to part of the heart), a pulmonary embolus (by cutting off blood to part of the lungs), or other problems. Any of these conditions may cause death or serious long-term disability. Call your doctor immediately if you suspect you have any of these conditions. He or she may advise you to stop using this drug.
PRECAUTIONS
Be alert for unusual signs and symptoms. If any of these warning signals (or any other unusual symptoms) ha-ppen while you are using Prochieve®, call your doctor immediately:
- Abnormal bleeding from the vagina.
- Pains in the calves or chest, a sudden shortness of breath or coughing blood indicating possible clots in the legs, heart, or lungs.
- Severe headache or vomiting, dizziness, faintness, or changes in vision or speech, weakness or numbness of an arm or leg indicating possible clots in the brain or eye.
- Breast lumps, which could be associated with fibrocystic disorders, fibroadenoma, or breast cancer. (Ask your doctor or health-care provider to show you how to examine your breasts monthly.)
- Yellowing of the skin and/or white of the eyes indicating possible liver problems.
You should also notify your doctor if you experience depression, worsening of your diabetic condition, or fluid retention.
Possible side effects of Prochieve®
In addition to the risks listed above, the following side effects have been reported in studies with Prochieve® used for the treatment of menstrual irregularities due to progesterone deficiency. In these studies, women were treated with estrogen prior to and during Prochieve® therapy. All side effects reported at a frequency of 5% or greater after Prochieve® was added to estrogen therapy also were reported with estrogen therapy alone. Consult your doctor if you experience any of the side effects mentioned below, or other side effects.
SIDE EFFECTS REPORTED AT A FREQUENCY OF 5% OR GREATER
- abdominal pain; increased appetite; bloating; cramps; fatigue
- headache
- nausea
- back pain
- depression; mood swings; sleep disorder
- vaginal discharge
- upper respiratory tract infection
SIDE EFFECTS REPORTED AT A FREQUENCY RANGING FROM 1% TO 5%
- increased sweating
- allergy; flu-like symptoms; hot flushes; pain
- dizziness *
- migraine; tremor
- gas; gastrointestinal discomfort
- thirst
- leg pain; muscle pain
- insomnia; nervousness; sleepiness *
- breast pain; painful menstruation
- infection; genital yeast infection
- acne; genital itching; rash; skin disorder
- frequent urination
SIDE EFFECTS REPORTED AT A FREQUENCY OF LESS THAN 1%
- dry mouth
- abnormal crying; allergic reaction; decreased appetite; dry eyes; swelling; face swelling; perineal pain (the perineum is the area between the vagina and the rectum); water retention † ; weakness
- fainting
- abdominal swelling; gastritis; toothache
- joint pain; leg cramps; skeletal pain
- non-cancerous cyst
- bruising
- aggressive reaction; forgetfulness
- anemia
- premenstrual syndrome; vaginal dryness
- sore throat
- rapid, shallow breathing; shortness of breath; runny nose
- hives; itching; oily or dry scaly skin; skin discoloration
- bladder inflammation; painful or difficult urination
- "pink eye"
*If you experience dizziness or sleepiness, do not drive or operate machinery.
† This may worsen some conditions such as asthma, epilepsy, migraine, heart disease, or kidney disease.
How Prochieve® works
Prochieve® has been formulated to be administered through the vagina. The moisturizing gel in Prochieve® forms a coating on the walls of the vagina which allows for absorption of progesterone through the vaginal tissue. Small, white globules may appear as a vaginal discharge possibly due to gel accumulation, even several days after usage. Prochieve® contains no irritating perfumes or dyes.
Other information
- Your doctor has prescribed this drug for you and you alone. Do not give this drug to anyone else.
- This medication was prescribed for your particular medical condition. Do not use it for another condition.
- Keep this and all drugs out of the reach of children.
How to use Prochieve®
The dosage is one application of the 4% gel (45 mg of progesterone), vaginally, every other day as directed by your doctor, for a total of six doses. In some cases, your doctor may prescribe the 8% gel (90 mg of progesterone) every other day, for a total of six doses.
It is important to note that a dosage increase from the 4% gel can only be accomplished by using the 8% gel. Increasing the volume of gel administered does not increase the amount of progesterone absorbed.
Prochieve® is to be applied directly from the specially designed sealed applicator into the vagina. The applicator is designed to deliver a premeasured dose of Prochieve®. A small amount of gel will be left in the tube after usage. Do not be concerned because you will still be receiving the appropriate, measured dosage.
-
Remove the applicator from the sealed wrapper. DO NOT remove the twist-off tab at this time.
For use at altitudes above 2500 feet, see special instructions below.
-
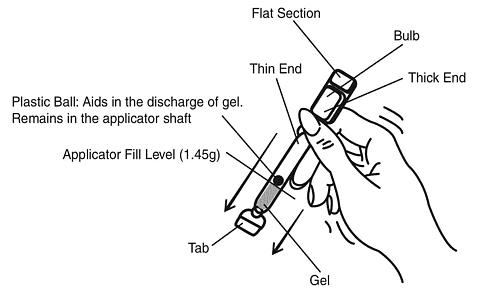
Hold the applicator between the thumb and forefinger along the seam on the sides of the bulb. Shake down vigorously 3 to 4 times (like a thermometer) to ensure that the contents are at the thin end of the applicator.
-
Hold the applicator by the flat section of the bulb. Twist off the tab at the thin end and throw away. DO NOT squeeze the bulb while twisting the tab. This could force some gel to be released before it is inserted.
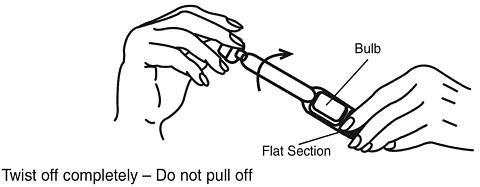
-
The applicator may be inserted into the vagina while you are in a sitting position or when lying on your back with your knees bent.
Gently insert the thin end well into the vagina.
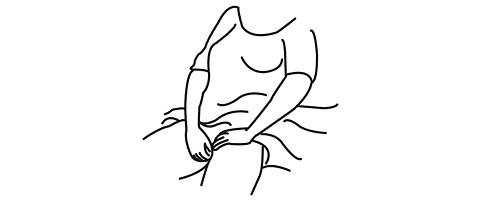
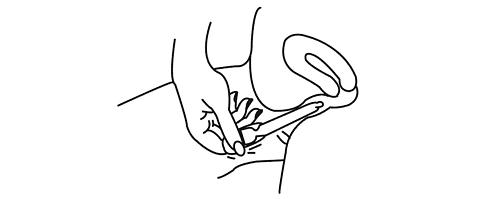
-
Squeeze the bulb of the applicator to deposit the gel into the vagina. Remove the applicator and throw it away in a waste container. Do not be concerned if a small amount of gel is left in the applicator. You will still be receiving the appropriate, measured dosage.
SPECIAL INSTRUCTIONS FOR USE AT ALTITUDES ABOVE 2500 FEET
-
Remove the applicator from the sealed wrapper. DO NOT remove the twist-off tab at this time.
Hold the applicator on both sides of the bulb, as shown. Using a lancet or a stick pin, make a single puncture in the flat part of the bulb. This will relieve the difference in air pressure between the inside and outside of the applicator caused by high altitudes. It will not affect the amount of gel administered.
You will still be receiving the appropriate, measured dosage.
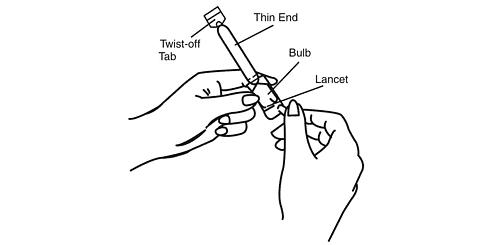
-
See Step 2 above.
-
See Step 3 above.
-
See Step 4 above.
-
Place your thumb or finger over the puncture that you made in the bulb of the applicator.
Squeeze the
bulb
of the applicator to deposit the gel into the vagina. Remove the applicator and throw it away in a waste container. Do not be concerned if a small amount of gel is left in the applicator. You will still be receiving the appropriate, measured dosage.
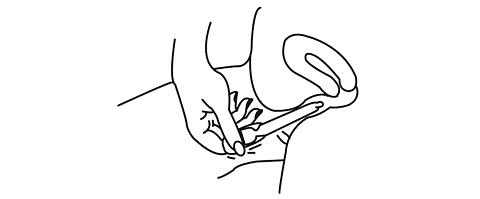
Prochieve® coats the vaginal lining to provide long-lasting release of progesterone. Small, white globules may appear as a vaginal discharge possibly due to gel accumulation, even several days after usage. It is not unusual, but if you are concerned, discuss this with your doctor.
If you forget a dose of Prochieve®, use it as soon as you remember, but do not use more than the recommended daily dose. Prochieve® should not be used at the same time that you are using other vaginal therapy.
This leaflet provides the most important information about Prochieve®. If you want to read more, ask your doctor or pharmacist about the professional leaflet. You may need their help to understand some of the information.
How Supplied
Prochieve® is available in two strengths: 4% gel (45 mg of progesterone) and 8% gel (90 mg of progesterone).
Each box of the 4% gel contains six single use, disposable vaginal applicators with a twist-off tab. Each box of the 8% gel contains either six or eighteen single use, disposable vaginal applicators with a twistoff tab. Each applicator is wrapped and sealed in a foil overwrap.
Prochieve® should be stored at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Do not use Prochieve® after the expiration date which is printed on the box.
Manufactured for: Columbia Laboratories, Inc., Livingston, NJ 07039
Manufactured by: Fleet Laboratories Ltd., Watford, United Kingdom
40905010004 Revised November 2004
Subscribe to the "News" RSS Feed 
TOP ۞
