-
Prograf Capsules and Injection (Astellas)
WARNING
Increased susceptibility to infection and the possible development of lymphoma may result from immunosuppression. Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe Prograf. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient.
DESCRIPTION
Prograf is available for oral administration as capsules (tacrolimus capsules) containing the equivalent of 0.5 mg, 1 mg or 5 mg of anhydrous tacrolimus. Inactive ingredients include lactose, hydroxypropyl methylcellulose, croscarmellose sodium, and magnesium stearate. The 0.5 mg capsule shell contains gelatin, titanium dioxide and ferric oxide, the 1 mg capsule shell contains gelatin and titanium dioxide, and the 5 mg capsule shell contains gelatin, titanium dioxide and ferric oxide.
Prograf is also available as a sterile solution (tacrolimus injection) containing the equivalent of 5 mg anhydrous tacrolimus in 1 mL for administration by intravenous infusion only. Each mL contains polyoxyl 60 hydrogenated castor oil (HCO-60), 200 mg, and dehydrated alcohol, USP, 80.0% v/v. Prograf injection must be diluted with 0.9% Sodium Chloride Injection or 5% Dextrose Injection before use.
Tacrolimus, previously known as FK506, is the active ingredient in Prograf. Tacrolimus is a macrolide immunosuppressant produced by Streptomyces tsukubaensis . Chemically, tacrolimus is designated as [3 S -[3 R* [ E (1 S* ,3 S *,4 S* )],4 S* ,5 R* ,8 S* ,9 E ,12 R* ,14 R* ,15 S* ,16 R* ,18 S* ,19 S* ,26a R* ]]-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-15,19-epoxy-3H-pyrido [2,1- c ][1,4] oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, monohydrate.
The chemical structure of tacrolimus is:
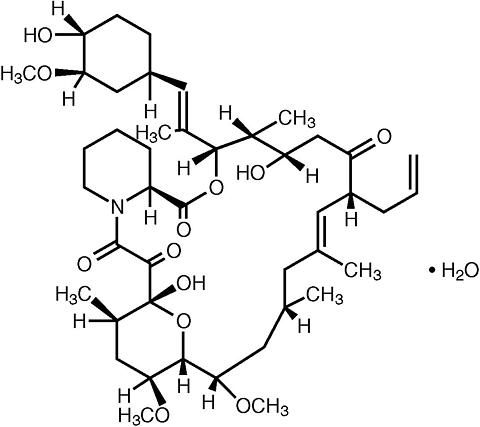
Tacrolimus has an empirical formula of C 44 H 69 NO 12 ·H 2 O and a formula weight of 822.03. Tacrolimus appears as white crystals or crystalline powder. It is practically insoluble in water, freely soluble in ethanol, and very soluble in methanol and chloroform.
CLINICAL PHARMACOLOGY
Mechanism of Action
Tacrolimus prolongs the survival of the host and transplanted graft in animal transplant models of liver, kidney, heart, bone marrow, small bowel and pancreas, lung and trachea, skin, cornea, and limb.
In animals, tacrolimus has been demonstrated to suppress some humoral immunity and, to a greater extent, cell-mediated reactions such as allograft rejection, delayed type hypersensitivity, collagen-induced arthritis, experimental allergic encephalomyelitis, and graft versus host disease.
Tacrolimus inhibits T-lymphocyte activation, although the exact mechanism of action is not known. Experimental evidence suggests that tacrolimus binds to an intracellular protein, FKBP-12. A complex of tacrolimus-FKBP-12, calcium, calmodulin, and calcineurin is then formed and the phosphatase activity of calcineurin inhibited. This effect may prevent the dephosphorylation and translocation of nuclear factor of activated T-cells (NF-AT), a nuclear component thought to initiate gene transcription for the formation of lymphokines (such as interleukin-2, gamma interferon). The net result is the inhibition of T-lymphocyte activation (i.e., immunosuppression).
Pharmacokinetics
Tacrolimus activity is primarily due to the parent drug. The pharmacokinetic parameters (means ± S.D.) of tacrolimus have been determined following intravenous (IV) and oral (PO) administration in healthy volunteers, kidney transplant and liver transplant patients. (See table below.)
Population NRoute
(Dose)Parameters C max
(ng/mL)T max
(hr)AUC
(ng·hr/mL)t 1/2
(hr)Cl
(L/hr/kg)V
(L/kg)Healthy
Volunteers8IV
(0.025 mg/kg/4hr)-- -- 598 *
± 12534.2
± 7.70.040
± 0.0091.91
± 0.3116PO
(5 mg)29.7
± 7.21.6
± 0.7243 **
± 7334.8
± 11.40.041 **/*
± 0.0081.94 **/*
± 0.53Kidney
Transplant
Pts26IV
(0.02 mg/kg/12hr)-- -- 294 ***
± 26218.8
± 16.70.083
± 0.0501.41
± 0.66PO
(0.2 mg/kg/day)19.2
± 10.33.0 203 ***
± 42# # # PO
(0.3 mg/kg/day)24.2
± 15.81.5 288 ***
± 93# # # Liver
Transplant
Pts17IV
(0.05 mg/kg/12 hr)-- -- 3300 ***
± 213011.7
± 3.90.053
± 0.0170.85
± 0.30PO
(0.3 mg/kg/day)68.5
± 30.02.3
± 1.5519 ***
± 179# # # **/* Corrected for individual bioavailability *AUC 0-120 **AUC 0-72 ***AUC 0-inf --not applicable #not available Due to intersubject variability in tacrolimus pharmacokinetics, individualization of dosing regimen is necessary for optimal therapy. (See DOSAGE AND ADMINISTRATION ) . Pharmacokinetic data indicate that whole blood concentrations rather than plasma concentrations serve as the more appropriate sampling compartment to describe tacrolimus pharmacokinetics.
Absorption
Absorption of tacrolimus from the gastrointestinal tract after oral administration is incomplete and variable. The absolute bioavailability of tacrolimus was 17 ± 10% in adult kidney transplant patients (N=26), 22 ± 6% in adult liver transplant patients (N=17), and 18 ± 5% in healthy volunteers (N=16).
A single dose study conducted in 32 healthy volunteers established the bioequivalence of the 1 mg and 5 mg capsules. Another single dose study in 32 healthy volunteers established the bioequivalence of the 0.5 mg and 1 mg capsules. Tacrolimus maximum blood concentrations (C max ) and area under the curve (AUC) appeared to increase in a dose-proportional fashion in 18 fasted healthy volunteers receiving a single oral dose of 3, 7 and 10 mg.
In 18 kidney transplant patients, tacrolimus trough concentrations from 3 to 30 ng/mL measured at 10-12 hours post-dose (C min ) correlated well with the AUC (correlation coefficient 0.93). In 24 liver transplant patients over a concentration range of 10 to 60 ng/mL, the correlation coefficient was 0.94.
Food Effects: The rate and extent of tacrolimus absorption were greatest under fasted conditions. The presence and composition of food decreased both the rate and extent of tacrolimus absorption when administered to 15 healthy volunteers.
The effect was most pronounced with a high-fat meal (848 kcal, 46% fat): mean AUC and C max were decreased 37% and 77%, respectively; T max was lengthened 5-fold. A high-carbohydrate meal (668 kcal, 85% carbohydrate) decreased mean AUC and mean C max by 28% and 65%, respectively.
In healthy volunteers (N=16), the time of the meal also affected tacrolimus bioavailability. When given immediately following the meal, mean C max was reduced 71%, and mean AUC was reduced 39%, relative to the fasted condition. When administered 1.5 hours following the meal, mean C max was reduced 63%, and mean AUC was reduced 39%, relative to the fasted condition.
In 11 liver transplant patients, Prograf administered 15 minutes after a high fat (400 kcal, 34% fat) breakfast, resulted in decreased AUC (27 ± 18%) and C max (50 ± 19%), as compared to a fasted state.
Distribution
The plasma protein binding of tacrolimus is approximately 99% and is independent of concentration over a range of 5-50 ng/mL. Tacrolimus is bound mainly to albumin and alpha-1-acid glycoprotein, and has a high level of association with erythrocytes. The distribution of tacrolimus between whole blood and plasma depends on several factors, such as hematocrit, temperature at the time of plasma separation, drug concentration, and plasma protein concentration. In a U.S. study, the ratio of whole blood concentration to plasma concentration averaged 35 (range 12 to 67).
Metabolism
Tacrolimus is extensively metabolized by the mixed-function oxidase system, primarily the cytochrome P-450 system (CYP3A). A metabolic pathway leading to the formation of 8 possible metabolites has been proposed. Demethylation and hydroxylation were identified as the primary mechanisms of biotransformation in vitro. The major metabolite identified in incubations with human liver microsomes is 13-demethyl tacrolimus. In in vitro studies, a 31-demethyl metabolite has been reported to have the same activity as tacrolimus.
Excretion
The mean clearance following IV administration of tacrolimus is 0.040, 0.083 and 0.053 L/hr/kg in healthy volunteers, adult kidney transplant patients and adult liver transplant patients, respectively. In man, less than 1% of the dose administered is excreted unchanged in urine.
In a mass balance study of IV administered radiolabeled tacrolimus to 6 healthy volunteers, the mean recovery of radiolabel was 77.8 ± 12.7%. Fecal elimination accounted for 92.4 ± 1.0% and the elimination half-life based on radioactivity was 48.1 ± 15.9 hours whereas it was 43.5 ± 11.6 hours based on tacrolimus concentrations. The mean clearance of radiolabel was 0.029 ± 0.015 L/hr/kg and clearance of tacrolimus was 0.029 ± 0.009 L/hr/kg.
When administered PO, the mean recovery of the radiolabel was 94.9 ± 30.7%. Fecal elimination accounted for 92.6 ± 30.7%, urinary elimination accounted for 2.3 ± 1.1% and the elimination half-life based on radioactivity was 31.9 ± 10.5 hours whereas it was 48.4 ± 12.3 hours based on tacrolimus concentrations. The mean clearance of radiolabel was 0.226 ± 0.116 L/hr/kg and clearance of tacrolimus 0.172 ± 0.088 L/hr/kg.
Special Populations
Pediatric
Pharmacokinetics of tacrolimus have been studied in liver transplantation patients, 0.7 to 13.2 years of age. Following IV administration of a 0.037 mg/kg/day dose to 12 pediatric patients, mean terminal half-life, volume of distribution and clearance were 11.5 ± 3.8 hours, 2.6 ± 2.1 L/kg and 0.138 ± 0.071 L/hr/kg, respectively. Following oral administration to 9 patients, mean AUC and C max were 337 ± 167 ng·hr/mL and 43.4 ± 27.9 ng/mL, respectively. The absolute bioavailability was 31 ± 21%.
Whole blood trough concentrations from 31 patients less than 12 years old showed that pediatric patients needed higher doses than adults to achieve similar tacrolimus trough concentrations. (See DOSAGE AND ADMINISTRATION ).
Renal and Hepatic Insufficiency
The mean pharmacokinetic parameters for tacrolimus following single administrations to patients with renal and hepatic impairment are given in the following table.
Population
(No. of Patients)Dose AUC 0-t
(ng·hr/mL)t 1/2
(hr)V
(L/kg)Cl
(L/hr/kg)Renal Impairment(n=12) 0.02
mg/kg/4hr
IV
393±123
(t=60 hr)
26.3±9.2 1.07
±0.20
0.038
±0.014
Mild Hepatic Impairment(n=6) 0.02
mg/kg/4hr
IV
7.7 mg
PO
367±107
(t=72 hr)
488±320
(t=72 hr)
60.6±43.8
Range:
27.8-141
66.1±44.8
Range: 29.5-138
3.1
±1.6
3.7
±4.7 *
0.042
±0.02
0.034
±0.019 *
Severe Hepatic Impairment
(n=6, IV)
(n=5, PO) **/*
0.02
mg/kg/4hr
IV (n=2)
0.01
mg/kg/8hr
IV (n=4)
8 mg PO
(n=1)
5 mg PO
(n=4)
4 mg PO
(n=1)
762±204
(t=120 hr)
289±117
(t=144 hr)
658
(t=120 hr)
533±156
(t=144 hr)
198±158
Range: 81-436
119±35
Range: 85-178
3.9
±1.0
3.1
±3.4 *
0.017
±0.013
0.016
±0.011 *
*corrected for bioavailability **/* 1 patient did not receive the PO dose Renal Insufficiency:
Tacrolimus pharmacokinetics following a single IV administration were determined in 12 patients (7 not on dialysis and 5 on dialysis, serum creatinine of 3.9 ± 1.6 and 12.0 ± 2.4 mg/dL, respectively) prior to their kidney transplant. The pharmacokinetic parameters obtained were similar for both groups.
The mean clearance of tacrolimus in patients with renal dysfunction was similar to that in normal volunteers (see previous table).
Hepatic Insufficiency:
Tacrolimus pharmacokinetics have been determined in six patients with mild hepatic dysfunction (mean Pugh score: 6.2) following single IV and oral administrations. The mean clearance of tacrolimus in patients with mild hepatic dysfunction was not substantially different from that in normal volunteers (see previous table). Tacrolimus pharmacokinetics were studied in 6 patients with severe hepatic dysfunction (mean Pugh score: >10) The mean clearance was substantially lower in patients with severe hepatic dysfunction, irrespective of the route of administration.
Race
A formal study to evaluate the pharmacokinetic disposition of tacrolimus in Black transplant patients has not been conducted. However, a retrospective comparison of Black and Caucasian kidney transplant patients indicated that Black patients required higher tacrolimus doses to attain similar trough concentrations. (See DOSAGE AND ADMINISTRATION ).
Gender
A formal study to evaluate the effect of gender on tacrolimus pharmacokinetics has not been conducted, however, there was no difference in dosing by gender in the kidney transplant trial. A retrospective comparison of pharmacokinetics in healthy volunteers, and in kidney and liver transplant patients indicated no gender-based differences.
Clinical Studies
Liver Transplantation
The safety and efficacy of Prograf-based immunosuppression following orthotopic liver transplantation were assessed in two prospective, randomized, non-blinded multicenter studies. The active control groups were treated with a cyclosporine-based immunosuppressive regimen. Both studies used concomitant adrenal corticosteroids as part of the immunosuppressive regimens. These studies were designed to evaluate whether the two regimens were therapeutically equivalent, with patient and graft survival at 12 months following transplantation as the primary endpoints. The Prograf-based immunosuppressive regimen was found to be equivalent to the cyclosporine-based immunosuppressive regimens.
In one trial, 529 patients were enrolled at 12 clinical sites in the United States; prior to surgery, 263 were randomized to the Prograf-based immunosuppressive regimen and 266 to a cyclosporine-based immunosuppressive regimen (CBIR). In 10 of the 12 sites, the same CBIR protocol was used, while 2 sites used different control protocols. This trial excluded patients with renal dysfunction, fulminant hepatic failure with Stage IV encephalopathy, and cancers; pediatric patients (</= 12 years old) were allowed.
In the second trial, 545 patients were enrolled at 8 clinical sites in Europe; prior to surgery, 270 were randomized to the Prograf-based immunosuppressive regimen and 275 to CBIR. In this study, each center used its local standard CBIR protocol in the active-control arm. This trial excluded pediatric patients, but did allow enrollment of subjects with renal dysfunction, fulminant hepatic failure in Stage IV encephalopathy, and cancers other than primary hepatic with metastases.
One-year patient survival and graft survival in the Prograf-based treatment groups were equivalent to those in the CBIR treatment groups in both studies. The overall one-year patient survival (CBIR and Prograf-based treatment groups combined) was 88% in the U.S. study and 78% in the European study. The overall one-year graft survival (CBIR and Prograf-based treatment groups combined) was 81% in the U.S. study and 73% in the European study. In both studies, the median time to convert from IV to oral Prograf dosing was 2 days.
Because of the nature of the study design, comparisons of differences in secondary endpoints, such as incidence of acute rejection, refractory rejection or use of OKT3 for steroid-resistant rejection, could not be reliably made.
Kidney Transplantation
Prograf-based immunosuppression following kidney transplantation was assessed in a Phase III randomized, multicenter, non-blinded, prospective study. There were 412 kidney transplant patients enrolled at 19 clinical sites in the United States. Study therapy was initiated when renal function was stable as indicated by a serum creatinine </= 4 mg/dL (median of 4 days after transplantation, range 1 to 14 days). Patients less than 6 years of age were excluded.
There were 205 patients randomized to Prograf-based immunosuppression and 207 patients were randomized to cyclosporine-based immunosuppression. All patients received prophylactic induction therapy consisting of an antilymphocyte antibody preparation, corticosteroids and azathioprine. Overall one year patient and graft survival was 96.1% and 89.6%, respectively and was equivalent between treatment arms.
Because of the nature of the study design, comparisons of differences in secondary endpoints, such as incidence of acute rejection, refractory rejection or use of OKT3 for steroid-resistant rejection, could not be reliably made.
INDICATIONS AND USAGE
Prograf is indicated for the prophylaxis of organ rejection in patients receiving allogeneic liver or kidney transplants. It is recommended that Prograf be used concomitantly with adrenal corticosteroids. Because of the risk of anaphylaxis, Prograf injection should be reserved for patients unable to take Prograf capsules orally.
CONTRAINDICATIONS
Prograf is contraindicated in patients with a hypersensitivity to tacrolimus. Prograf injection is contraindicated in patients with a hypersensitivity to HCO-60 (polyoxyl 60 hydrogenated castor oil).
WARNINGS
(See boxed WARNING .)
Insulin-dependent post-transplant diabetes mellitus (PTDM) was reported in 20% of Prograf-treated kidney transplant patients without pretransplant history of diabetes mellitus in the Phase III study (See Tables Below). The median time to onset of PTDM was 68 days. Insulin dependence was reversible in 15% of these PTDM patients at one year and in 50% at two years post transplant. Black and Hispanic kidney transplant patients were at an increased risk of development of PTDM.
Incidence of Post Transplant Diabetes Mellitus
and Insulin Use at 2 Years in Kidney Transplant Recipients in the Phase III studyStatus of PTDM *Prograf CBIR Patients without pretransplant history of diabetes mellitus151 151 New onset PTDM * , 1st Year30/151 (20%) 6/151 (4%) Still insulin dependent at one year in those without prior history of diabetes.25/151 (17%) 5/151 (3%) New onset PTDM * post
1 year1 0 Patients with PTDM * at 2 years16/151 (11%) 5/151 (3%) *use of insulin for 30 or more consecutive days, with < 5 day gap, without a prior history of insulin dependent diabetes mellitus or non insulin dependent diabetes mellitus.Development of Post Transplant Diabetes Mellitus by Race and
by Treatment Group during First Year Post Kidney Transplantation in the Phase III studyPatient RacePrograf CBIR No. of Patients
at RiskPatients Who
Developed PTDM *No. of Patients
at RiskPatients Who
Developed PTDM *Black41 15 (37%) 36 3 (8%) Hispanic17 5 (29%) 18 1 (6%) Caucasian82 10 (12%) 87 1 (1%) Other11 0 (0%) 10 1 (10%) Total151 30 (20%) 151 6 (4%) *use of insulin for 30 or more consecutive days, with < 5 day gap, without a prior history of insulin dependent diabetes mellitus or non insulin dependent diabetes mellitus.Insulin-dependent post-transplant diabetes mellitus was reported in 18% and 11% of Prograf-treated liver transplant patients and was reversible in 45% and 31% of these patients at one year post transplant, in the U.S. and European randomized studies, respectively (See Table below). Hyperglycemia was associated with the use of Prograf in 47% and 33% of liver transplant recipients in the U.S. and European randomized studies, respectively, and may require treatment (see ADVERSE REACTIONS ).
Incidence of Post Transplant Diabetes Mellitus and Insulin Use at One Year in Liver Transplant Recipients Status of PTDM *US Study European Study Prograf CBIR Prograf CBIR Patients at risk **239 236 239 249 New Onset PTDM *42 (18%) 30 (13%) 26 (11%) 12 (5%) Patients still on insulin at 1 year23 (10%) 19 (8%) 18 (8%) 6 (2%) *use of insulin for 30 or more consecutive days, with < 5 day gap, without a prior history of insulin dependent diabetes mellitus or non insulin dependent diabetes mellitus.**Patients without pretransplant history of diabetes mellitus.Prograf can cause neurotoxicity and nephrotoxicity, particularly when used in high doses. Nephrotoxicity was reported in approximately 52% of kidney transplantation patients and in 40% and 36% of liver transplantation patients receiving Prograf in the U.S. and European randomized trials, respectively (see ADVERSE REACTIONS ). More overt nephrotoxicity is seen early after transplantation, characterized by increasing serum creatinine and a decrease in urine output. Patients with impaired renal function should be monitored closely as the dosage of Prograf may need to be reduced. In patients with persistent elevations of serum creatinine who are unresponsive to dosage adjustments, consideration should be given to changing to another immunosuppressive therapy. Care should be taken in using tacrolimus with other nephrotoxic drugs. In particular, to avoid excess nephrotoxicity, Prograf should not be used simultaneously with cyclosporine. Prograf or cyclosporine should be discontinued at least 24 hours prior to initiating the other. In the presence of elevated Prograf or cyclosporine concentrations, dosing with the other drug usually should be further delayed.
Mild to severe hyperkalemia was reported in 31% of kidney transplant recipients and in 45% and 13% of liver transplant recipients treated with Prograf in the U.S. and European randomized trials, respectively, and may require treatment (see ADVERSE REACTIONS ). Serum potassium levels should be monitored and potassium-sparing diuretics should not be used during Prograf therapy (see PRECAUTIONS ).
Neurotoxicity, including tremor, headache, and other changes in motor function, mental status, and sensory function were reported in approximately 55% of liver transplant recipients in the two randomized studies. Tremor occurred more often in Prograf-treated kidney transplant patients (54%) compared to cyclosporine-treated patients. The incidence of other neurological events in kidney transplant patients was similar in the two treatment groups (see ADVERSE REACTIONS ). Tremor and headache have been associated with high whole-blood concentrations of tacrolimus and may respond to dosage adjustment. Seizures have occurred in adult and pediatric patients receiving Prograf (see ADVERSE REACTIONS ). Coma and delirium also have been associated with high plasma concentrations of tacrolimus.
As in patients receiving other immunosuppressants, patients receiving Prograf are at increased risk of developing lymphomas and other malignancies, particularly of the skin. The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent. A lymphoproliferative disorder (LPD) related to Epstein-Barr Virus (EBV) infection has been reported in immunosuppressed organ transplant recipients. The risk of LPD appears greatest in young children who are at risk for primary EBV infection while immunosuppressed or who are switched to Prograf following long-term immunosuppression therapy. Because of the danger of oversuppression of the immune system which can increase susceptibility to infection, combination immunosuppressant therapy should be used with caution.
A few patients receiving Prograf injection have experienced anaphylactic reactions. Although the exact cause of these reactions is not known, other drugs with castor oil derivatives in the formulation have been associated with anaphylaxis in a small percentage of patients. Because of this potential risk of anaphylaxis, Prograf injection should be reserved for patients who are unable to take Prograf capsules.
Patients receiving Prograf injection should be under continuous observation for at least the first 30 minutes following the start of the infusion and at frequent intervals thereafter. If signs or symptoms of anaphylaxis occur, the infusion should be stopped. An aqueous solution of epinephrine should be available at the bedside as well as a source of oxygen.
PRECAUTIONS
General
Hypertension is a common adverse effect of Prograf therapy (see ADVERSE REACTIONS ). Mild or moderate hypertension is more frequently reported than severe hypertension. Antihypertensive therapy may be required; the control of blood pressure can be accomplished with any of the common antihypertensive agents. Since tacrolimus may cause hyperkalemia, potassium-sparing diuretics should be avoided. While calcium-channel blocking agents can be effective in treating Prograf-associated hypertension, care should be taken since interference with tacrolimus metabolism may require a dosage reduction (see Drug Interactions ).
Renally and Hepatically Impaired Patients
For patients with renal insufficiency some evidence suggests that lower doses should be used (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION ).
The use of Prograf in liver transplant recipients experiencing post-transplant hepatic impairment may be associated with increased risk of developing renal insufficiency related to high whole-blood levels of tacrolimus. These patients should be monitored closely and dosage adjustments should be considered. Some evidence suggests that lower doses should be used in these patients (see DOSAGE AND ADMINISTRATION ).
Myocardial Hypertrophy
Myocardial hypertrophy has been reported in association with the administration of Prograf, and is generally manifested by echocardiographically demonstrated concentric increases in left ventricular posterior wall and interventricular septum thickness. Hypertrophy has been observed in infants, children and adults. This condition appears reversible in most cases following dose reduction or discontinuance of therapy. In a group of 20 patients with pre- and post-treatment echocardiograms who showed evidence of myocardial hypertrophy, mean tacrolimus whole blood concentrations during the period prior to diagnosis of myocardial hypertrophy ranged from 11 to 53 ng/mL in infants (N=10, age 0.4 to 2 years), 4 to 46 ng/mL in children (N=7, age 2 to 15 years) and 11 to 24 ng/mL in adults (N=3, age 37 to 53 years).
In patients who develop renal failure or clinical manifestations of ventricular dysfunction while receiving Prograf therapy, echocardiographic evaluation should be considered. If myocardial hypertrophy is diagnosed, dosage reduction or discontinuation of Prograf should be considered.
Information for Patients
Patients should be informed of the need for repeated appropriate laboratory tests while they are receiving Prograf. They should be given complete dosage instructions, advised of the potential risks during pregnancy, and informed of the increased risk of neoplasia. Patients should be informed that changes in dosage should not be undertaken without first consulting their physician.
Patients should be informed that Prograf can cause diabetes mellitus and should be advised of the need to see their physician if they develop frequent urination, increased thirst or hunger. As with other immunosuppressive agents, owing to the potential risk of malignant skin changes, exposure to sunlight and ultraviolet (UV) light should be limited by wearing protective clothing and using a sunscreen with a high protection factor.
Laboratory Tests
Serum creatinine, potassium, and fasting glucose should be assessed regularly. Routine monitoring of metabolic and hematologic systems should be performed as clinically warranted.
Drug Interactions
Due to the potential for additive or synergistic impairment of renal function, care should be taken when administering Prograf with drugs that may be associated with renal dysfunction. These include, but are not limited to, aminoglycosides, amphotericin B, and cisplatin. Initial clinical experience with the co-administration of Prograf and cyclosporine resulted in additive/synergistic nephrotoxicity. Patients switched from cyclosporine to Prograf should receive the first Prograf dose no sooner than 24 hours after the last cyclosporine dose. Dosing may be further delayed in the presence of elevated cyclosporine levels.
Drugs That May Alter Tacrolimus Concentrations
Since tacrolimus is metabolized mainly by the CYP3A enzyme systems, substances known to inhibit these enzymes may decrease the metabolism or increase bioavailability of tacrolimus as indicated by increased whole blood or plasma concentrations. Drugs known to induce these enzyme systems may result in an increased metabolism of tacrolimus or decreased bioavailability as indicated by decreased whole blood or plasma concentrations. Monitoring of blood concentrations and appropriate dosage adjustments are essential when such drugs are used concomitantly.
*Drugs That May Increase Tacrolimus Blood Concentrations:Calcium
Channel
Blockers
Antifungal
Agents
Macrolide
Antibiotics
Gastrointestinal
Prokinetic
Agents
Other
Drugs
clotrimazoleclarithromycinbromocriptinediltiazemfluconazoleerythromycincisapridechloramphenicolnicardipineitraconazoletroleandomycinmetoclopramidecimetidinenifedipineketoconazolecyclosporineverapamilvoriconazoledanazolethinyl estradiolmethylprednisoloneomeprazoleprotease inhibitorsnefazodonemagnesium-aluminum-hydroxideIn a study of 6 normal volunteers, a significant increase in tacrolimus oral bioavailability (14±5% vs. 30±8%) was observed with concomitant ketoconazole administration (200 mg). The apparent oral clearance of tacrolimus during ketoconazole administration was significantly decreased compared to tacrolimus alone (0.430±0.129 L/hr/kg vs. 0.148±0.043 L/hr/kg). Overall, IV clearance of tacrolimus was not significantly changed by ketoconazole co-administration, although it was highly variable between patients.
* Drugs That May Decrease Tacrolimus Blood Concentrations:AnticonvulsantsAntimicrobialsHerbalPreparationsOther DrugscarbamazepinerifabutinsirolimusphenobarbitalcaspofunginSt. John's Wortphenytoinrifampin*This table is not all inclusive.St. John's Wort ( Hypericum perforatum ) induces CYP3A4 and P-glycoprotein. Since tacrolimus is a substrate for CYP3A4, there is the potential that the use of St. John's Wort in patients receiving Prograf could result in reduced tacrolimus levels.
In a single-dose crossover study in healthy volunteers, co-administration of tacrolimus and magnesium-aluminum-hydroxide resulted in a 21% increase in the mean tacrolimus AUC and a 10% decrease in the mean tacrolimus C max relative to tacrolimus administration alone.
In a study of 6 normal volunteers, a significant decrease in tacrolimus oral bioavailability (14±6% vs. 7±3%) was observed with concomitant rifampin administration (600 mg). In addition, there was a significant increase in tacrolimus clearance (0.036±0.008 L/hr/kg vs. 0.053±0.010 L/hr/kg) with concomitant rifampin administration.
Interaction studies with drugs used in HIV therapy have not been conducted. However, care should be exercised when drugs that are nephrotoxic (e.g., ganciclovir) or that are metabolized by CYP3A (e.g., nelfinavir, ritonavir) are administered concomitantly with tacrolimus. Based on a clinical study of 5 liver transplant recipients co-administration of tacrolimus with nelfinavir increased blood concentrations of tacrolimus significantly and, as a result, a reduction in the tacrolimus dose by an average of 16-fold was needed to maintain mean trough tacrolimus blood concentrations of 9.7 ng/mL. Thus frequent monitoring of tacrolimus blood concentrations and appropriate dosage adjustments are essential when nelfinavir is used concomitantly. Tacrolimus may affect the pharmacokinetics of other drugs (e.g., phenytoin) and increase their concentration. Grapefruit juice affects CYP3A-mediated metabolism and should be avoided (see DOSAGE AND ADMINISTRATION ).
Following co-administration of tacrolimus and sirolimus (2 or 5 mg/day) in stable renal transplant patients, mean tacrolimus AUC 0-12 and C min decreased approximately by 30% relative to tacrolimus alone. Mean tacrolimus AUC 0-12 and C min following co-administration of 1 mg/day of sirolimus decreased approximately 3% and 11% respectively. The safety and efficacy of tacrolimus used in combination with sirolimus for the prevention of graft rejection has not been established and is not recommended.
Other Drug Interactions
Immunosuppressants may affect vaccination. Therefore, during treatment with Prograf, vaccination may be less effective. The use of live vaccines should be avoided; live vaccines may include, but are not limited to measles, mumps, rubella, oral polio, BCG, yellow fever, and TY 21a typhoid. 1
Carcinogenesis, Mutagenesis and Impairment of Fertility
An increased incidence of malignancy is a recognized complication of immunosuppression in recipients of organ transplants. The most common forms of neoplasms are non-Hodgkin's lymphomas and carcinomas of the skin. As with other immunosuppressive therapies, the risk of malignancies in Prograf recipients may be higher than in the normal, healthy population. Lymphoproliferative disorders associated with Epstein-Barr Virus infection have been seen. It has been reported that reduction or discontinuation of immunosuppression may cause the lesions to regress.
No evidence of genotoxicity was seen in bacterial ( Salmonella and E. coli ) or mammalian (Chinese hamster lung-derived cells) in vitro assays of mutagenicity, the in vitro CHO/HGPRT assay of mutagenicity, or in vivo clastogenicity assays performed in mice; tacrolimus did not cause unscheduled DNA synthesis in rodent hepatocytes.
Carcinogenicity studies were carried out in male and female rats and mice. In the 80-week mouse study and in the 104-week rat study no relationship of tumor incidence to tacrolimus dosage was found. The highest doses used in the mouse and rat studies were 0.8-2.5 times (mice) and 3.5-7.1 times (rats) the recommended clinical dose range of 0.1-0.2 mg/kg/day when corrected for body surface area.
No impairment of fertility was demonstrated in studies of male and female rats. Tacrolimus, given orally at 1.0 mg/kg (0.7-1.4 × the recommended clinical dose range of 0.1-0.2 mg/kg/day based on body surface area corrections) to male and female rats, prior to and during mating, as well as to dams during gestation and lactation, was associated with embryolethality and with adverse effects on female reproduction. Effects on female reproductive function (parturition) and embryolethal effects were indicated by a higher rate of pre-implantation loss and increased numbers of undelivered and nonviable pups. When given at 3.2 mg/kg (2.3-4.6 × the recommended clinical dose range based on body surface area correction), tacrolimus was associated with maternal and paternal toxicity as well as reproductive toxicity including marked adverse effects on estrus cycles, parturition, pup viability, and pup malformations.
Pregnancy: Category C
In reproduction studies in rats and rabbits, adverse effects on the fetus were observed mainly at dose levels that were toxic to dams. Tacrolimus at oral doses of 0.32 and 1.0 mg/kg during organogenesis in rabbits was associated with maternal toxicity as well as an increase in incidence of abortions; these doses are equivalent to 0.5-1 × and 1.6-3.3 × the recommended clinical dose range (0.1-0.2 mg/kg) based on body surface area corrections. At the higher dose only, an increased incidence of malformations and developmental variations was also seen. Tacrolimus, at oral doses of 3.2 mg/kg during organogenesis in rats, was associated with maternal toxicity and caused an increase in late resorptions, decreased numbers of live births, and decreased pup weight and viability. Tacrolimus, given orally at 1.0 and 3.2 mg/kg (equivalent to 0.7-1.4 × and 2.3-4.6 × the recommended clinical dose range based on body surface area corrections) to pregnant rats after organogenesis and during lactation, was associated with reduced pup weights.
No reduction in male or female fertility was evident.
There are no adequate and well-controlled studies in pregnant women. Tacrolimus is transferred across the placenta. The use of tacrolimus during pregnancy has been associated with neonatal hyperkalemia and renal dysfunction. Prograf should be used during pregnancy only if the potential benefit to the mother justifies potential risk to the fetus.
Nursing Mothers
Since tacrolimus is excreted in human milk, nursing should be avoided.
Pediatric Patients
Experience with Prograf in pediatric kidney transplant patients is limited. Successful liver transplants have been performed in pediatric patients (ages up to 16 years) using Prograf. Two randomized active-controlled trials of Prograf in primary liver transplantation included 56 pediatric patients. Thirty-one patients were randomized to Prograf-based and 25 to cyclosporine-based therapies. Additionally, a minimum of 122 pediatric patients were studied in an uncontrolled trial of tacrolimus in living related donor liver transplantation. Pediatric patients generally required higher doses of Prograf to maintain blood trough concentrations of tacrolimus similar to adult patients (see DOSAGE AND ADMINISTRATION ).
ADVERSE REACTIONS
Liver Transplantation
The principal adverse reactions of Prograf are tremor, headache, diarrhea, hypertension, nausea, and renal dysfunction. These occur with oral and IV administration of Prograf and may respond to a reduction in dosing. Diarrhea was sometimes associated with other gastrointestinal complaints such as nausea and vomiting.
Hyperkalemia and hypomagnesemia have occurred in patients receiving Prograf therapy. Hyperglycemia has been noted in many patients; some may require insulin therapy (see WARNINGS ).
The incidence of adverse events was determined in two randomized comparative liver transplant trials among 514 patients receiving tacrolimus and steroids and 515 patients receiving a cyclosporine-based regimen (CBIR). The proportion of patients reporting more than one adverse event was 99.8% in the tacrolimus group and 99.6% in the CBIR group. Precautions must be taken when comparing the incidence of adverse events in the U.S. study to that in the European study. The 12-month posttransplant information from the U.S. study and from the European study is presented below. The two studies also included different patient populations and patients were treated with immunosuppressive regimens of differing intensities. Adverse events reported in >/= 15% in tacrolimus patients (combined study results) are presented below for the two controlled trials in liver transplantation:
LIVER TRANSPLANTATION: ADVERSE EVENTS OCCURRING IN >/= 15% OF PROGRAF-TREATED PATIENTS EUROPEAN EUROPEAN U.S. STUDY (%) STUDY (%) U.S. STUDY (%) STUDY (%) Prograf
(N=250)CBIR
(N=250)Prograf
(N=264)CBIR
(N=265)Prograf
(N=250)CBIR
(N=250)Prograf
(N=264)CBIR
(N=265)Nervous SystemMetabolic and NutritionalHeadache (see WARNINGS )
64 60 37 26 Hyperkalemia (see WARNINGS )45 26 13 9 Tremor (see WARNINGS )Hypokalemia29 34 13 16 56 46 48 32 Hyperglycemia(see WARNINGS )Insomnia64 68 32 23 47 38 33 22 Paresthesia40 30 17 17 Hypomagnesemia48 45 16 9 GastrointestinalHemic and LymphaticDiarrhea72 47 37 27 Anemia47 38 5 1 Nausea46 37 32 27 Leukocytosis32 26 8 8 Constipation24 27 23 21 Thrombocytopenia24 20 14 19 LFT Abnormal36 30 6 5 MiscellaneousAnorexia34 24 7 5 Abdominal Pain59 54 29 22 Vomiting27 15 14 11 Pain63 57 24 22 CardiovascularHypertension (seePRECAUTIONS )Fever48 56 19 22 Asthenia52 48 11 7 47 56 38 43 Back Pain30 29 17 17 UrogenitalAscites27 22 7 8 Kidney FunctionAbnormal (seeWARNINGS )Peripheral Edema26 26 12 14 Respiratory System40 27 36 23 Pleural Effusion30 32 36 35 CreatinineIncreased (seeWARNINGS )Atelectasis28 30 5 4 Dyspnea29 23 5 4 39 25 24 19 Skin and AppendagesBUN Increased(see WARNINGS )Pruritus36 20 15 7 30 22 12 9 Rash24 19 10 4 Urinary TractInfection16 18 21 19 Oliguria18 15 19 12 Less frequently observed adverse reactions in both liver transplantation and kidney transplantation patients are described under the subsection Less Frequently Reported Adverse Reactions below.
Kidney Transplantation
The most common adverse reactions reported were infection, tremor, hypertension, decreased renal function, constipation, diarrhea, headache, abdominal pain and insomnia.
Adverse events that occurred in >/= 15% of Prograf-treated kidney transplant patients are presented below:
KIDNEY TRANSPLANTATION: ADVERSE EVENTS OCCURRING IN >/= 15% OF PROGRAF-TREATED PATIENTS Nervous System Tremor (See WARNINGS )Prograf
(N=205)CBIR
(N=207)Prograf
(N=205)CBIR
(N=207)Prograf
(N=205)CBIR
(N=207)5434 Urogenital Creatinine increased (See WARNINGS )45 42 Hemic and LymphaticAnemia30 24 Leukopenia15 17 Headache(See WARNINGS )44 38 Urinary Tract Infection34 35 MiscellaneousInfection45 49 Insomnia32 30 Peripheral Edema36 48 Paresthesia23 16 Metabolic and NutritionalAsthenia34 30 Dizziness19 16 Abdominal Pain33 31 GastrointestinalHypophosphatemia49 53 Pain32 30 Diarrhea44 41 Hypomagnesemia34 17 Fever29 29 Nausea38 36 Hyperlipemia31 38 Back Pain24 20 Constipation35 43 Hyperkalemia (SeeWARNINGS )Vomiting29 23 31 32 Respiratory SystemDyspepsia28 20 Diabetes Mellitus (See WARNINGS )Dyspnea22 18 Cardiovascular24 9 Cough increased18 15 Hypertension (See PRECAUTIONS )Hypokalemia22 25 50 52 Hyperglycemia (See WARNINGS )MusculoskeletalChest Pain19 13 22 16 Arthralgia25 24 Edema18 19 SkinRash17 12 Pruritus15 7 Less frequently observed adverse reactions in both liver transplantation and kidney transplantation patients are described under the subsection Less Frequently Reported Adverse Reactions shown below.
Less Frequently Reported Adverse Reactions
The following adverse events were reported in the range of 3% to less than 15% incidence in either liver or kidney transplant recipients who were treated with tacrolimus in the Phase 3 comparative trials.
NERVOUS SYSTEM: (see WARNINGS ) abnormal dreams, agitation, amnesia, anxiety, confusion, convulsion, depression, dizziness, emotional lability, encephalopathy, hallucinations, hypertonia, incoordination, myoclonus, nervousness, neuropathy, psychosis, somnolence, thinking abnormal; SPECIAL SENSES: abnormal vision, amblyopia, ear pain, otitis media, tinnitus; GASTROINTESTINAL: anorexia, cholangitis, cholestatic jaundice, dyspepsia, dysphagia, esophagitis, flatulence, gastritis, gastrointestinal hemorrhage, GGT increase, GI perforation, hepatitis, ileus, increased appetite, jaundice, liver damage, liver function test abnormal, oral moniliasis, rectal disorder, stomatitis; CARDIOVASCULAR: angina pectoris, chest pain, deep thrombophlebitis, abnormal ECG, hemorrhage, hypotension, postural hypotension, peripheral vascular disorder, phlebitis, tachycardia, thrombosis, vasodilatation; UROGENITAL: (see WARNINGS ) albuminuria, cystitis, dysuria, hematuria, hydronephrosis, kidney failure, kidney tubular necrosis, nocturia, pyuria, toxic nephropathy, oliguria, urinary frequency, urinary incontinence, vaginitis; METABOLIC/NUTRITIONAL: acidosis, alkaline phosphatase increased, alkalosis, ALT (SGPT) increased, AST (SGOT) increased, bicarbonate decreased, bilirubinemia, BUN increased, dehydration, GGT increased, healing abnormal, hypercalcemia, hypercholesterolemia, hyperlipemia, hyperphosphatemia, hyperuricemia, hypervolemia, hypocalcemia, hypoglycemia, hyponatremia, hypophosphatemia, hypoproteinemia, lactic dehydrogenase increase, weight gain; ENDOCRINE: (see PRECAUTIONS ) Cushing's syndrome, diabetes mellitus; HEMIC/LYMPHATIC: coagulation disorder, ecchymosis, hypochromic anemia, leukocytosis, leukopenia, polycythemia, prothrombin decreased, serum iron decreased, thrombocytopenia; MISCELLANEOUS: abdomen enlarged, abscess, accidental injury, allergic reaction, cellulitis, chills, flu syndrome, generalized edema, hernia, peritonitis, photosensitivity reaction, sepsis; MUSCULOSKELETAL: arthralgia, cramps, generalized spasm, joint disorder, leg cramps, myalgia, myasthenia, osteoporosis; RESPIRATORY: asthma, bronchitis, cough increased, lung disorder, pneumothorax, pulmonary edema, pharyngitis, pneumonia, respiratory disorder, rhinitis, sinusitis, voice alteration; SKIN: acne, alopecia, exfoliative dermatitis, fungal dermatitis, herpes simplex, hirsutism, skin discoloration, skin disorder, skin ulcer, sweating.
There have been rare spontaneous reports of myocardial hypertrophy associated with clinically manifested ventricular dysfunction in patients receiving Prograf therapy (see PRECAUTIONS -- Myocardial Hypertrophy ) .
Post Marketing
The following have been reported: increased amylase including pancreatitis, hearing loss including deafness, leukoencephalopathy, thrombocytopenic purpura, hemolytic-uremia syndrome, acute renal failure, Stevens-Johnson syndrome, stomach ulcer, glycosuria, cardiac arrhythmia, QT prolongation, Torsades de Pointes and gastroenteritis.
OVERDOSAGE
Limited overdosage experience is available. Acute overdosages of up to 30 times the intended dose have been reported. Almost all cases have been asymptomatic and all patients recovered with no sequelae. Occasionally, acute overdosage has been followed by adverse reactions consistent with those listed in the ADVERSE REACTIONS section except in one case where transient urticaria and lethargy were observed. Based on the poor aqueous solubility and extensive erythrocyte and plasma protein binding, it is anticipated that tacrolimus is not dialyzable to any significant extent; there is no experience with charcoal hemoperfusion. The oral use of activated charcoal has been reported in treating acute overdoses, but experience has not been sufficient to warrant recommending its use. General supportive measures and treatment of specific symptoms should be followed in all cases of overdosage.
In acute oral and IV toxicity studies, mortalities were seen at or above the following doses: in adult rats, 52 × the recommended human oral dose; in immature rats, 16 × the recommended oral dose; and in adult rats, 16 × the recommended human IV dose (all based on body surface area corrections).
DOSAGE AND ADMINISTRATION
Prograf injection (tacrolimus injection)
For IV Infusion Only
NOTE: Anaphylactic reactions have occurred with injectables containing castor oil derivatives. See WARNINGS.
In patients unable to take oral Prograf capsules, therapy may be initiated with Prograf injection. The initial dose of Prograf should be administered no sooner than 6 hours after transplantation. The recommended starting dose of Prograf injection is 0.03-0.05 mg/kg/day as a continuous IV infusion. Adult patients should receive doses at the lower end of the dosing range. Concomitant adrenal corticosteroid therapy is recommended early post-transplantation. Continuous IV infusion of Prograf injection should be continued only until the patient can tolerate oral administration of Prograf capsules.
Preparation for Administration/Stability
Prograf injection must be diluted with 0.9% Sodium Chloride Injection or 5% Dextrose Injection to a concentration between 0.004 mg/mL and 0.02 mg/mL prior to use. Diluted infusion solution should be stored in glass or polyethylene containers and should be discarded after 24 hours. The diluted infusion solution should not be stored in a PVC container due to decreased stability and the potential for extraction of phthalates. In situations where more dilute solutions are utilized (e.g., pediatric dosing, etc.), PVC-free tubing should likewise be used to minimize the potential for significant drug adsorption onto the tubing. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Due to the chemical instability of tacrolimus in alkaline media, Prograf injection should not be mixed or co-infused with solutions of pH 9 or greater (e.g., ganciclovir or acyclovir).
Prograf capsules (tacrolimus capsules)
Summary of Initial Oral Dosage Recommendations and Typical Whole Blood Trough Concentrations Patient PopulationRecommended Initial
Oral Dose *Typical Whole Blood Trough
ConcentrationsAdult kidney transplant patients0.2 mg/kg/day month 1-3 : 7-20 ng/mL
month 4-12 : 5-15 ng/mLAdult liver transplant patients0.10-0.15 mg/kg/day month 1-12 : 5-20 ng/mL Pediatric liver transplant patients0.15-0.20 mg/kg/day month 1-12 : 5-20 ng/mL *Note: two divided doses, q12hLiver Transplantation
It is recommended that patients initiate oral therapy with Prograf capsules if possible. If IV therapy is necessary, conversion from IV to oral Prograf is recommended as soon as oral therapy can be tolerated. This usually occurs within 2-3 days. The initial dose of Prograf should be administered no sooner than 6 hours after transplantation. In a patient receiving an IV infusion, the first dose of oral therapy should be given 8-12 hours after discontinuing the IV infusion. The recommended starting oral dose of Prograf capsules is 0.10-0.15 mg/kg/day administered in two divided daily doses every 12 hours. Co-administered grapefruit juice has been reported to increase tacrolimus blood trough concentrations in liver transplant patients. (See Drugs That May Alter Tacrolimus Concentrations .)
Dosing should be titrated based on clinical assessments of rejection and tolerability. Lower Prograf dosages may be sufficient as maintenance therapy. Adjunct therapy with adrenal corticosteroids is recommended early post transplant.
Dosage and typical tacrolimus whole blood trough concentrations are shown in the table above; blood concentration details are described in Blood Concentration Monitoring: Liver Transplantation below.
Kidney Transplantation
The recommended starting oral dose of Prograf is 0.2 mg/kg/day administered every 12 hours in two divided doses. The initial dose of Prograf may be administered within 24 hours of transplantation, but should be delayed until renal function has recovered (as indicated for example by a serum creatinine </= 4 mg/dL). Black patients may require higher doses to achieve comparable blood concentrations. Dosage and typical tacrolimus whole blood trough concentrations are shown in the table above; blood concentration details are described in Blood Concentration Monitoring: Kidney Transplantation below.
The data in kidney transplant patients indicate that the Black patients required a higher dose to attain comparable trough concentrations compared to Caucasian patients.
Time After
TransplantCaucasian
n = 114Black
n = 56Dose
(mg/kg)Trough
Concentrations
(ng/mL)Dose
(mg/kg)Trough
Concentrations
(ng/mL)Day 70.18 12.0 0.23 10.9 Month 10.17 12.8 0.26 12.9 Month 60.14 11.8 0.24 11.5 Month 120.13 10.1 0.19 11.0 Pediatric Patients
Pediatric liver transplantation patients without pre-existing renal or hepatic dysfunction have required and tolerated higher doses than adults to achieve similar blood concentrations. Therefore, it is recommended that therapy be initiated in pediatric patients at a starting IV dose of 0.03-0.05 mg/kg/day and a starting oral dose of 0.15-0.20 mg/kg/day. Dose adjustments may be required. Experience in pediatric kidney transplantation patients is limited.
Patients with Hepatic or Renal Dysfunction
Due to the reduced clearance and prolonged half-life, patients with severe hepatic impairment (Pugh >/= 10) may require lower doses of Prograf. Close monitoring of blood concentrations is warranted.
Due to the potential for nephrotoxicity, patients with renal or hepatic impairment should receive doses at the lowest value of the recommended IV and oral dosing ranges. Further reductions in dose below these ranges may be required. Prograf therapy usually should be delayed up to 48 hours or longer in patients with post-operative oliguria.
Conversion from One Immunosuppressive Regimen to Another
Prograf should not be used simultaneously with cyclosporine. Prograf or cyclosporine should be discontinued at least 24 hours before initiating the other. In the presence of elevated Prograf or cyclosporine concentrations, dosing with the other drug usually should be further delayed.
Blood Concentration Monitoring
Monitoring of tacrolimus blood concentrations in conjunction with other laboratory and clinical parameters is considered an essential aid to patient management for the evaluation of rejection, toxicity, dose adjustments and compliance. Factors influencing frequency of monitoring include but are not limited to hepatic or renal dysfunction, the addition or discontinuation of potentially interacting drugs and the posttransplant time. Blood concentration monitoring is not a replacement for renal and liver function monitoring and tissue biopsies.
Two methods have been used for the assay of tacrolimus, a microparticle enzyme immunoassay (MEIA) and an ELISA. Both methods have the same monoclonal antibody for tacrolimus. Comparison of the concentrations in published literature to patient concentrations using the current assays must be made with detailed knowledge of the assay methods and biological matrices employed. Whole blood is the matrix of choice and specimens should be collected into tubes containing ethylene diamine tetraacetic acid (EDTA) anti-coagulant. Heparin anti-coagulation is not recommended because of the tendency to form clots on storage. Samples which are not analyzed immediately should be stored at room temperature or in a refrigerator and assayed within 7 days; if samples are to be kept longer they should be deep frozen at -20°C for up to 12 months.
Liver Transplantation
Although there is a lack of direct correlation between tacrolimus concentrations and drug efficacy, data from Phase II and III studies of liver transplant patients have shown an increasing incidence of adverse events with increasing trough blood concentrations. Most patients are stable when trough whole blood concentrations are maintained between 5 to 20 ng/mL. Long term posttransplant patients often are maintained at the low end of this target range.
Data from the U.S. clinical trial show that tacrolimus whole blood concentrations, as measured by ELISA, were most variable during the first week post-transplantation. After this early period, the median trough blood concentrations, measured at intervals from the second week to one year post-transplantation, ranged from 9.8 ng/mL to 19.4 ng/mL.
Therapeutic Drug Monitoring, 1995, Volume 17, Number 6 contains a consensus document and several position papers regarding the therapeutic monitoring of tacrolimus from the 1995 International Consensus Conference on Immunosuppressive Drugs. Refer to these manuscripts for further discussions of tacrolimus monitoring.
Kidney Transplantation
Data from the Phase III study indicates that trough concentrations of tacrolimus in whole blood, as measured by IMx®, were most variable during the first week of dosing. During the first three months, 80% of the patients maintained trough concentrations between 7-20 ng/mL, and then between 5-15 ng/mL, through one-year.
The relative risk of toxicity is increased with higher trough concentrations. Therefore, monitoring of whole blood trough concentrations is recommended to assist in the clinical evaluation of toxicity.
HOW SUPPLIED
Prograf capsules (tacrolimus capsules) 0.5 mg
Oblong, light yellow, branded with red "0.5 mg" on the capsule cap and "
607" on the capsule body, supplied in 100-count bottles (NDC 0469-0607-73).
Prograf capsules (tacrolimus capsules) 1 mg
Oblong, white, branded with red "1 mg" on the capsule cap and "
617" on the capsule body, supplied in 100-count bottles (NDC 0469-0617-73) and 10 blister cards of 10 capsules (NDC 0469-0617-11), containing the equivalent of 1 mg anhydrous tacrolimus.
Prograf capsules (tacrolimus capsules) 5 mg
Oblong, grayish/red, branded with white "5 mg" on the capsule cap and "
657" on the capsule body, supplied in 100-count bottles (NDC 0469-0657-73) and 10 blister cards of 10 capsules (NDC 0469-0657-11), containing the equivalent of 5 mg anhydrous tacrolimus.
Made in Japan
Store and Dispense
Store at 25°C (77°F); excursions permitted to 15°C-30°C (59°F-86°F).
Prograf injection (tacrolimus injection) 5 mg (for IV infusion only)
Supplied as a sterile solution in 1 mL ampules containing the equivalent of 5 mg of anhydrous tacrolimus per mL, in boxes of 10 ampules (NDC 0469-3016-01).
Made in Ireland
Store and Dispense
Store between 5°C and 25°C (41°F and 77°F).
Rx only
Marketed by:
Astellas Pharma US, Inc
Deerfield, IL 60015-2548
REFERENCE
- CDC: Recommendations of the Advisory Committee on Immunization Practices: Use of vaccines and immune globulins in persons with altered immunocompetence. MMWR 1993;42(RR-4):1-18.
Revised, April 2005
Subscribe to the "News" RSS Feed 
TOP ۞
