-
Relafen Tablets (Glaxosmithkline)
DESCRIPTION
RELAFEN (nabumetone) is a naphthylalkanone designated chemically as 4-(6-methoxy-2-naphthalenyl)-2-butanone.
Nabumetone is a white to off-white crystalline substance with a molecular weight of 228.3. It is nonacidic and practically insoluble in water, but soluble in alcohol and most organic solvents. It has an n-octanol:phosphate buffer partition coefficient of 2400 at pH 7.4.
Tablets for Oral Administration: Each oval-shaped, film-coated tablet contains 500 mg or 750 mg of nabumetone. Inactive ingredients consist of hypromellose, microcrystalline cellulose, polyethylene glycol, polysorbate 80, sodium lauryl sulfate, sodium starch glycolate, and titanium dioxide. The 750-mg tablets also contain iron oxides.
CLINICAL PHARMACOLOGY
RELAFEN is a nonsteroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic, and antipyretic properties in pharmacologic studies. As with other nonsteroidal anti-inflammatory agents, its mode of action is not known; however, the ability to inhibit prostaglandin synthesis may be involved in the anti-inflammatory effect.
The parent compound is a prodrug, which undergoes hepatic biotransformation to the active component, 6-methoxy-2-naphthylacetic acid (6MNA), that is a potent inhibitor of prostaglandin synthesis.
It is acidic and has an n-octanol:phosphate buffer partition coefficient of 0.5 at pH 7.4.
Pharmacokinetics: After oral administration, approximately 80% of a radiolabelled dose of nabumetone is found in the urine, indicating that nabumetone is well absorbed from the gastrointestinal tract. Nabumetone itself is not detected in the plasma because, after absorption, it undergoes rapid biotransformation to the principal active metabolite, 6-methoxy-2-naphthylacetic acid (6MNA). Approximately 35% of a 1,000-mg oral dose of nabumetone is converted to 6MNA and 50% is converted into unidentified metabolites which are subsequently excreted in the urine. Following oral administration of RELAFEN, 6MNA exhibits pharmacokinetic characteristics that generally follow a one-compartment model with first order input and first order elimination.
6MNA is more than 99% bound to plasma proteins. The free fraction is dependent on total concentration of 6MNA and is proportional to dose over the range of 1,000 mg to 2,000 mg. It is 0.2% to 0.3% at concentrations typically achieved following administration of 1,000 mg of RELAFEN and is approximately 0.6% to 0.8% of the total concentrations at steady state following daily administration of 2,000 mg.
Steady-state plasma concentrations of 6MNA are slightly lower than predicted from single-dose data. This may result from the higher fraction of unbound 6MNA which undergoes greater hepatic clearance.
Coadministration of food increases the rate of absorption and subsequent appearance of 6MNA in the plasma but does not affect the extent of conversion of nabumetone into 6MNA. Peak plasma concentrations of 6MNA are increased by approximately one third.
Coadministration with an aluminum-containing antacid had no significant effect on the bioavailability of 6MNA.
Table 1. Mean Pharmacokinetic Parameters of Nabumetone Active Metabolite (6MNA) at Steady State Following Oral Administration of 1,000-mg or 2,000-mg Doses of RELAFENAbbreviation
(units)Young Adults
Mean ± SD
1,000 mg
n = 31Young Adults
Mean ± SD
2,000 mg
n = 12Elderly
Mean ± SD
1,000 mg
n = 27T max (hr)3.0 (1.0 to 12.0) 2.5 (1.0 to 8.0) 4.0 (1.0 to 10.0) t 1/2 (hr)22.5 ± 3.7 26.2 ± 3.7 29.8 ± 8.1 CL SS /F (mL/min)26.1 ± 17.3 21.0 ± 4.0 18.6 ± 13.4 Vd SS /F (L)55.4 ± 26.4 53.4 ± 11.3 50.2 ± 25.3
The simulated curves in the graph below illustrate the range of active metabolite plasma concentrations that would be expected from 95% of patients following 1,000-mg to 2,000-mg doses to steady state. The cross-hatched area represents the expected overlap in plasma concentrations due to intersubject variation following oral administration of 1,000 mg to 2,000 mg of RELAFEN.
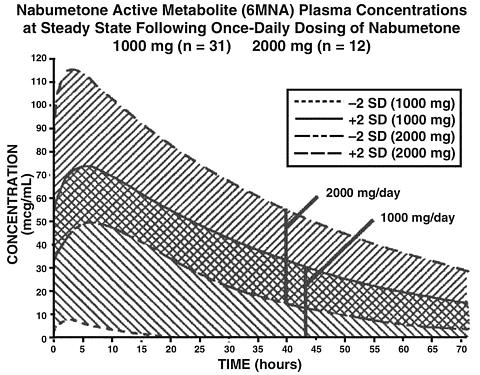
6MNA undergoes biotransformation in the liver, producing inactive metabolites that are eliminated as both free metabolites and conjugates. None of the known metabolites of 6MNA has been detected in plasma. Preliminary in vivo and in vitro studies suggest that unlike other NSAIDs, there is no evidence of enterohepatic recirculation of the active metabolite. Approximately 75% of a radiolabelled dose was recovered in urine in 48 hours. Approximately 80% was recovered in 168 hours. A further 9% appeared in the feces. In the first 48 hours, metabolites consisted of:
--nabumetone, unchangednot detectable --6-methoxy-2-naphthylacetic acid<1% (6MNA), unchanged--6MNA, conjugated11% --6-hydroxy-2-naphthylacetic acid5% (6HNA), unchanged--6HNA, conjugated7% --4-(6-hydroxy-2-naphthyl)-butan-2-ol,9% Conjugated-- O -desmethyl-nabumetone, conjugated7% --unidentified minor metabolites34% Total % Dose:73%
Following oral administration of dosages of 1,000 mg to 2,000 mg to steady state, the mean plasma clearance of 6MNA is 20 to 30 mL/min and the elimination half-life is approximately 24 hours.
Elderly Patients: Steady-state plasma concentrations in elderly patients were generally higher than in young healthy subjects (see Table 1 for summary of pharmacokinetic parameters).
Renal Insufficiency: In studies of patients with renal insufficiency, the mean terminal half-life of 6MNA was increased in patients with severe renal dysfunction (creatinine clearance <30 mL/min/1.73 m 2 ). In moderate renal insufficiency (creatinine clearance 30 to 49 mL/min), the terminal half-life of 6MNA is increased and there is a 50% increase in the plasma levels of unbound 6MNA. In patients undergoing hemodialysis, steady-state plasma concentrations of the active metabolite were similar to those observed in healthy subjects. Due to extensive protein binding, 6MNA is not dialyzable (see PRECAUTIONS : Renal Effects ).
Hepatic Impairment: Data in patients with severe hepatic impairment are limited. Biotransformation of nabumetone to 6MNA and the further metabolism of 6MNA to inactive metabolites is dependent on hepatic function and could be reduced in patients with severe hepatic impairment (history of or biopsy-proven cirrhosis).
Special Studies: Gastrointestinal: RELAFEN was compared to aspirin in inducing gastrointestinal blood loss. Food intake was not monitored. Studies utilizing 51 Cr-tagged red blood cells in healthy males showed no difference in fecal blood loss after 3 or 4 weeks' administration of 1,000 mg or 2,000 mg of RELAFEN daily when compared to either placebo-treated or nontreated subjects. In contrast, aspirin 3,600 mg daily produced an increase in fecal blood loss when compared to subjects who received RELAFEN, placebo, or treatment. The clinical relevance of the data is unknown.
The following endoscopy trials entered patients who had been previously treated with NSAIDs. These patients had varying baseline scores and different courses of treatment. The trials were not designed to correlate symptoms and endoscopy scores. The clinical relevance of these endoscopy trials, i.e., either G.I. symptoms or serious G.I. events, is not known.
Ten endoscopy studies were conducted in 488 patients who had baseline and post-treatment endoscopy. In 5 clinical trials that compared a total of 194 patients on 1,000 mg of RELAFEN daily or naproxen 250 mg or 500 mg twice daily for 3 to 12 weeks, treatment with RELAFEN resulted in fewer patients with endoscopically detected lesions (>3 mm). In 2 trials a total of 101 patients administered 1,000 mg or 2,000 mg of RELAFEN daily or piroxicam 10 mg to 20 mg for 7 to 10 days, there were fewer patients treated with RELAFEN with endoscopically detected lesions. In 3 trials of a total of 47 patients on 1,000 mg of RELAFEN daily or indomethacin 100 mg to 150 mg daily for 3 to 4 weeks, the endoscopy scores were higher with indomethacin. Another 12-week trial in a total of 171 patients compared the results of treatment with 1,000 mg of RELAFEN daily to ibuprofen 2,400 mg/day and ibuprofen 2,400 mg/day plus misoprostol 800 mcg/day. The results showed that patients treated with RELAFEN had a lower number of endoscopically detected lesions (>5 mm) than patients treated with ibuprofen alone but comparable to the combination of ibuprofen plus misoprostol. The results did not correlate with abdominal pain.
Other: In 1-week, repeat-dose studies in healthy volunteers, 1,000 mg of RELAFEN daily had little effect on collagen-induced platelet aggregation and no effect on bleeding time. In comparison, naproxen 500 mg daily suppressed collagen-induced platelet aggregation and significantly increased bleeding time.
CLINICAL TRIALS
Osteoarthritis: The use of RELAFEN in relieving the signs and symptoms of osteoarthritis (OA) was assessed in double-blind, controlled trials in which 1,047 patients were treated for 6 weeks to 6 months. In these trials, RELAFEN in a dose of 1,000 mg/day administered at night was comparable to naproxen 500 mg/day and to aspirin 3,600 mg/day.
Rheumatoid Arthritis: The use of RELAFEN in relieving the signs and symptoms of rheumatoid arthritis (RA) was assessed in double-blind, randomized, controlled trials in which 770 patients were treated for 3 weeks to 6 months. RELAFEN, in a dose of 1,000 mg/day administered at night, was comparable to naproxen 500 mg/day and to aspirin 3,600 mg/day.
In controlled clinical trials of rheumatoid arthritis patients, RELAFEN has been used in combination with gold, d-penicillamine, and corticosteroids.
Individualization of Dosing: There is considerable interpatient variation in response to RELAFEN. Therapy is usually initiated at 1,000 mg daily, then adjusted, if needed, based on clinical response.
In clinical trials with osteoarthritis and rheumatoid arthritis patients, most patients responded to RELAFEN in doses of 1,000 mg/day administered nightly; total daily dosages up to 2,000 mg were used. In open-labelled studies, 1,490 patients were permitted dosage increases and were followed for approximately 1 year (mode). Twenty percent of patients (n = 294) were withdrawn for lack of effectiveness during the first year of these open-labelled studies. The following table provides patient-exposure to doses used in the US clinical trials:
Table 2. Clinical Double-Blinded and Open-Labelled Trials of RELAFEN in Osteoarthritis and Rheumatoid Arthritis Dose of
RELAFENNumber of Patients Mean/Mode
Duration of
Treatment (yr)OA RA OA RA 500 mg17 6 0.4/- 0.2/- 1,000 mg917 701 1.2/1 1.4/1 1,500 mg645 224 2.3/1 1.7/1 2,000 mg15 100 0.6/1 1.3/1
As with other NSAIDs, the lowest dose should be sought for each patient. Patients weighing under 50 kg may be less likely to require dosages beyond 1,000 mg; therefore, after observing the response to initial therapy, the dose should be adjusted to meet individual patients' requirements.
INDICATIONS AND USAGE
RELAFEN is indicated for acute and chronic treatment of signs and symptoms of osteoarthritis and rheumatoid arthritis.
CONTRAINDICATIONS
RELAFEN is contraindicated in patients who have previously exhibited hypersensitivity to it.
RELAFEN is contraindicated in patients in whom RELAFEN, aspirin, or other NSAIDs induce asthma, urticaria, or other allergic-type reactions. Fatal asthmatic reactions have been reported in such patients receiving NSAIDs.
WARNINGS
Risk of G.I. Ulceration, Bleeding, and Perforation with NSAID Therapy: Serious gastrointestinal toxicity such as bleeding, ulceration, and perforation can occur at any time, with or without warning symptoms, in patients treated chronically with NSAID therapy. Although minor upper gastrointestinal problems, such as dyspepsia, are common, usually developing early in therapy, physicians should remain alert for ulceration and bleeding in patients treated chronically with NSAIDs even in the absence of previous G.I. tract symptoms.
In controlled clinical trials involving 1,677 patients treated with RELAFEN (1,140 followed for 1 year and 927 for 2 years), the cumulative incidence of peptic ulcers was 0.3% (95% CI; 0%, 0.6%) at 3 to 6 months, 0.5% (95% CI; 0.1%, 0.9%) at 1 year and 0.8% (95% CI; 0.3%, 1.3%) at 2 years. Physicians should inform patients about the signs and symptoms of serious G.I. toxicity and what steps to take if they occur. In patients with active peptic ulcer, physicians must weigh the benefits of therapy with RELAFEN against possible hazards, institute an appropriate ulcer treatment regimen and monitor the patients' progress carefully.
Studies to date have not identified any subset of patients not at risk of developing peptic ulceration and bleeding. Except for a prior history of serious G.I. events and other risk factors known to be associated with peptic ulcer disease, such as alcoholism, smoking, other medications known to increase the risk of gastrointestinal ulcer (e.g., oral corticosteroids), etc., no risk factors (e.g., age, sex) have been associated with increased risk. Elderly or debilitated patients seem to tolerate ulceration or bleeding less well than other individuals and most spontaneous reports of fatal G.I. events are in this population.
High doses of any NSAID probably carry a greater risk of these reactions, although controlled clinical trials showing this do not exist in most cases. In considering the use of relatively large doses (within the recommended dosage range), sufficient benefit should be anticipated to offset the potential increased risk of G.I. toxicity.
PRECAUTIONS
General: Renal Effects: As a class, NSAIDs have been associated with renal papillary necrosis and other abnormal renal pathology during long-term administration to animals.
A second form of renal toxicity often associated with NSAIDs is seen in patients with conditions leading to a reduction in renal blood flow or blood volume, where renal prostaglandins have a supportive role in the maintenance of renal perfusion. In these patients, administration of an NSAID results in a dose-dependent decrease in prostaglandin synthesis and, secondarily, in a reduction of renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics, and the elderly. Discontinuation of NSAID therapy is typically followed by recovery to the pretreatment state.
Because nabumetone undergoes extensive hepatic metabolism, no adjustment of the dosage of RELAFEN is generally necessary in patients with renal insufficiency; however, as with all NSAIDs, patients with impaired renal function should be monitored more closely than patients with normal renal function (see CLINICAL PHARMACOLOGY : Renal Insufficiency ). In patients with severe renal impairment (creatinine clearance </=30 mL/min), laboratory tests should be performed at baseline and within weeks of starting therapy. Further tests should be carried out as necessary; if the impairment worsens, discontinuation of therapy may be warranted. In subjects with moderate renal impairment (creatinine clearance 30 to 49 mL/min) there is a 50% increase in unbound plasma 6MNA and dose adjustment may be warranted. The oxidized and conjugated metabolites of 6MNA are eliminated primarily by the kidneys. The extent to which these largely inactive metabolites may accumulate in patients with renal failure has not been studied. As with other drugs whose metabolites are excreted by the kidneys, the possibility that adverse reactions (not listed in ADVERSE REACTIONS ) may be attributable to these metabolites should be considered (see CLINICAL PHARMACOLOGY : Renal Insufficiency ).
Hepatic Function: As with other NSAIDs, borderline elevations of 1 or more liver function tests may occur in up to 15% of patients. These abnormalities may progress, may remain essentially unchanged, or may return to normal with continued therapy. The ALT (SGPT) test is probably the most sensitive indicator of liver dysfunction. Meaningful (3 times the upper limit of normal) elevations of ALT (SGPT) or AST (SGOT) have occurred in controlled clinical trials of RELAFEN in less than 1% of patients. A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with RELAFEN. Severe hepatic reactions, including jaundice and fatal hepatitis, have been reported with RELAFEN and other NSAIDs. Although such reactions are rare, if abnormal liver tests persist or worsen, if clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), RELAFEN should be discontinued. Because nabumetone's biotransformation to 6MNA is dependent upon hepatic function, the biotransformation could be decreased in patients with severe hepatic dysfunction; therefore, RELAFEN should be used with caution in patients with severe hepatic impairment (see Pharmacokinetics : Hepatic Impairment ).
Fluid Retention and Edema: Fluid retention and edema have been observed in some patients taking RELAFEN; therefore, as with other NSAIDs, RELAFEN should be used cautiously in patients with a history of congestive heart failure, hypertension, or other conditions predisposing to fluid retention.
Photosensitivity: Based on ultraviolet (U.V.) light photosensitivity testing, RELAFEN may be associated with more reactions to sun exposure than might be expected based on skin tanning types.
Information for Patients: RELAFEN, like other drugs of its class, is not free of side effects. The side effects of these drugs can cause discomfort and, rarely, there are more serious side effects, such as gastrointestinal bleeding, which may result in hospitalization and even fatal outcome.
NSAIDs are often essential agents in the management of arthritis, but they also may be commonly employed for conditions that are less serious. Physicians may wish to discuss with their patients the potential risks (see WARNINGS , PRECAUTIONS and ADVERSE REACTIONS ) and likely benefits of NSAID treatment, particularly when the drugs are used for less serious conditions where treatment without NSAIDs may represent an acceptable alternative to both the patient and the physician.
Laboratory Tests: Because severe G.I. tract ulceration and bleeding can occur without warning symptoms, physicians should follow chronically-treated patients for signs and symptoms of ulceration and bleeding, and should inform them of the importance of this follow-up (see WARNINGS ; Risk of G.I. Ulceration, Bleeding, and Perforation with NSAID Therapy ).
Drug Interactions: In vitro studies have shown that, because of its affinity for protein, 6MNA may displace other protein-bound drugs from their binding site. Caution should be exercised when administering RELAFEN with warfarin since interactions have been seen with other NSAIDs.
Concomitant administration of an aluminum-containing antacid had no significant effect on the bioavailability of 6MNA. When administered with food or milk, there is more rapid absorption; however, the total amount of 6MNA in the plasma is unchanged (see CLINICAL PHARMACOLOGY : Pharmacokinetics ).
Carcinogenesis, Mutagenesis: In 2-year studies conducted in mice and rats, nabumetone had no statistically significant tumorigenic effect. Nabumetone did not show mutagenic potential in the Ames test and mouse micronucleus test in vivo; however, nabumetone- and 6MNA-treated lymphocytes in culture showed chromosomal aberrations at 80 mcg/mL and higher concentrations (equal to the average human exposure to RELAFEN at the maximum recommended dose).
Impairment of Fertility: Nabumetone did not impair fertility of male or female rats treated orally at doses of 320 mg/kg/day (1,888 mg/m 2 ) before mating.
Pregnancy: Teratogenic Effects: Pregnancy Category C. Nabumetone did not cause any teratogenic effect in rats given up to 400 mg/kg (2,360 mg/m 2 ) and in rabbits up to 300 mg/kg (3,540 mg/m 2 ) orally; however, increased post-implantation loss was observed in rats at 100 mg/kg (590 mg/m 2 ) orally and at higher doses (equal to the average human exposure to 6MNA at the maximum recommended human dose). There are no adequate, well-controlled studies in pregnant women. This drug should be used during pregnancy only if clearly needed.
Because of the known effect of prostaglandin-synthesis-inhibiting drugs on the human fetal cardiovascular system (closure of ductus arteriosus), use of RELAFEN during the third trimester of pregnancy is not recommended.
Labor and Delivery: The effects of RELAFEN on labor and delivery in women are not known. As with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia and delayed parturition occurred in rats treated throughout pregnancy.
Nursing Mothers: RELAFEN is not recommended for use in nursing mothers because of the possible adverse effects of prostaglandin-synthesis-drugs on neonates. It is not known whether nabumetone or its metabolites are excreted in human milk; however, 6MNA is excreted in the milk of lactating rats.
Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Geriatric Use: Of the 1,677 patients in US clinical studies who were treated with RELAFEN, 411 patients (24%) were 65 years or older; 22 patients (1%) were 75 years or older. No overall differences in efficacy or safety were observed between these older patients and younger ones. Similar results were observed in a 1-year, non-US postmarketing surveillance study of 10,800 patients treated with RELAFEN, of whom 4,577 patients (42%) were 65 years or older.
ADVERSE REACTIONS
Adverse reaction information was derived from blinded-controlled and open-labelled clinical trials and from worldwide marketing experience. In the description below, rates of the more common events (greater than 1%) and many of the less common events (less than 1%) represent results of US clinical studies.
Of the 1,677 patients who received RELAFEN during US clinical trials, 1,524 were treated for at least 1 month, 1,327 for at least 3 months, 929 for at least a year, and 750 for at least 2 years. More than 300 patients have been treated for 5 years or longer.
The most frequently reported adverse reactions were related to the gastrointestinal tract and included diarrhea, dyspepsia, and abdominal pain.
Incidence >/=1%--Probably Causally Related
Gastrointestinal: Diarrhea (14%), dyspepsia (13%), abdominal pain (12%), constipation * , flatulence * , nausea * , positive stool guaiac * , dry mouth, gastritis, stomatitis, vomiting.
Central Nervous System: Dizziness * , headache * , fatigue, increased sweating, insomnia, nervousness, somnolence.
Dermatologic: Pruritus * , rash * .
Special Senses: Tinnitus * .
Miscellaneous: Edema * .
*Incidence of reported reaction between 3% and 9%. Reactions occurring in 1% to 3% of the patients are unmarked.
Incidence <1%--Probably Causally Related **/*
Gastrointestinal: Anorexia, jaundice, duodenal ulcer, dysphagia, gastric ulcer, gastroenteritis, gastrointestinal bleeding, increased appetite, liver function abnormalities, melena, hepatic failure.
Central Nervous System: Asthenia, agitation, anxiety, confusion, depression, malaise, paresthesia, tremor, vertigo.
Dermatologic: Bullous eruptions, photosensitivity, urticaria, pseudoporphyria cutanea tarda, toxic epidermal necrolysis, erythema multiforme, Stevens-Johnson syndrome.
Cardiovascular: Vasculitis.
Metabolic: Weight gain.
Respiratory: Dyspnea, eosinophilic pneumonia, hypersensitivity pneumonitis, idiopathic interstitial pneumonitis.
Genitourinary: Albuminuria, azotemia, hyperuricemia, interstitial nephritis, nephrotic syndrome, vaginal bleeding, renal failure.
Special Senses: Abnormal vision.
Hematologic/Lymphatic: Thrombocytopenia.
Hypersensitivity: Anaphylactoid reaction, anaphylaxis, angioneurotic edema.
**/* Adverse reactions reported only in worldwide postmarketing experience or in the literature, not seen in clinical trials, are considered rarer and are italicized.
Incidence <1%--Causal Relationship Unknown
Gastrointestinal: Bilirubinuria, duodenitis, eructation, gallstones, gingivitis, glossitis, pancreatitis, rectal bleeding.
Central Nervous System: Nightmares.
Dermatologic: Acne, alopecia.
Cardiovascular: Angina, arrhythmia, hypertension, myocardial infarction, palpitations, syncope, thrombophlebitis.
Respiratory: Asthma, cough.
Genitourinary: Dysuria, hematuria, impotence, renal stones.
Special Senses: Taste disorder.
Body as a Whole: Fever, chills.
Hematologic/Lymphatic: Anemia, leukopenia, granulocytopenia.
Metabolic/Nutritional: Hyperglycemia, hypokalemia, weight loss.
OVERDOSAGE
Symptoms following acute NSAIDs overdoses are usually limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression, and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose.
Patients should be managed by symptomatic and supportive care following a NSAIDs overdose. There are no specific antidotes. Emesis and/or activated charcoal (60 to 100 grams in adults, 1 to 2 g/kg in children), and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose (5 to 10 times the usual dose). Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
There have been overdoses of up to 25 grams of RELAFEN reported with no long-term sequelae following standard emergency treatment (i.e., activated charcoal, gastric lavage, IV H 2 -blockers, etc.).
DOSAGE AND ADMINISTRATION
Osteoarthritis and Rheumatoid Arthritis: The recommended starting dose is 1,000 mg taken as a single dose with or without food. Some patients may obtain more symptomatic relief from 1,500 mg to 2,000 mg per day. RELAFEN can be given in either a single or twice-daily dose. Dosages greater than 2,000 mg per day have not been studied. The lowest effective dose should be used for chronic treatment (see PRECAUTIONS : Renal Effects ).
HOW SUPPLIED
Tablets: Oval-shaped, film-coated: 500 mg-white, imprinted with the product name RELAFEN and 500, in bottles of 100, and in Single-Unit Packages of 100 (intended for institutional use only). 750 mg-beige, imprinted with the product name RELAFEN and 750, in bottles of 100, and in Single-Unit Packages of 100 (intended for institutional use only).
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) in well-closed container; dispense in light-resistant container.
500 mg 100's: NDC 0029-4851-20
500 mg SUP 100's: NDC 0029-4851-21
750 mg 100's: NDC 0029-4852-20
Glaxosmithkline, Research Triangle Park, NC 27709
©2004, Glaxosmithkline. All rights reserved.
February 2005/RL:L14
Subscribe to the "News" RSS Feed 
TOP ۞
