-
VESIcare Tablets (Astellas)
DESCRIPTION
VESIcare® (solifenacin succinate) is a muscarinic receptor antagonist. Chemically, solifenacin succinate is butanedioic acid, compounded with (1 S )-(3 R )-1-azabicyclo[2.2.2]oct-3-yl 3,4-dihydro-1-phenyl-2(1 H )-iso-quinolinecarboxylate (1:1) having an empirical formula of C 23 H 26 N 2 O 2 ·C 4 H 6 O 4 , and a molecular weight of 480.55. The structural formula of solifenacin succinate is:
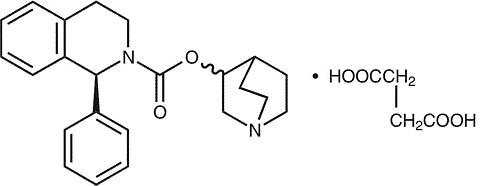
Solifenacin succinate is a white to pale-yellowish-white crystal or crystalline powder. It is freely soluble at room temperature in water, glacial acetic acid, dimethyl sulfoxide, and methanol. Each VESIcare tablet contains 5 or 10 mg of solifenacin succinate and is formulated for oral administration. In addition to the active ingredient solifenacin succinate, each VESIcare tablet also contains the following inert ingredients: lactose monohydrate, corn starch, hypromellose 2910, magnesium stearate, talc, polyethylene glycol 8000 and titanium dioxide with yellow ferric oxide (5 mg VESIcare tablet) or red ferric oxide (10 mg VESIcare tablet).
CLINICAL PHARMACOLOGY
Solifenacin is a competitive muscarinic receptor antagonist. Muscarinic receptors play an important role in several major cholinergically mediated functions, including contractions of urinary bladder smooth muscle and stimulation of salivary secretion.
Pharmacokinetics
Absorption
After oral administration of VESIcare to healthy volunteers, peak plasma levels (C max ) of solifenacin are reached within 3 to 8 hours after administration, and at steady state ranged from 32.3 to 62.9 ng/mL for the 5 and 10 mg VESIcare tablets, respectively. The absolute bioavailability of solifenacin is approximately 90%, and plasma con-centrations of solifenacin are proportional to the dose administered.
Effect of food
There is no significant effect of food on the pharmacokinetics of solifenacin.
Distribution
Solifenacin is approximately 98% ( in vivo ) bound to human plasma proteins, principally to (alpha) 1 -acid glycoprotein. Solifenacin is highly distributed to non-CNS tissues, having a mean steady-state volume of distribution of 600L.
Metabolism
Solifenacin is extensively metabolized in the liver. The primary pathway for elimination is by way of CYP3A4; however, alternate metabolic pathways exist. The primary metabolic routes of solifenacin are through N-oxidation of the quinuclidin ring and 4R-hydroxylation of tetrahydroisoquinoline ring. One pharmacologically active metabolite (4R-hydroxy solifenacin), occurring at low concentrations and unlikely to contribute significantly to clinical activity, and three pharmacologically inactive metabolites (N-glucuronide and the N-oxide and 4R-hydroxy-N-oxide of solifenacin) have been found in human plasma after oral dosing.
Excretion
Following the administration of 10 mg of 14 C-solifenacin succinate to healthy volunteers, 69.2% of the radioactivity was recovered in the urine and 22.5% in the feces over 26 days. Less than 15% (as mean value) of the dose was recovered in the urine as intact solifenacin. The major metabolites identified in urine were N-oxide of solifenacin, 4R-hydroxy solifenacin and 4R-hydroxy-N-oxide of solifenacin and in feces 4R-hydroxy solifenacin. The elimination half-life of solifenacin following chronic dosing is approximately 45-68 hours.
Pharmacokinetics in Special Populations
Age
Multiple dose studies of VESIcare in elderly volunteers (65 to 80 years) showed that C max , AUC and t 1/2 values were 20-25% higher as compared to the younger volunteers (18 to 55 years). (See PRECAUTIONS , Geriatric Use ).
Pediatric
The pharmacokinetics of solifenacin has not been established in pediatric patients.
Gender
The pharmacokinetics of solifenacin is not significantly influenced by gender.
Race
The number of subjects of different races studied is not adequate to make any conclusions on the effect of race on the pharmacokinetics of solifenacin.
Renal Impairment
VESIcare should be used with caution in patients with renal impairment. There is a 2.1-fold increase in AUC and 1.6-fold increase in t 1/2 of solifenacin in patients with severe renal impairment. Doses of VESIcare greater than 5 mg are not recommended in patients with severe renal impairment (CL cr < 30 mL/min) (see PRECAUTIONS , DOSAGE AND ADMINISTRATION ).
Hepatic Impairment
VESIcare should be used with caution in patients with reduced hepatic function. There is a 2-fold increase in the t 1/2 and 35% increase in AUC of solifenacin in patients with moderate hepatic impairment. Doses of VESIcare greater than 5 mg are not recommended in patients with moderate hepatic impairment (Child-Pugh B). VESIcare is not re-commended for patients with severe hepatic impairment (Child-Pugh C) (see PRECAUTIONS , DOSAGE AND ADMINISTRATION ).
Drug-Drug Interactions
Drugs Metabolized by Cytochrome P450
At therapeutic concentrations, solifenacin does not inhibit CYP1A1/2, 2C9, 2C19, 2D6, or 3A4 derived from human liver microsomes.
CYP3A4 Inhibitors
In vitro drug metabolism studies have shown that solifenacin is a substrate of CYP3A4. Inducers or inhibitors of CYP3A4 may alter solifenacin pharmacokinetics.
Ketoconazole Interaction Study
Following the administration of 10 mg of VESIcare in the presence of 400 mg of ketoconazole, a potent inhibitor of CYP3A4, the mean C max and AUC of solifenacin increased by 1.5 and 2.7-fold, respectively. Therefore, it is recommended not to exceed a 5 mg daily dose of VESIcare when administered with therapeutic doses of ketoconazole or other potent CYP3A4 inhibitors (see PRECAUTIONS , DOSAGE AND ADMINISTRATION ).
Oral Contraceptives
In the presence of solifenacin there are no significant changes in the plasma concentrations of combined oral contraceptives (ethinyl estradiol/levogestrel).
Warfarin
Solifenacin has no significant effect on the pharmacokinetics of R -warfarin or S -warfarin.
Digoxin
Solifenacin had no significant effect on the pharmacokinetics of digoxin (0.125 mg/day) in healthy subjects.
Cardiac Electrophysiology
The effect of 10 mg and 30 mg solifenacin succinate on the QT interval was evaluated at the time of peak plasma concentration of solifenacin in a multi-dose, randomized, double-blind, placebo and positive-controlled (moxifloxacin 400 mg) trial. Subjects were randomized to one of two treatment groups after receiving placebo and moxifloxacin sequentially. One group (n=51) went on to complete 3 additional sequential periods of dosing with solifenacin 10, 20, and 30 mg while the second group (n=25) in parallel completed a sequence of placebo and moxifloxacin. Study subjects were female volunteers aged 19 to 79 years. The 30 mg dose of solifenacin succinate (three times the highest recommended dose) was chosen for use in this study because this dose results in a solifenacin exposure that covers those observed upon co-administration of 10 mg VESIcare with potent CYP3A4 inhibitors (e.g. ketoconazole, 400 mg). Due to the sequential dose escalating nature of the study, baseline EKG measurements were separated from the final QT assessment (of the 30 mg dose level) by 33 days.
The median difference from baseline in heart rate associated with the 10 and 30 mg doses of solifenacin succinate compared to placebo was -2 and 0 beats/minute, respectively. Because a significant period effect on QTc was observed, the QTc effects were analyzed utilizing the parallel placebo control arm rather than the pre-specified intra-patient analysis. Representative results are shown in Table 1.
Table 1. QTc changes in msec (90%CI) from baseline at T max (relative to placebo) * Drug/DoseFridericia method
(using mean difference)Solifenacin 10 mg2 (-3,6)Solifenacin 30 mg8 (4, 13)*Results displayed are those derived from the parallel design portion of the study and represent the comparison of Group 1 to time-matched placebo effects in Group 2 Moxifloxacin was included as a positive control in this study and, given the length of study, its effect on the QT interval was evaluated in 3 different sessions. The placebo subtracted mean changes (90% CI) in QTcF for moxifloxacin in the three sessions were 11 (7, 14), 12 (8, 17), and 16 (12, 21), respectively.
The QT interval prolonging effect appeared greater for the 30 mg compared to the 10 mg dose of solifenacin. Although the effect of the highest solifenacin dose (three times the maximum therapeutic dose) studied did not appear as large as that of the positive control moxifloxacin at its therapeutic dose, the confidence intervals overlapped. This study was not designed to draw direct statistical conclusions between the drugs or the dose levels.
CLINICAL STUDIES
VESIcare was evaluated in four twelve-week, double-blind, randomized, placebo-controlled, parallel group, multicenter clinical trials for the treatment of overactive bladder in patients having symptoms of urinary frequency, urgency, and/or urge or mixed incontinence (with a predominance of urge). Entry criteria required that patients have symptoms of overactive bladder for >/= 3 months duration. These studies involved 3027 patients (1811 on VESIcare and 1216 on placebo), and approximately 90% of these patients completed the 12-week studies. Two of the four studies evaluated the 5 and 10 mg VESIcare doses and the other two evaluated only the 10 mg dose. All patients completing the 12-week studies were eligible to enter an open label, long term extension study and 81% of patients enrolling completed the additional 40-week treatment period. The majority of patients were Caucasian (93%) and female (80%) with a mean age of 58 years.
The primary endpoint in all four trials was the mean change from baseline to 12 weeks in number of micturitions/24 hours. Secondary endpoints included mean change from baseline to 12 weeks in number of incontinence episodes/24 hours, and mean volume voided per micturition. The efficacy of VESIcare was similar across patient age and gender. The mean reduction in the number of mictruitions per 24 hours was significantly greater with VESIcare 5 mg (2.3; p<0.001) and VESIcare 10 mg (2.7; p<0.001) compared to placebo, (1.4).
The mean reduction in the number of incontinence episodes per 24 hours was significantly greater with VESIcare 5 mg (1.5; p<0.001) and VESIcare 10 mg (1.8; p<0.001) treatment groups compared to placebo (1.1). The mean increase in the volume voided per micturition was significantly greater with VESIcare 5 mg (32.3 mL; p<0.001) and VESIcare 10 mg (42.5 mL; p<0.001) compared with placebo (8.5 mL).
The results for the primary and secondary endpoints in the four individual 12-week clinical studies of VESIcare are reported in Tables 2 through 5.
Table 2. Mean Change from Baseline to Endpoint for VESIcare (5 mg and 10 mg daily) and Placebo: 905-CL-015 ParameterPlacebo
(N=253)
Mean (SE)VESIcare
5 mg
(N=266)
Mean (SE)VESIcare
10 mg
(N=264)
Mean (SE)Urinary Frequency (Number of Micturitions/24 hours) *
Baseline
Reduction
P value vs. placebo12.2 (0.26)
1.2 (0.21)12.1 (0.24)
2.2 (0.18)
<0.00112.3 (0.24)
2.6 (0.20)
<0.001Number of Incontinence Episodes/24 hours **
Baseline
Reduction
P value vs. placebo2.7 (0.23)
0.8 (0.18)2.6 (0.22)
1.4 (0.15)
<0.012.6 (0.23)
1.5 (0.18)
<0.01Volume Voided per micturition [mL] **
Baseline
Increase
P value vs. placebo143.8 (3.37)
7.4 (2.28)149.6 (3.35)
32.9 (2.92)
<0.001147.2 (3.15)
39.2 (3.11)
<0.001* Primary endpoint ** Secondary endpoint Table 3. Mean Change from Baseline to Endpoint for VESIcare (5 mg and 10 mg daily) and Placebo: 905-CL-018 ParameterPlacebo
(N=281)
Mean (SE)VESIcare
5 mg
(N=286)
Mean (SE)VESIcare
10 mg
(N=290)
Mean (SE)Urinary Frequency (Number of Micturitions/24 hours) *
Baseline
Reduction
P value vs. placebo12.3 (0.23)
1.7 (0.19)12.1 (0.23)
2.4 (0.17)
<0.00112.1 (0.21)
2.9 (0.18)
<0.001Number of Incontinence Episodes/24 hours **
Baseline
Reduction
P value vs. placebo3.2 (0.24)
1.3 (0.19)2.6 (0.18)
1.6 (0.16)
<0.012.8 (0.20)
1.6 (0.18)
0.016Volume Voided per micturition [mL] **
Baseline
Increase
P value vs. placebo147.2 (3.18)
11.3 (2.52)148.5 (3.16)
31.8 (2.94)
<0.001145.9 (3.42)
36.6 (3.04)
<0.001* Primary endpoint ** Secondary endpoint Table 4. Mean Change from Baseline to Endpoint for VESIcare (10 mg daily) and Placebo: 905-CL-013 ParameterPlacebo
(N=309)
Mean (SE)VESIcare
10 mg
(N=306)
Mean (SE)Urinary Frequency (Number of Micturitions/24 hours) *
Baseline
Reduction
P value vs. placebo11.5 (0.18)
1.5 (0.15)11.7 (0.18)
3.0 (0.15)
<0.001Number of Incontinence Episodes/24 hours **
Baseline
Reduction
P value vs. placebo3.0 (0.20)
1.1 (0.16)3.1 (0.22)
2.0 (0.19)
<0.001Volume Voided per micturition [mL] **
Baseline
Increase
P value vs. placebo190.3 (5.48)
2.7 (3.15)183.5 (4.97)
47.2 (3.79)
<0.001* Primary endpoint ** Secondary endpoint
Table 5. Mean Change from Baseline to Endpoint for VESIcare (10 mg daily) and Placebo: 905-CL-014 ParameterPlacebo
(N=295)
Mean (SE)VESIcare
10 mg
(N=298)
Mean (SE)Urinary Frequency (Number of Micturitions/24 hours) *
Baseline
Reduction
P value vs. placebo11.8 (0.18)
1.3 (0.16)11.5 (0.18)
2.4 (0.15)
<0.001Number of Incontinence Episodes/24 hours **
Baseline
Reduction
P value vs. placebo2.9 (0.18)
1.2 (0.15)2.9 (0.17)
2.0 (0.15)
<0.001Volume Voided per micturition [mL] **
Baseline
Increase
P value vs. placebo175.7 (4.44)
13.0 (3.45)174.1 (4.15)
46.4 (3.73)
<0.001* Primary endpoint ** Secondary endpoint INDICATIONS AND USAGE
VESIcare is indicated for the treatment of overactive bladder with symptions of urge urinary incontinence, urgency, and urinary frequency.
CONTRAINDICATIONS
VESIcare is contraindicated in patients with urinary retention, gastric retention, uncontrolled narrow-angle glaucoma, and in patients who have demonstrated hypersensitivity to the drug substance or other components of the product.
PRECAUTIONS
Bladder Outflow Obstruction
VESIcare, like other anticholinergic drugs, should be administered with caution to patients with clinically significant bladder outflow obstruction because of the risk of urinary retention.
Gastrointestinal Obstructive Disorders and Decreased GI Motility
VESIcare, like other anticholinergics, should be used with caution in patients with decreased gastrointestinal motility.
Controlled Narrow-Angle Glaucoma
VESIcare should be used with caution in patients being treated for narrow-angle glaucoma. (See CONTRAINDICATIONS )
Reduced Renal Function
VESIcare should be used with caution in patients with reduced renal function. Doses of VESIcare greater than 5 mg are not recommended in patients with severe renal impairment (CL cr <30 mL/min). (See CLINICAL PHARMACOLOGY , DOSAGE AND ADMINISTRATION .)
Reduced Hepatic Function
VESIcare should be used with caution in patients with reduced hepatic function. Doses of VESIcare greater than 5 mg are not recommended in patients with moderate hepatic impairment (Child-Pugh B). VESIcare is not recommended for patients with severe hepatic impairment (Child-Pugh C). (See CLINICAL PHARMACOLOGY , DOSAGE AND ADMINISTRATION .)
Drug-Drug Interactions
Do not exceed a 5 mg daily dose of VESIcare when administered with therapeutic doses of ketoconazole or other potent CYP3A4 inhibitors. (See CLINICAL PHARMACOLOGY , DOSAGE AND ADMINISTRATION .)
Patients with Congenital or Acquired QT Prolongation
In a study of the effect of solifenacin on the QT interval in 76 healthy women (See CLINICAL PHARMACOLOGY , Cardiac Electrophysiology ), the QT prolonging effect appeared less with solifenacin 10 mg than with 30 mg (three times the maximum recommended dose), and the effect of solifenacin 30 mg did not appear as large as that of the positive control moxifloxacin at its therapeutic dose. This observation should be considered in clinical decisions to prescribe VESIcare for patients with a known history of QT prolongation or patients who are taking medications known to prolong the QT interval.
Information for Patients
Patients should be informed that antimuscarinic agents such as VESIcare have been associated with constipation and blurred vision. Patients should be advised to contact their physician if they experience severe abdominal pain or become constipated for 3 or more days. Because VESIcare may cause blurred vision, patients should be advised to exercise caution in decisions to engage in potentially dangerous activities until the drug's effect on the patient's vision has been determined. Heat prostration (due to decreased sweating) can occur when anticholinergic drugs, such as VESIcare, are used in a hot environment. Patients should read the patient leaflet entitled "Patient Information VESIcare" before starting therapy with VESIcare.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Solifenacin succinate was not mutagenic in the in vitro Salmonella typhimurium or Escherichia coli microbial mutagenicity test or chromosomal aberration test in human peripheral blood lymphocytes with or without metabolic activation, or in the in vivo micronucleus test in rats.
No increase in tumors was found following the administration of solifenacin succinate to male and female mice for 104 weeks at doses up to 200 mg/kg/day (5 and 9 times human exposure at the maximum recommended human dose [MRHD], respectively), and male and female rats for 104 weeks at doses up to 20 and 15 mg/kg/day, respectively (<1 times exposure at the MRHD).
Solifenacin succinate had no effect on reproductive function, fertility or early embryonic development of the fetus in male and female mice treated with 250 mg/kg/day (13 times exposure at the MRHD) of solifenacin succinate, and in male rats treated with 50 mg/kg/day (<1 times exposure at the MRHD) and female rats treated with 100 mg/kg/day (1.7 times exposure at the MRHD) of solifenacin succinate.
Pregnancy, Teratogenic Effects, Pregnancy Category
Pregnancy Category C
Reproduction studies have been performed in mice, rats and rabbits. After oral administration of 14 C-solifenacin succinate to pregnant mice, drug-related material has shown to cross the placental barrier. No embryotoxicity or teratogenecity was observed in mice treated with 30 mg/kg/day (1.2 times exposure at the maximum recommended human dose [MRHD]). Administration of solifenacin succinate to pregnant mice at doses of 100 mg/kg and greater (3.6 times exposure at the MRDH), during the major period of organ development resulted in reduced fetal body weights. Administration of 250 mg/kg (7.9 times exposure at the MRHD) to pregnant mice resulted in an increased incidence of cleft palate. In utero and lactational exposures to maternal doses of solifenacin succinate of 100 mg/kg/day and greater (3.6 times exposure at the MRHD) resulted in reduced peripartum and postnatal survival, reductions in body weight gain, and delayed physical development (eye opening and vaginal patency). An increase in the percentage of male offspring was also observed in litters from offspring exposed to maternal doses of 250 mg/kg/day. No embryotoxic effects were observed in rats at up to 50 mg/kg/day (<1 times exposure at the MRHD) or in rabbits at up to 50 mg/kg/day (1.8 times exposure at the MRHD). There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, VESIcare should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
The effect of VESIcare on labor and delivery in humans has not been studied.
There were no effects on natural delivery in mice treated with 30 mg/kg/day (1.2 times exposure at the maximum recommended human dose [MRHD]). Administration of soli-fenacin succinate at 100 mg/kg/day (3.6 times exposure at the MRHD) or greater increased peripartum pup mortality.
Nursing Mothers
After oral administration of 14 C-solifenacin succinate to lactating mice, radioactivity was detected in maternal milk. There were no adverse observations in mice treated with 30 mg/kg/day (1.2 times exposure at the maximum recommended human dose [MRHD]). Pups of female mice treated with 100 mg/kg/day (3.6 times exposure at the MRHD) or greater revealed reduced body weights, postpartum pup mortality or delays in the onset of reflex and physical development during the lactation period.
It is not known whether solifenacin is excreted in human milk. Because many drugs are excreted in human milk, VESIcare should not be administered during nursing. A decision should be made whether to discontinue nursing or to discontinue VESIcare in nursing mothers.
Pediatric Use
The safety and effectiveness of VESIcare in pediatric patients have not been established.
Geriatric Use
In placebo controlled clinical studies, similar safety and effectiveness were observed between older (623 patients >/= 65 years and 189 patients >/= 75 years) and younger patients (1188 patients < 65 years) treated with VESIcare (See CLINICAL PHARMACOLOGY , Pharmacokinetics in Special Populations ).
ADVERSE REACTIONS
VESIcare has been evaluated for safety in 1811 patients in randomized, placebo-controlled trials. Expected side effects of antimuscarinic agents are dry mouth, constipation, blurred vision (accommodation abnormalities), urinary retention, and dry eyes. The most common adverse events reported in patients treated with VESIcare were dry mouth and constipation and the incidence of these side effects was higher in the 10 mg compared to the 5 mg dose group. In the four 12-week double-blind clinical trials there were three intestinal serious adverse events in patients, all treated with VESIcare 10 mg (one fecal impaction, one colonic obstruction, and one intestinal obstruction). The overall rate of serious adverse events in the double-blind trials was 2%. Angioneurotic edema has been reported in one patient taking VESIcare 5 mg. Compared to twelve weeks of treatment with VESIcare, the incidence and severity of adverse events were similar in patients who remained on drug for up to 12 months. The most frequent reason for discontinuation due to an adverse event was dry mouth, 1.5%. Table 6 lists adverse events, regardless of causality, that were reported in randomized, placebo-controlled trials at an incidence greater than placebo and in 1% or more of patients treated with VESIcare 5 or 10 mg once daily for up to 12 weeks.
Table 6. Percentage of Patients with Treatment-emergent Adverse Events Exceeding Placebo Rate and Reported by 1% or More Patients for Combined Pivotal StudiesSYSTEM ORGAN CLASS
MedDRA Preferred TermPlacebo
(%)VESIcare
5 mg
(%)VESIcare
10 mg
(%)Number of Patients1216 578 1233 Number of Patients with Treatment-emergent AE634 265 773 GASTROINTESTINAL DISORDERSDry Mouth4.2 10.9 27.6 Constipation2.9 5.4 13.4 Nausea2.0 1.7 3.3 Dyspepsia1.0 1.4 3.9 Abdominal Pain Upper1.0 1.9 1.2 Vomiting NOS0.9 0.2 1.1 INFECTIONS AND INFESTATIONSUrinary Tract Infection NOS2.8 2.8 4.8 Influenza1.3 2.2 0.9 Pharyngitis NOS1.0 0.3 1.1 NERVOUS SYSTEM DISORDERSDizziness1.8 1.9 1.8 EYE DISORDERSVision Blurred1.8 3.8 4.8 Dry Eyes NOS0.6 0.3 1.6 RENAL AND URINARY DISORDERSUrinay Retention0.6 0 1.4 GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONSEdema Lower Limb0.7 0.3 1.1 Fatigue1.1 1.0 2.1 PSYCHIATRIC DISORDERSDepression NOS0.8 1.2 0.8 RESPIRATORY, THORACIC AND MEDIASTINAL DISORDERSCough0.2 0.2 1.1 VASCULAR DISORDERSHypertension NOS0.6 1.4 0.5 OVERDOSAGE
Acute: Overdosage with VESIcare can potentially result in severe anticholinergic effects and should be treated accordingly. The highest VESIcare dose given to human volunteers was a single 100 mg dose.
Chronic: Intolerable anticholinergic side effects (fixed and dilated pupils, blurred vision, failure of heel-to-toe exam, tremors and dry skin) occurred on day 3 in normal volunteers taking 50 mg daily (5 times the maximum recommended therapeutic dose) and resolved within 7 days following discontinuation of drug.
Treatment of Overdosage
No cases of acute overdosage have been reported, but in the event of overdose with VESIcare treat with gastric lavage and appropriate supportive measures.
DOSAGE AND ADMINISTRATION
The recommended dose of VESIcare is 5 mg once daily. If the 5 mg dose is well tolerated, the dose may be increased to 10 mg once daily.
VESIcare should be taken with liquids and swallowed whole. VESIcare can be administered with or without food.
Dose Adjustment in Renal Impairment
For patients with severe renal impairment (CL cr <30 mL/min), a daily dose of VESIcare greater than 5 mg is not recommended.
Dose Adjustment in Hepatic Impairment
For patients with moderate hepatic impairment (Child-Pugh B), a daily dose of VESIcare greater than 5 mg is not recommended. Use of VESIcare in patients with severe hepatic impairment (Child-Pugh C) is not recommended.
Dose Adjustment CYP3A4 Inhibitors
When administered with therapeutic doses of ketoconazole or other potent CYP3A4 inhibitors, a daily dose of VESIcare greater than 5 mg is not recommended.
HOW SUPPLIED
VESIcare is supplied as round, film-coated tablets, available in bottles and unit dose blister packages as follows:
strength 5 mg 10 mg color light yellow light pink debossed logo, 150 logo, 151 Bottle of 30 NDC 51248-150-01 NDC 51248-151-01 Bottle of 90 NDC 51248-150-03 NDC 51248-151-03 Unit Dose Pack of 100
NDC 51248-150-52 NDC 51248-151-52 Store at 25°C (77°F) with excursions permitted from 15°C to 30°C (59-86°F) [see USP Controlled Room Temperature]
Rx Only
Manufactured by:
Astellas Pharma Technologies Inc.
Norman, Oklahoma 73072
Marketed by:
Astellas Pharma US, Inc.
Deerfield, IL 60015-2548
Marketed and Distributed by:
Research Triangle Park
North Carolina 27709
©2005 Astellas Pharma US, Inc.
PRT26 March 2005
Subscribe to the "News" RSS Feed 
TOP ۞
