-
Voltaren Tablets (Novartis)
Rx only
Prescribing Information
The following prescribing information is based on official labeling in effect July 2005
DESCRIPTION
Voltaren ® (diclofenac sodium enteric-coated tablets), is a benzene-acetic acid derivative. Voltaren is available as Delayed-Release (enteric-coated) Tablets of 25 mg (yellow), 50 mg (light brown), and 75 mg (light pink) for oral administration. The chemical name is 2-[(2,6-dichlorophenyl)amino] benzeneacetic acid, monosodium salt. The molecular weight is 318.14. Its molecular formula is C 14 H 10 Cl 2 NNaO 2 , and it has the following structural formula
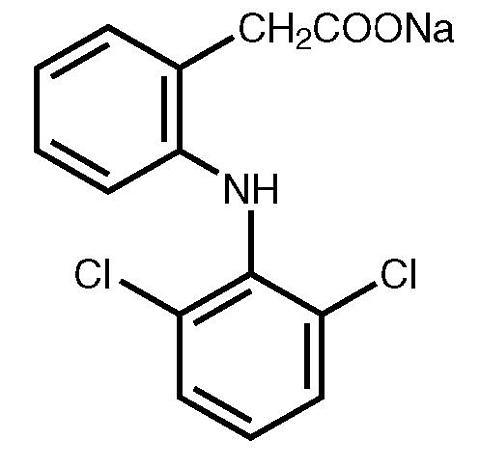
The inactive ingredients in Voltaren include: hydroxypropyl methylcellulose, iron oxide, lactose, magnesium stearate, methacrylic acid copolymer, microcrystalline cellulose, polyethylene glycol, povidone, propylene glycol, sodium hydroxide, sodium starch glycolate, talc, titanium dioxide, D&C Yellow No. 10 Aluminum Lake (25-mg tablet only), FD&C Blue No. 1 Aluminum Lake (50-mg tablet only).
CLINICAL PHARMACOLOGY
Pharmacodynamics
Voltaren ® (diclofenac sodium enteric-coated tablets), is a nonsteroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic, and antipyretic activities in animal models. The mechanism of action of Voltaren, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
Pharmacokinetics
Absorption
Diclofenac is 100% absorbed after oral administration compared to IV administration as measured by urine recovery. However, due to first-pass metabolism, only about 50% of the absorbed dose is systemically available (see Table 1). Food has no significant effect on the extent of diclofenac absorption. However, there is usually a delay in the onset of absorption of 1 to 4.5 hours and a reduction in peak plasma levels of < 20%.
Table 1. Pharmacokinetic Parameters for Diclofenac PK ParameterNormal Healthy Adults
(20-48 yrs.)Mean Coefficient of Variation (%) Absolute
Bioavailability (%)
[N = 7]55 40 T max (hr)
[N = 56]2.3 69 Oral Clearance (CL/F;
mL/min) [N = 56]582 23 Renal Clearance
(% unchanged drug in
urine) [N = 7]<1 -- Apparent Volume of
Distribution (V/F; L/kg)
[N = 56]1.4 58 Terminal Half-life (hr)
[N = 56]2.3 48 Distribution
The apparent volume of distribution (V/F) of diclofenac sodium is 1.4 L/kg.
Diclofenac is more than 99% bound to human serum proteins, primarily to albumin. Serum protein binding is constant over the concentration range (0.15-105 µg/mL) achieved with recommended doses.
Diclofenac diffuses into and out of the synovial fluid. Diffusion into the joint occurs when plasma levels are higher than those in the synovial fluid, after which the process reverses and synovial fluid levels are higher than plasma levels. It is not known whether diffusion into the joint plays a role in the effectiveness of diclofenac.
Metabolism
Five diclofenac metabolites have been identified in human plasma and urine. The metabolites include 4'-hydroxy-, 5-hydroxy-, 3'-hydroxy-, 4',5-dihydroxy- and 3'-hydroxy-4'-methoxy diclofenac. In patients with renal dysfunction, peak concentrations of metabolites 4'-hydroxy- and 5-hydroxy-diclofenac were approximately 50% and 4% of the parent compound after single oral dosing compared to 27% and 1% in normal healthy subjects. However, diclofenac metabolites undergo further glucuronidation and sulfation followed by biliary excretion.
One diclofenac metabolite 4'-hydroxy-diclofenac has very weak pharmacologic activity.
Excretion
Diclofenac is eliminated through metabolism and subsequent urinary and biliary excretion of the glucuronide and the sulfate conjugates of the metabolites. Little or no free unchanged diclofenac is excreted in the urine. Approximately 65% of the dose is excreted in the urine and approximately 35% in the bile as conjugates of unchanged diclofenac plus metabolites. Because renal elimination is not a significant pathway of elimination for unchanged diclofenac, dosing adjustment in patients with mild to moderate renal dysfunction is not necessary. The terminal half-life of unchanged diclofenac is approximately 2 hours.
Special Populations
Pediatric: The pharmacokinetics of Voltaren has not been investigated in pediatric patients.
Race: Pharmacokinetics differences due to race have not been identified.
Hepatic Insufficiency: Hepatic metabolism accounts for almost 100% of Voltaren elimination, so patients with hepatic disease may require reduced doses of Voltaren compared to patients with normal hepatic function.
Renal Insufficiency: Diclofenac pharmacokinetics has been investigated in subjects with renal insufficiency. No differences in the pharmacokinetics of diclofenac have been detected in studies of patients with renal impairment. In patients with renal impairment (inulin clearance 60-90, 30-60, and <30 mL/min; N=6 in each group), AUC values and elimination rate were comparable to those in healthy subjects.
INDICATIONS AND USAGE
Voltaren ® (diclofenac sodium enteric-coated tablets), is indicated:
- For relief of signs and symptoms of osteoarthritis
- For relief of signs and symptoms of rheumatoid arthritis
- For acute or long-term use in the relief of signs and symptoms of ankylosing spondylitis
CONTRAINDICATIONS
Voltaren ® (diclofenac sodium enteric-coated tablets), is contraindicated in patients with known hypersensitivity to diclofenac. Voltaren should not be given to patients who have experienced asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS - Anaphylactoid Reactions, and PRECAUTIONS - Preexisting Asthma ).
WARNINGS
Gastrointestinal (GI) Effects - Risk of GI Ulceration, Bleeding, and Perforation:
Serious gastrointestinal toxicity such as inflammation, bleeding, ulceration, and perforation of the stomach, small intestine or large intestine, can occur at any time, with or without warning symptoms, in patients treated with nonsteroidal anti-inflammatory drugs (NSAIDs). Minor upper gastrointestinal problems, such as dyspepsia, are common and may also occur at any time during NSAID therapy. Therefore, physicians and patients should remain alert for ulceration and bleeding even in the absence of previous GI tract symptoms. Patients should be informed about the signs and/or symptoms of serious GI toxicity and the steps to take if they occur. The utility of periodic laboratory monitoring has not been demonstrated, nor has it been adequately assessed. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. It has been demonstrated that upper GI ulcers, gross bleeding or perforation, caused by NSAIDs, appear to occur in approximately 1% of patients treated for 3-6 months, and in about 2%-4% of patients treated for one year. These trends continue thus, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore special care should be taken in treating this population. To minimize the potential risk for an adverse GI event, the lowest effective dose should be used for the shortest possible duration. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Studies have shown that patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding and who use NSAIDs, have a greater than 10-fold risk for developing a GI bleed than patients with neither of these risk factors. In addition to a past history of ulcer disease, pharmacoepidemiological studies have identified several other co-therapies or co-morbid conditions that may increase the risk for GI bleeding such as: treatment with oral corticosteroids, treatment with anticoagulants, longer duration of NSAID therapy, smoking, alcoholism, older age, and poor general health status.
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to Voltaren ® (diclofenac sodium enteric-coated tablets). Voltaren should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs. (See CONTRAINDICATIONS and PRECAUTIONS - Preexisting Asthma .) Emergency help should be sought in cases where an anaphylactoid reaction occurs.
Advanced Renal Disease
In cases with advanced kidney disease, treatment with Voltaren is not recommended. If NSAID therapy, however, must be initiated, close monitoring of the patient's kidney function is advisable (see PRECAUTIONS - Renal Effects ).
Pregnancy
In late pregnancy, as with other NSAIDs, Voltaren should be avoided because it may cause premature closure of the ductus arteriosus.
PRECAUTIONS
General
Voltaren ® (diclofenac sodium enteric-coated tablets), cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of Voltaren in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
Hepatic Effects
Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs including Voltaren. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continued therapy. Based on this experience, in patients on chronic treatment with Voltaren, periodic monitoring of transaminases is recommended (see PRECAUTIONS - Laboratory Tests ). Notable elevations of ALT or AST (three or more times the upper limit of normal) have been reported in approximately 2%-4% of patients, including marked elevations (eight or more times the upper limit of normal) in about 1% of patients in clinical trials with diclofenac. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with Voltaren. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), Voltaren should be discontinued.
Renal Effects
Caution should be used when initiating treatment with Voltaren in patients with considerable dehydration. It is advisable to rehydrate patients first and then start therapy with Voltaren. Caution is also recommended in patients with preexisting kidney disease (see WARNINGS - Advanced Renal Disease ).
As with other NSAIDs, long-term administration of diclofenac has resulted in renal papillary necrosis and other renal medullary changes. Renal toxicity has also been seen in patients in which renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a nonsteroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of nonsteroidal anti-inflammatory drug therapy is usually followed by recovery to the pretreatment state.
Voltaren metabolites are eliminated primarily by the kidneys. The extent to which the metabolites may accumulate in patients with renal failure has not been studied. As with other NSAIDs, metabolites of which are excreted by the kidney, patients with significantly impaired renal function should be more closely monitored.
Hematological Effects
Anemia is sometimes seen in patients receiving NSAIDs, including Voltaren. This may be due to fluid retention, GI loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including Voltaren, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
All drugs which inhibit the biosynthesis of prostaglandins may interfere to some extent with platelet function and vascular responses to bleeding.
NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Voltaren does not generally affect platelet counts, prothrombin time (PT), or partial thromboplastin time (PTT). Patients receiving Voltaren who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
Fluid Retention and Edema
Fluid retention and edema have been observed in some patients taking NSAIDs. Therefore, as with other NSAIDs, Voltaren should be used with caution in patients with fluid retention, hypertension, or heart failure.
Preexisting Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross-reactivity, including bronchospasm, between aspirin and other nonsteroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, Voltaren should not be administered to patients with this form of aspirin sensitivity and should be used with caution in all patients with preexisting asthma.
Information for Patients
Voltaren, like other drugs of its class, can cause discomfort and, rarely, more serious side effects, such as gastrointestinal bleeding, which may result in hospitalization and even fatal outcomes. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS - Risk of Gastrointestinal Ulceration, Bleeding and Perforation ).
Patients should report to their physicians' signs or symptoms of gastrointestinal ulceration or bleeding, skin rash, weight gain, or edema.
Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
Patients should also be instructed to seek immediate emergency help in the case of an anaphylactoid reaction (see WARNINGS ).
In late pregnancy, as with other NSAIDs, Voltaren should be avoided because it will cause premature closure of the ductus arteriosus.
Laboratory Tests
Patients on long-term treatment with NSAIDs, should have their CBC and a chemistry profile (including transaminases) checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, Voltaren should be discontinued.
Drug Interactions
Aspirin: When Voltaren is administered with aspirin, its protein binding is reduced. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of diclofenac and aspirin is not generally recommended because of the potential of increased adverse effects.
Methotrexate: NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
Cyclosporine: Voltaren, like other NSAIDs, may affect renal prostaglandins and increase the toxicity of certain drugs. Therefore, concomitant therapy with Voltaren may increase cyclosporine's nephrotoxicity. Caution should be used when Voltaren is administered concomitantly with cyclosporine.
ACE-inhibitors: Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors.
Furosemide: Clinical studies, as well as post-marketing observations, have shown that Voltaren can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see PRECAUTIONS - Renal Effects ), as well as to assure diuretic efficacy.
Lithium: NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
Warfarin: The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
Pregnancy
Teratogenic Effects: Pregnancy Category C
Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women.
Nonteratogenic Effects: Because of the known effects of nonsteroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided.
Labor and Delivery
In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of Voltaren on labor and delivery in pregnant women are unknown.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Voltaren, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
As with any NSAIDs, caution should be exercised in treating the elderly (65 years and older).
ADVERSE REACTIONS
In patients taking Voltaren ® (diclofenac sodium enteric-coated tablets), or other NSAIDs, the most frequently reported adverse experiences occurring in approximately 1%-10% of patients are:
Gastrointestinal experiences including: abdominal pain, constipation, diarrhea, dyspepsia, flatulence, gross bleeding/perforation, heartburn, nausea, GI ulcers (gastric/duodenal) and vomiting.
Abnormal renal function, anemia, dizziness, edema, elevated liver enzymes, headaches, increased bleeding time, pruritus, rashes and tinnitus.
Additional adverse experiences reported occasionally include:
Body as a Whole: fever, infection, sepsis
Cardiovascular System: congestive heart failure, hypertension, tachycardia, syncope
Digestive System: dry mouth, esophagitis, gastric/peptic ulcers, gastritis, gastrointestinal bleeding, glossitis, hematemesis, hepatitis, jaundice
Hemic and Lymphatic System: ecchymosis, eosinophilia, leukopenia, melena, purpura, rectal bleeding, stomatitis, thrombocytopenia
Metabolic and Nutritional: weight changes
Nervous System: anxiety, asthenia, confusion, depression, dream abnormalities, drowsiness, insomnia, malaise, nervousness, paresthesia, somnolence, tremors, vertigo
Respiratory System: asthma, dyspnea
Skin and Appendages: alopecia, photosensitivity, sweating increased
Special Senses: blurred vision
Urogenital System: cystitis, dysuria, hematuria, interstitial nephritis, oliguria/polyuria, proteinuria, renal failure.
Other adverse reactions, which occur rarely are:
Body as a Whole: anaphylactic reactions, appetite changes, death
Cardiovascular System: arrhythmia, hypotension, myocardial infarction, palpitations, vasculitis
Digestive System: colitis, eructation, liver failure, pancreatitis
Hemic and Lymphatic System: agranulocytosis, hemolytic anemia, aplastic anemia, lymphadenopathy, pancytopenia
Metabolic and Nutritional: hyperglycemia
Nervous System: convulsions, coma, hallucinations, meningitis
Respiratory System: respiratory depression, pneumonia
Skin and Appendages: angioedema, toxic epidermal necrolysis, erythema multiforme, exfoliative dermatitis, Stevens-Johnson syndrome, urticaria
Special Senses: conjunctivitis, hearing impairment.
OVERDOSAGE
Symptoms following acute NSAID overdoses are usually limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose.
Patients should be managed by symptomatic and supportive care following a NSAID overdose. There are no specific antidotes. Emesis and/or activated charcoal (60 to 100 g in adults, 1 to 2 g/kg in children) and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose (5 to 10 times the usual dose). Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
DOSAGE AND ADMINISTRATION
As with other NSAIDs, the lowest dose should be sought for each patient. Therefore, after observing the response to initial therapy with Voltaren ® (diclofenac sodium enteric-coated tablets), the dose and frequency should be adjusted to suit an individual patient's needs.
For the relief of osteoarthritis, the recommended dosage is 100-150 mg/day in divided doses (50 mg b.i.d. or t.i.d., or 75 mg b.i.d.).
For the relief of rheumatoid arthritis, the recommended dosage is 150-200 mg/day in divided doses (50 mg t.i.d. or q.i.d., or 75 mg b.i.d.).
For the relief of ankylosing spondylitis, the recommended dosage is 100-125 mg/day, administered as 25 mg q.i.d., with an extra 25-mg dose at bedtime if necessary.
Different formulations of diclofenac [Voltaren ® (diclofenac sodium enteric-coated tablets); Voltaren ® -XR (diclofenac sodium extended-release tablets); Cataflam ® (diclofenac potassium immediate-release tablets)] are not necessarily bioequivalent even if the milligram strength is the same.
HOW SUPPLIED
Voltaren ® Tablets
25 mg - yellow, biconvex, triangular-shaped, enteric-coated tablets (imprinted VOLTAREN 25 on one side in black ink)
Bottles of 100 ......................................................... NDC 0028-0258-01
50 mg - light brown, biconvex, triangular-shaped, enteric-coated tablets (imprinted VOLTAREN 50 on one side in black ink)
Bottles of 100 ......................................................... NDC 0028-0262-01
75 mg - light pink, biconvex, triangular-shaped, enteric-coated tablets (imprinted VOLTAREN 75 on one side in black ink)
Bottles of 100 ......................................................... NDC 0028-0264-01
Do not store above 30°C (86°F). Protect from moisture.
Dispense in tight container (USP).
REV: FEBRUARY 2003 T2003-20
89016302
Distributed by:
Manufactured by:
Mova Pharmaceuticals Corp.
Caguas, Puerto Rico 00726
Novartis Pharmaceuticals Corp.
East Hanover, NJ 07936
Subscribe to the "News" RSS Feed 
TOP ۞
